Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vikas Jain | -- | 3151 | 2023-05-09 11:04:31 | | | |
| 2 | Camila Xu | Meta information modification | 3151 | 2023-05-10 02:58:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/44026 (accessed on 07 February 2026).
Kumar RM, Kumar H, Bhatt T, Jain R, Panchal K, Chaurasiya A, et al. Fisetin in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/44026. Accessed February 07, 2026.
Kumar, Rachna M., Hitesh Kumar, Tanvi Bhatt, Rupshee Jain, Kanan Panchal, Akash Chaurasiya, Vikas Jain. "Fisetin in Cancer" Encyclopedia, https://encyclopedia.pub/entry/44026 (accessed February 07, 2026).
Kumar, R.M., Kumar, H., Bhatt, T., Jain, R., Panchal, K., Chaurasiya, A., & Jain, V. (2023, May 09). Fisetin in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/44026
Kumar, Rachna M., et al. "Fisetin in Cancer." Encyclopedia. Web. 09 May, 2023.
Copy Citation
Fisetin is a flavonoid naturally occurring in various plants that possesses anticancer activity. It has the power to stop cancers from growing quickly, becoming invasive, and spreading to multiple tissues.
cancer
fisetin
flavonoids
nanocarrier
phytoconstituent
1. Introduction
Cancer is one of the most challenging diseases that has a major global health impact. In 2020, there were 19.3 million new instances of cancer and 10 million cancer-related deaths, worldwide [1]. The most common type of cancer in both men and women is lung cancer, which is followed by breast, prostate, and colorectal cancer, in terms of incidence; and colorectal, stomach, and liver cancer in terms of death [2].
A series of genetic changes that affect biological functions, such as cell division, proliferation, transcription, and gene expression, lead to cancer. Cancer cells become somewhat self-sufficient, which causes uncontrollable cell proliferation and division, resulting in the spread of malignant cells throughout the body. Cancer cells continue to divide actively because they lack the ability to influence a cell’s homeostatic system. Instead, they produce oncogenic proteins that mimic the growth signals seen in healthy cells [3]. Carcinogenesis is the process by which healthy cells transform into self-sufficient cancer cells. These processes may take place at the genome, epigenome, or cellular levels, among other levels [4].
Oncogenic transition causes malignant cells to become independent of growth cues, resulting in uncontrolled growth. As a result, they generate their own signals and transmit them via a process known as the signal transduction pathway to other signaling proteins [5]. The intracellular signaling molecules, receptors, and extracellular growth signals, all exhibit significant alterations. The proto-oncogenes that produce proteins to promote cell division along with signaling molecules promote the expression of oncogenes, and encode for multiple factors that promote tumor progression [6][7].
Numerous studies have shown that flavonoids can prevent the development of cancer [8][9]. Flavonoids are secondary metabolites classified under the group of polyphenolic compounds. These naturally existing compounds are commonly dispersed in a plant’s leaf, stem, and root [10][11]. They exhibit remarkable outcomes in the human body, including anti-allergic, antioxidant, anti-inflammatory, and antiviral activities [12][13]. Moreover, flavonoids have been found to inhibit the proliferation, growth, and metastasis of breast cancer in vitro as well as in animal models [14][15]. Its use as an anti-carcinogenic agent is attributed to its antioxidant and anti-inflammatory properties [16]. Flavonoids interfere with multiple signal transduction pathways that occur during carcinogenesis, thereby reducing proliferation, angiogenesis, and metastasis, or increasing apoptosis, making them potential anticancer agents. However, its dose-dependent pharmacokinetics and low-dose potency contribute to some therapeutic obstacles [17][18]. Based on their structure, flavonoids are classified into six categories, viz., flavonol, flavanone, flavanol, flavone, anthocyanidin, and isoflavonoid. Quercetin, kaempferol, fisetin, myricetin, galangin, and casticin are flavonols with anticancer properties (Figure 1).
