Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Li Wang | -- | 1318 | 2023-05-09 13:26:22 | | | |
| 2 | Catherine Yang | Meta information modification | 1318 | 2023-05-10 02:08:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, W.; Li, N.; Long, M. YAP/TAZ May Bridge Microgravity and Liver Dysfunction. Encyclopedia. Available online: https://encyclopedia.pub/entry/44039 (accessed on 13 January 2026).
Li W, Li N, Long M. YAP/TAZ May Bridge Microgravity and Liver Dysfunction. Encyclopedia. Available at: https://encyclopedia.pub/entry/44039. Accessed January 13, 2026.
Li, Wang, Ning Li, Mian Long. "YAP/TAZ May Bridge Microgravity and Liver Dysfunction" Encyclopedia, https://encyclopedia.pub/entry/44039 (accessed January 13, 2026).
Li, W., Li, N., & Long, M. (2023, May 09). YAP/TAZ May Bridge Microgravity and Liver Dysfunction. In Encyclopedia. https://encyclopedia.pub/entry/44039
Li, Wang, et al. "YAP/TAZ May Bridge Microgravity and Liver Dysfunction." Encyclopedia. Web. 09 May, 2023.
Copy Citation
Microgravity exposure during spaceflight causes the disordered regulation of liver function, presenting a specialized mechano-biological coupling process. While YAP/TAZ serves as a typical mechanosensitive pathway involved in hepatocyte metabolism, it remains unclear whether and how it is correlated with microgravity-induced liver dysfunction. Whether or not the data in liver functions are derived from infight or ground-based studies, or what types of observations are presented at the organism or cellular level, it is still critical to map out the potential gravity-sensitive signaling pathways from the above functional or phenotypic cues.
microgravity
liver metabolism
mechanosensing
cytoskeleton
nuclear deformation
1. YAP/TAZ Is Essential for Liver Metabolism