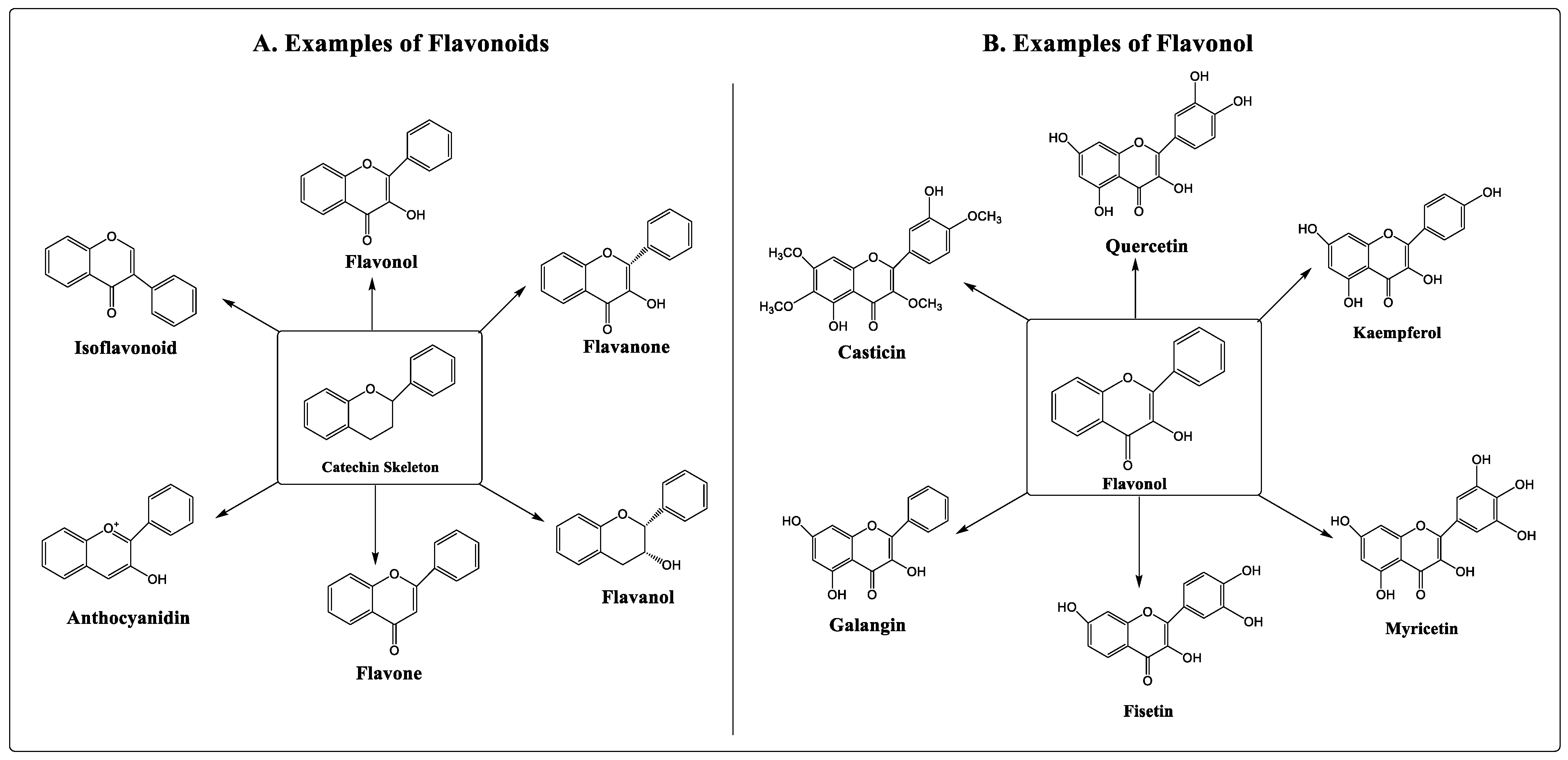
Figure 1. Types and examples of flavonoids (A) and flavonols (B).
Fisetin is a flavonoid naturally occurring in various plants that possesses anticancer activity [10][19]. It has the power to stop cancers from growing quickly, becoming invasive, and spreading to multiple tissues. Similar outcomes of fisetin were reported in various preclinical studies with melanoma, and with pancreatic, prostate, and colorectal cancer [20][21][22]. Fisetin chemically is a 3,3′,4′,7-tetrahydroxyflavone, and offers multiple pharmacological benefits, which include antioxidant, anti-inflammatory, antiangiogenic, and anticancer activities (Figure 2) [23][24][25][26]. Moreover, fisetin also inhibits tumor proliferation by repressing tumor mass multiplication, invasion, migration, and autophagy. It also promotes cell cycle averting and cell death in many types of cancers, which include prostate, breast, lung, bladder, melanoma, and hepatocellular cancers, and nasopharyngeal carcinoma [27][28][29].
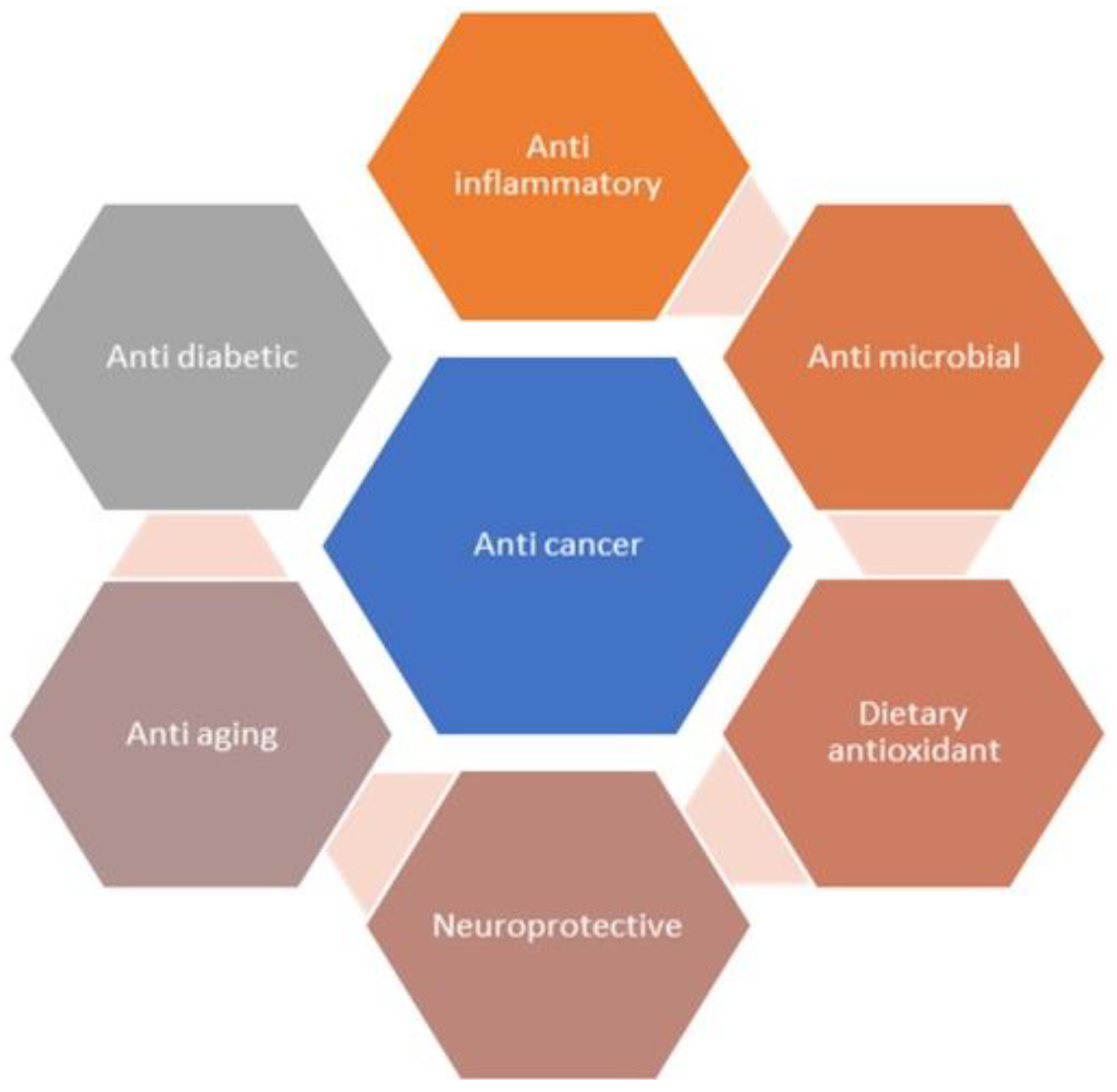
Figure 2. Pharmacological effects of fisetin.