Typically, YAP and TAZ are regarded as the effectors of the Hippo cascade of kinases, the core components of which comprise the mammalian STE20-like protein 1/2 (MST1/2) kinase and large tumor suppressor 1/2 (LATS1/2) kinase (Figure 1) [1]. Activated MST1/2 induces the phosphorylation and activation of LATS1/2. In turn, LATS1/2 promotes the phosphorylation of YAP/TAZ, resulting in cytoplasmic retention by binding to 14-3-3 protein and proteasomal degradation [2][3][4]. Conversely, those YAP/TAZ proteins not phosphorylated can enter the nucleus and initiate gene transcriptions, predominantly depending on their interaction with the TEA domain (TEAD) family [5].
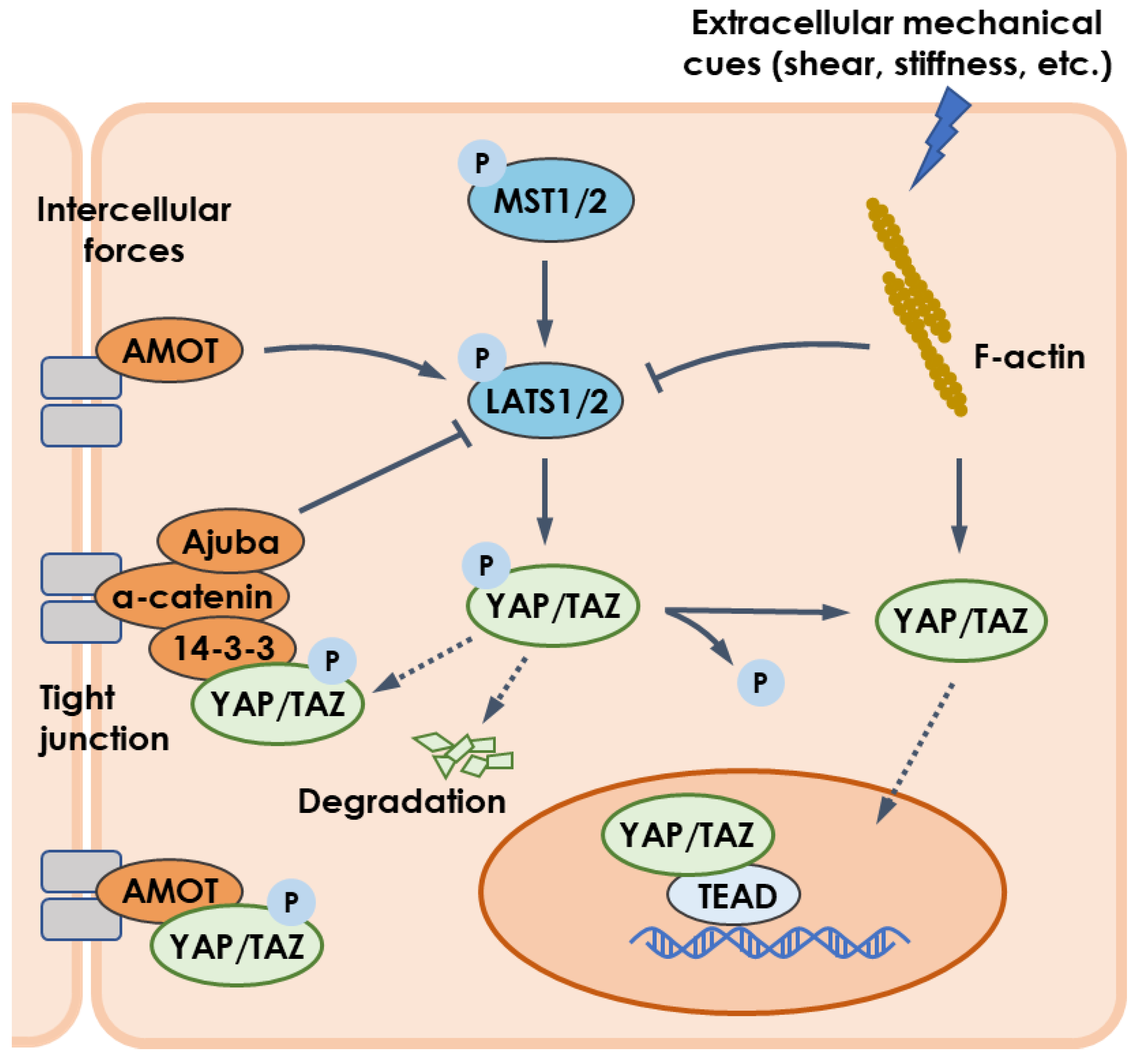
Figure 1. YAP/TAZ pathway regulated by mechanical cues. Intercellular forces enable LATS1/2 to be upregulated by AMOT but inhibited by Ajuba. Mechanical cues such as flow shear and substrate stiffness regulate actin cytoskeleton to activate YAP/TAZ pathway via downregulating LAST1/2 or upregulating YAP/TAZ. Besides, YAP/TAZ is sequestrated in cytoplasm by physical interaction with AMOT. Arrows, blunt ends, and dashed lines indicate activation, inhibition, and translocation, respectively.
Although the main issues of YAP/TAZ signaling concentrate on cell proliferation and differentiation, increasing evidence indicates their key roles in hepatocyte metabolism (Figure 2) [6][7]. For example, YAP knockdown reduced lipid droplets deposition in liver tumor cells induced by anti-PD-1 treatment, suggesting YAP promoted lipid uptake and synthesis [8]. This is in line with a study using the dexamethasone-induced hepatomegaly model, where the pregnane X receptor/YAP pathway was activated together with enhanced uptake of fatty acids and suppressed lipolysis and fatty acid β-oxidation [9]. Furthermore, overexpressed YAP in HCC cells promoted lipid peroxides production by transcriptionally upregulating the arachidonate lipoxygenase 3, which sensitized HCC cells to ferroptosis [10]. Given that YAP inhibition in mammary epithelial cells or prostate cancer cells reduced free fatty acid contents but increased triglyceride and cholesterol levels within cells [11][12], YAP-mediated lipid metabolism may vary with cell types.
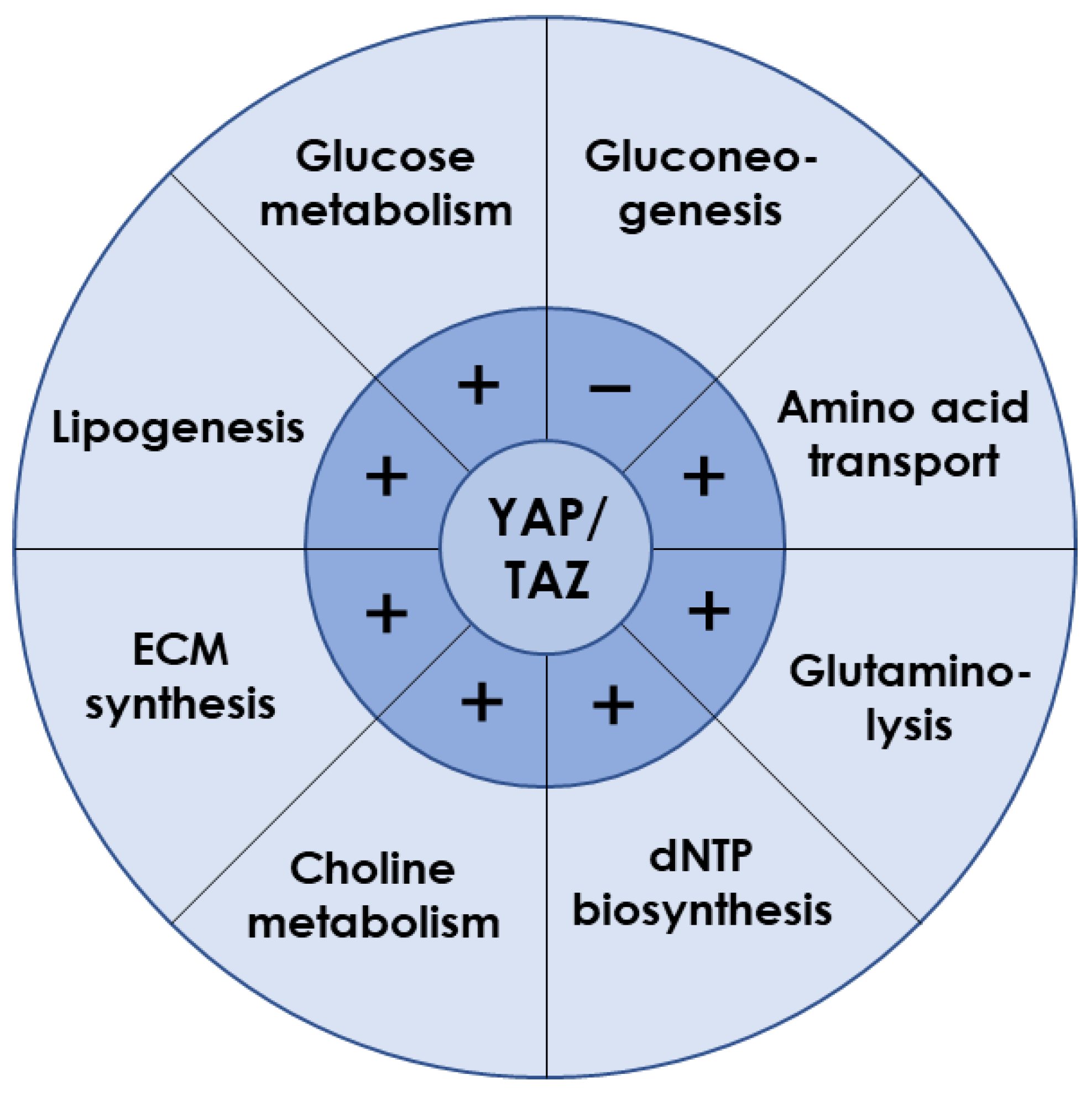
Figure 2. Liver metabolism regulated by YAP/TAZ. YAP/TAZ is required for the expression of key transporters or enzymes involved in various metabolic processes. Those plus and minus signs indicate the positive and negative regulation, respectively.
Glucose metabolism is a major energy source for supporting cellular activities in liver. As the exclusive pathway for initiating glucose metabolism, glycolysis was found to be prevalent in energy-consuming tumor cells, along with frequent YAP nuclear translocation [13]. Indeed, YAP activation increased ATP content, glucose consumption, lactic acid production, extracellular acidification rate, and glycolysis-related enzymes expression [14][15][16], indicating the accelerated glycolytic rate. Hexokinase 2, lactate dehydrogenase A, glucose transporter 1 (GLUT1), and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3/4 (PFKFB3/4) contribute to glucose uptake [17][18]. GLUT1 was identified as the direct transcriptional target of YAP/TEAD1 to promote the glycolysis process [14][19][20]. In addition, the hypoxic microenvironment also helped YAP to be liberated from cytoplasm and stabilized in the nucleus [21][22][23][24]. Glycolysis was enhanced when YAP bound the promoters of pyruvate kinase M2 or PFKFB3 with the assistance of hypoxia-inducible factor 1α or TEAD1, respectively [25][26]. When blood glucose is too low to meet body requirements, gluconeogenesis is initiated by glucagon. In a capping actin protein of a muscle Z-line (CAPZ) liver-specific knockout mouse, YAP hyperactivation was observed to be associated with decreased gene expressions of glucose-6-phosphatase, phosphoenolpyruvate carboxylase 1, and fructose-1,6-bisphosphatase 1, serving as the key genes required for gluconeogenesis [27]. Loss-and-gain functional experiments in mouse primary hepatocytes demonstrated that active YAP inhibited gluconeogenic gene expression induced by glucagon or dexamethasone treatment, through preventing peroxisome proliferator–activated receptor-gamma coactivator 1 from binding to the promoters of gluconeogenic targets [28]. Overall, YAP plays a key role in promoting glucose uptake and consumption while inhibiting gluconeogenesis and, hence, is essential for maintaining glucose homeostasis in the whole organism [29][30].