2. Physicochemical Properties and Synthesis of Fisetin
Fisetin has a structure of diphenylpropane, containing two aromatic rings. Its molecular formula is C15H10O6, and its molecular mass is 286.239 g/mol. With a density of 1.688 g/mL, fisetin melts at 330 °C, indicating its crystalline nature. As a result of this property, it exhibits lower solubility in water and a positive log P of 0.151 mg/mL and 1.81, respectively [30]. It is essentially insoluble in benzene, chloroform, ether, and petroleum ether, in addition to water. However, it is soluble in organic solvents such as alcohol, acetone, acetic acid, DMF, and DMSO [31]. Quantitatively, the solubilities of fisetin in ethanol, DMSO, and DMF are approximately 5 mg/mL and 30 mg/mL, respectively [31]. The strong acidic and basic pKa values of fisetin are 6.32 and −3.9, respectively [30][32]. Thus, it also exhibits solubility in a solution of fixed alkali hydroxide [31]. The physicochemical properties of fisetin are summarized in Table 1. The concentration of fisetin in plant sources is measured by freeze-drying the plants, which is the acid hydrolyzed product of the parent glycosides. The daily uptake of fisetin was calculated to be an average of 0.4 milligrams. The highest amount of fisetin detected in strawberries was found to be approximately 160 µg/g, followed by apple and persimmon, which were approximately 26.9 µg/g and 10.5 µg/g, respectively [33].
Table 1. Physicochemical properties of fisetin.
| Characteristics | Description |
|---|---|
| Occurrence | Cucumber, Apple, Strawberry, Grape, Persimmon, Onion |
| Chemical class | Flavonoid |
| IUPAC | 2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one |
| Chemical formula | C15H10O6 |
| Molecular mass | 286.24 g/mol |
| Melting point | 330.0 °C |
| Density | 1.688 g/mL |
| Solubility | Soluble in ethanol, acetone, acetic acid, DMSO, solutions of fixed alkali hydroxides, methanol |
| BCS class | Class II (Low soluble and highly permeable) |
| Appearance | Crystalline powder |
| Log P | 3.2 |
| pKa | 7.42 |
| Chemical metabolites | Geraldol (3,4′,7-trihydroxy-3′-methoxyflavone), Fisetin-4′-glucoside |
| Stability | Stability ≥ 4 years at −20 °C |
Fisetin is available naturally in various plant sources; however, it was first extracted from venetian sumach (Rhus cotinus L.) in 1833. Fisetin is also extracted from strawberries and mulberry leaves, using methanol extraction followed by liquid-liquid extraction [34][35]. The extract of persimmon fruit also contains fisetin as one of its active constituents, which was extracted using a number of methods, and quantified using liquid chromatography [36].
Later, the chemical characteristics and structure of fisetin were elucidated by S. Kostanecki in 1890s. The first synthesis of fisetin was performed in 1904, which involved the preparation of partially protected chalcones, further cyclized to flavanone under acidic conditions. The stable structure of fisetin was synthesized through several steps, such as oxidation, hydrolysis, and demethylations of chalcone and flavanone counterparts [37][38].