YAP/TAZ is also required for amino acid metabolism in liver. For instance, the serine/glycine-producing enzymes were increased by YAP overexpression [31]. Activated YAP/TAZ upregulated the amino acid transporters of solute carrier 38A1 (SLC38A1), SLC7A5, and SLC3A2 to supply sources of essential amino acid synthesis [32][33]. Glutamine is the most abundant nonessential amino acid and can be used as the complement of intermediate products in the tricarboxylic acid after deamination by glutaminase and subsequent conversion into α-ketoglutarate [34]. Knockdown of YAP or TAZ could reduce intracellular glutamate concentrations with different mechanisms, where YAP deletion reduced expression of SLC1A5 but not glutaminase, while TAZ deletion decreased both SLC1A5 and glutaminase expression. Experiments in zebrafish indicated that YAP reprogramed glutamine metabolism by upregulating the expression and activity of glutamine synthetase to drive nucleotide biosynthesis [35].
Furthermore, YAP/TAZ participates in other metabolic processes in liver. It directly regulated the expression of key enzymes involved in deoxynucleoside triphosphates (dNTP) biosynthesis required for DNA replication and dNTP precursor pools maintenance, enabling cells to resist chemotherapeutics targeting dNTP synthesis and to avoid oncogene-induced senescence phenotype [36]. In addition, activated YAP promoted choline metabolism by inducing expression of those genes involved in apical extrusion during cell competition [37]. Moreover, YAP overexpression promoted the synthesis of ECM components such as aggrecan, type II collagen, and sulfated glycosaminoglycan [38].
Evidently, YAP/TAZ is known to positively regulate lipogenesis, glucose metabolism, amino acid metabolism, glutaminolysis, dNTP synthesis, choline metabolism, and ECM synthesis, but to negatively regulate gluconeogenesis (Figure 2). Interestingly, liver metabolism after YAP/TAZ inhibition is consistent with those functional changes induced by microgravity. Therefore, it reasonably anticipates that YAP/TAZ may act as a key modulator when it reacts to microgravity stimuli.
2. Microgravity Regulates YAP/TAZ Activation
Increasing knowledge suggests that YAP/TAZ can respond to microgravity, although such responses have been poorly understood in hepatocytes. When cardiovascular progenitor cells (CPCs) were cultured on the International Space Station or with 2D clinostat on the ground, both spaceflight and simulated effects of microgravity were able to induce gene expressions of YAP and its target, superoxide dismutase 2, a marker of cell survival [39]. However, this finding was limited to CPCs derived from adult humans, because the YAP gene was downregulated by spaceflight in neonatal CPCs [40]. In human colorectal cancer cell HCT116, simulated effects of microgravity using RWV promoted YAP nuclear localization, which was correlated with stemness marker expressions of octamer-binding transcription factor 4 (Oct4), SRY-box 2 (Sox2), homeobox protein nanog (Nanog), and NK2 homeobox 5 [41]. On the contrary, YAP nuclear entry was impaired in glioblastoma cells or mesenchymal stem cells (MSCs) when cells were cultured within RPM or clinostat, respectively [42][43]. By utilizing a 2D clinostat, the activation and expression of TAZ were hindered by depolymerizing F-actin, stemmed from the simulated effects of microgravity, which accounted for the inhibited osteogenic differentiation of MSCs [44][45]. Furthermore, human microvascular endothelial cells subjected to hypergravity by centrifuge (4 g for 15 min or 20 g for 15 min~6 h) presented a dose-dependent increase in phosphorylated focal adhesion kinase, YAP, and myosin fibers, as well as improved tube formation, indicating a seemingly opposite response to microgravity in the same cell type [46][47]. Taken together, the YAP/TAZ pathway is positively or negatively regulated by microgravity stimuli in an experimental condition- or cell type-specific manner. It is yet difficult to draw an unambiguous conclusion from those studies since YAP/TAZ activation has not been measured in different cell types upon identical microgravity exposure or in the identical cell type under various microgravity loading modes. Nevertheless, space microgravity is assumed to be sensed by the YAP/TAZ molecule at the cellular level, although the elaborative perception mechanism remains obscure. Further exploration of whether YAP/TAZ is affected and how it is exactly inhibited by microgravity loading in hepatocytes, finally inducing liver dysfunction, is still needed.
References
- Manning, S.A.; Kroeger, B.; Harvey, K.F. The regulation of Yorkie, YAP and TAZ: New insights into the Hippo pathway. Development 2020, 147, dev179069.
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133.
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by LATS and CK1 regulates YAP stability through SCF (beta-TrCP). Genes Dev. 2010, 24, 72–85.
- Liu, C.Y.; Zha, Z.Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF (beta-TrCP) E3 ligase. J. Biol. Chem. 2010, 285, 37159–37169.
- Park, J.; Hansen, C.G. Cellular feedback dynamics and multilevel regulation driven by the hippo pathway. Biochem. Soc. Trans. 2021, 49, 1515–1527.
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018, 28, 196–206.
- Ibar, C.; Irvine, K.D. Integration of Hippo-YAP signaling with metabolism. Dev. Cell 2020, 54, 256–267.
- Hao, L.; Guo, Y.; Peng, Q.; Zhang, Z.; Ji, J.; Liu, Y.; Xue, Y.; Li, C.; Zheng, K.; Shi, X. Dihydroartemisinin reduced lipid droplet deposition by YAP1 to promote the anti-PD-1 effect in hepatocellular carcinoma. Phytomedicine 2022, 96, 153913.
- Jiao, T.; Yao, X.; Zhao, Y.; Zhou, Y.; Gao, Y.; Fan, S.; Chen, P.; Li, X.; Jiang, Y.; Yang, X.; et al. Dexamethasone-induced liver enlargement is related to PXR/YAP activation and lipid accumulation but not hepatocyte proliferation. Drug Metab. Dispos. 2020, 48, 830–839.
- Qin, Y.; Pei, Z.; Feng, Z.; Lin, P.; Wang, S.; Li, Y.; Huo, F.; Wang, Q.; Wang, Z.; Chen, Z.-N.; et al. Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma via ALOXE3-mediated lipid peroxidation accumulation. Front. Cell Dev. Biol. 2021, 9, 751593.
- Chen, Z.; Qiu, H.; Ma, L.; Luo, J.; Sun, S.; Kang, K.; Gou, D.; Loor, J.J. miR-30e-5p and miR-15a synergistically regulate fatty acid metabolism in goat mammary epithelial cells via LRP6 and YAP1. Int. J. Mol. Sci. 2016, 17, 1909.
- Lee, H.-C.; Ou, C.-H.; Huang, Y.-C.; Hou, P.-C.; Creighton, C.J.; Lin, Y.-S.; Hu, C.-Y.; Lin, S.-C. YAP1 overexpression contributes to the development of enzalutamide resistance by induction of cancer stemness and lipid metabolism in prostate cancer. Oncogene 2021, 40, 2407–2421.
- Li, M.; Gao, Z.; Ding, H.; Wang, Z.; Mu, H.; Zhang, L.; Wei, J.; Ma, Z. FSCN1 promotes glycolysis and epithelial-mesenchymal transition in prostate cancer through a YAP/TAZ signaling pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 6245647.
- Li, H.; Fu, L.; Lin, B.; Lin, X.; Dong, Q.; Wang, E. Ajuba overexpression regulates mitochondrial potential and glucose uptake through YAP/Bcl-xL/GLUT1 in human gastric cancer. Gene 2019, 693, 16–24.
- Yu, T.; Liu, Y.; Xue, J.; Sun, X.; Zhu, D.; Ma, L.; Guo, Y.; Jin, T.; Cao, H.; Chen, Y.; et al. Gankyrin modulated non-small cell lung cancer progression via glycolysis metabolism in a YAP1-dependent manner. Cell Death Discov. 2022, 8, 312.
- Shen, Y.; Zhao, S.; Wang, S.; Pan, X.; Zhang, Y.; Xu, J.; Jiang, Y.; Li, H.; Zhang, Q.; Gao, J.; et al. S1P/S1PR3 axis promotes aerobic glycolysis by YAP/c-MYC/PGAM1 axis in osteosarcoma. Ebiomedicine 2019, 40, 210–223.
- Liu, Q.P.; Luo, Q.; Deng, B.; Ju, Y.; Song, G.B. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling. Cancers 2020, 12, 490.
- Li, Y.; Yang, S.; Liu, Y.; Yang, S. Deletion of Trp53 and Rb1 in Ctsk-expressing cells drives osteosarcoma progression by activating glucose metabolism and YAP signaling. MedComm 2022, 3, e131.
- Wang, L.; Sun, J.; Gao, P.; Su, K.; Wu, H.; Li, J.; Lou, W. Wnt1-inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. J. Cell. Physiol. 2019, 234, 15941–15950.
- Kashihara, T.; Mukai, R.; Oka, S.-i.; Zhai, P.; Nakada, Y.; Yang, Z.; Mizushima, W.; Nakahara, T.; Warren, J.S.; Abdellatif, M.; et al. YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J. Clin. Investig. 2022, 132, e150595.
- Sun, Z.; Zhang, Q.; Yuan, W.; Li, X.; Chen, C.; Guo, Y.; Shao, B.; Dang, Q.; Zhou, Q.; Wang, Q.; et al. MiR-103a-3p promotes tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J. Exp. Clin. Cancer Res. 2020, 39, 250.
- Wang, Y.; Liu, S. LncRNA GHET1 promotes hypoxia-induced glycolysis, proliferation, and invasion in triple-negative breast cancer through the Hippo/YAP signaling pathway. Front. Cell Dev. Biol. 2021, 9, 643515.
- Jia, Y.; Li, H.-Y.; Wang, J.; Wang, Y.; Zhang, P.; Ma, N.; Mo, S.-J. Phosphorylation of 14-3-3 zeta links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis 2019, 8, 31.
- Jia, Y.; Li, H.-Y.; Wang, Y.; Wang, J.; Zhu, J.-W.; Wei, Y.-Y.; Lou, L.; Chen, X.; Mo, S.-J. Crosstalk between hypoxia-sensing ULK1/2 and YAP-driven glycolysis fuels pancreatic ductal adenocarcinoma development. Int. J. Biol. Sci. 2021, 17, 2772–2794.
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1 alpha and sustains HIF-1 alpha protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 216.
- Feng, Y.; Zou, R.; Zhang, X.; Shen, M.; Chen, X.; Wang, J.; Niu, W.; Yuan, Y.; Yuan, F. YAP promotes ocular neovascularization by modifying PFKFB3-driven endothelial glycolysis. Angiogenesis 2021, 24, 489–504.
- Pocaterra, A.; Santinon, G.; Romani, P.; Brian, I.; Dimitracopoulos, A.; Ghisleni, A.; Carnicer-Lombarte, A.; Forcato, M.; Braghetta, P.; Montagner, M.; et al. F-actin dynamics regulates mammalian organ growth and cell fate maintenance. J. Hepatol. 2019, 71, 130–142.
- Hu, Y.; Shin, D.J.; Pan, H.; Lin, Z.; Dreyfuss, J.M.; Camargo, F.D.; Miao, J.; Biddinger, S.B. YAP suppresses gluconeogenic gene expression through PGC1α. Hepatology 2017, 66, 2029–2041.
- Han, D.J.; Aslam, R.; Misra, P.; Chiu, F.; Ojha, T.; Chowdhury, A.; Chan, C.K.; Sung, H.-K.; Yuen, D.A.; Luk, C.T. Disruption of adipocyte YAP improves glucose homeostasis in mice and decreases adipose tissue fibrosis. Mol. Metab. 2022, 66, 101594.
- Gao, Y.; Ma, K.; Kang, Y.; Liu, W.; Liu, X.; Long, X.; Hayashi, T.; Hattori, S.; Mizuno, K.; Fujisaki, H.; et al. Type I collagen reduces lipid accumulation during adipogenesis of preadipocytes 3T3-L1 via the YAP-mTOR-autophagy axis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159181.
- Duan, L.-M.; Liu, J.-Y.; Yu, C.-W.; Fan, J.-X.; Li, T.; Yang, J.-X.; Zheng, Y.-B.; Liu, F.-C.; He, Z.-T.; Yuan, H.-L.; et al. PLC epsilon knockdown prevents serine/glycine metabolism and proliferation of prostate cancer by suppressing YAP. Am. J. Cancer Res. 2020, 10, 196–210.
- Wu, Q.; Li, J.; Sun, S.; Chen, X.; Zhang, H.; Li, B.; Sun, S. YAP/TAZ-mediated activation of serine metabolism and methylation regulation is critical for LKB1-deficient breast cancer progression. Biosci. Rep. 2017, 37, BSR20171072.
- Jeon, H.Y.; Choi, J.; Kraaier, L.; Kim, Y.H.; Eisenbarth, D.; Yi, K.; Kang, J.-G.; Kim, J.W.; Shim, H.S.; Lee, J.-H.; et al. Airway secretory cell fate conversion via YAP-mTORC1-dependent essential amino acid metabolism. EMBO J. 2022, 41, e109365.
- Edwards, D.N.; Ngwa, V.M.; Wang, S.; Shiuan, E.; Brantley-Sieders, D.M.; Kim, L.C.; Reynolds, A.B.; Chen, J. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 2017, 10, eaan4667.
- Cox, A.G.; Hwang, K.L.; Brown, K.K.; Evason, K.J.; Beltz, S.; Tsomides, A.; O’Connor, K.; Galli, G.G.; Yimlamai, D.; Chhangawala, S.; et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat. Cell Biol. 2016, 18, 886–896.
- Santinon, G.; Brian, I.; Pocaterra, A.; Romani, P.; Franzolin, E.; Rampazzo, C.; Bicciato, S.; Dupont, S. dNTP metabolism links mechanical cues and YAP/TAZ to cell growth and oncogene-induced senescence. EMBO J. 2018, 37, e97780.
- Sunaga, S.; Kofuji, S.; Nishina, H. YAP drives cell competition by activating choline metabolism. Biochem. Biophys. Res. Commun. 2021, 572, 178–184.
- Li, S.; Huang, Z.; Zhu, Y.; Yan, J.; Li, J.; Chen, J.; Zhou, J.; Zhang, Y.; Chen, W.; Xu, K.; et al. Bromodomain-containing protein 7 regulates matrix metabolism and apoptosis in human nucleus pulposus cells through the BRD7-PI3K-YAP1 signaling axis. Exp. Cell Res. 2021, 405, 112658.
- Camberos, V.; Baio, J.; Bailey, L.; Hasaniya, N.; Lopez, L.V.; Kearns-Jonker, M. Effects of spaceflight and simulated microgravity on YAP1 expression in cardiovascular progenitors: Implications for cell-based repair. Int. J. Mol. Sci. 2019, 20, 2742.
- Baio, J.; Martinez, A.F.; Silva, I.; Hoehn, C.V.; Countryman, S.; Bailey, L.; Hasaniya, N.; Pecaut, M.J.; Kearns-Jonker, M. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. NPJ Microgravity 2018, 4, 13.
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684.
- Silvani, G.; Bradbury, P.; Basirun, C.; Mehner, C.; Zalli, D.; Poole, K.; Chou, J. Testing 3D printed biological platform for advancing simulated microgravity and space mechanobiology research. NPJ Microgravity 2022, 8, 19.
- Thompson, M.; Woods, K.; Newberg, J.; Oxford, J.T.; Uzer, G. Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity. NPJ Microgravity 2020, 6, 35.
- Chen, Z.; Luo, Q.; Lin, C.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 2015, 468, 21–26.
- Chen, Z.; Luo, Q.; Lin, C.; Kuang, D.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci. Rep. 2016, 6, 30322.
- De Cesari, C.; Barravecchia, I.; Pyankova, O.V.; Vezza, M.; Germani, M.M.; Scebba, F.; van Loon, J.J.W.A.; Angeloni, D. Hypergravity activates a pro-angiogenic homeostatic response by human capillary endothelial cells. Int. J. Mol. Sci. 2020, 21, 2354.
- Balsamo, M.; Barravecchia, I.; Mariotti, S.; Merenda, A.; De Cesari, C.; Vukich, M.; Angeloni, D. Molecular and cellular characterization of space flight effects on microvascular endothelial cell function—Preparatory work for the SFEF project. Microgravity Sci. Technol. 2014, 26, 351–363.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
10 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