3. Pharmacokinetics and Bioavailability of Fisetin
Fisetin exhibits a very short terminal half-life of approximately 3 hrs in its free form. This half-life is found to be less than that of its metabolites [39]. Fisetin and its metabolites were tested in rats, and their effects on hemolysis brought on by 2,2’-azobis (2-amidinopropane hydrochloride) (AAPH) were compared. The mean concentration–time profiles of metabolites in serum rapidly decreased with fisetin, at a dose of 10 mg/kg (intravenously). Higher concentrations of sulfates/glucuronides were present at all time points than the parent compounds, indicating liver-biotransformed fisetin by conjugation metabolism (sulfation). The level of fisetin was maintained on oral dosage (50 mg/kg weight of the body) after the first pass due to the existence of the parent component in serum. Fisetin was converted to sulphates and glucuronides, whereas enterocytes underwent sulfation less frequently than hepatocytes. Following treatment with 50 mg/kg of fisetin, the Cmax and AUC0-4320min (area under serum concentration–time curve 0 to 4320 min) values of the 5-OH-flavone sulfate/glucuronide were 27 and 59 times greater, respectively, than those of the 5-OH-flavone after 40 milligrams/kg of the body weight of 5-OH-flavone. The AUC0-4320 min of 7-OH-flavone sulfate/glucuronide was found to be significantly lower than that of 5-OH-flavone sulfate/glucuronide after an equivalent dosage. Fisetin and its serum metabolites prevented hemolysis brought on by AAPH, showing that the residual phenolic groups’ post-conjugation metabolism is responsible for their scavenging free-radical actions [40]. Following intraperitoneal delivery of the drug to mice at a dose of 223 mg/kg, the Cmax reached 2.5 µg/mL in 15 min. There was a biphasic decline in plasma concentration, with a short half-life of 0.09 h and a terminal half-life of 3.1 h.
The bioavailability of fisetin was enhanced by employing several formulation approaches, the majority of which are based on the application of nanotechnology. For instance, fisetin-loaded nanocochleates improved drug bioavailability up to 141 times following sustained release of the drug from the prepared complex [41]. Additionally, the drug solubility was also improved by 6.5-fold by complexation with cyclodextrin [42]. The fisetin-loaded liposomal system improved the drug bioavailability 47 times after intraperitoneal injection. At a dose of 21 mg/kg, liposomal fisetin inhibited tumor growth more than two-fold compared to pure drug alone [43]. Another approach to enhance the bioavailability and solubility of fisetin is to prepare crystalline nanosuspensions using Eudragit®EPO, stabilizers, surfactants, and polymers [44].
4. Novel Formulation Strategies and Drug Delivery System of Fisetin
Fisetin, being a phytopharmaceutical, has an advantage over synthetic drugs due to its safety profile and biocompatibility. Fisetin may be considered a prime candidate for use as an effective anticancer agent, due to its ability to affect various signaling pathways. Unfortunately, poor targeting and stability issues due to its undesirable hydrophobic nature and extremely poor aqueous solubility (<1 mg/mL) make it challenging to administer intravenously, leading to compromised bioavailability and limiting its use. To address this issue and overcome the hurdles related to drug delivery, it is crucial to develop novel delivery strategies to increase bioavailability and eventually increase the therapeutic outcome. Intensive research has been carried out to develop drug carriers for flavonoids. The use of biodegradable and biocompatible polymers in nanotechnology-based delivery systems helps overcome these challenges.
4.1. Complexation
Complexation improves the physicochemical stability, dissolution rate, solubility, and bioavailability of poor water-soluble drugs [45]. Cyclodextrins are highly versatile oligosaccharides that are widely used as pharmaceutical excipients for this purpose. Cyclodextrin derivatives can help substances with poor water solubility become more soluble. Additionally, P-glycoprotein (P-GP), which is in charge of drug efflux, and cytochrome P450, which is in charge of drug metabolism and improves oral bioavailability, are inhibited by cyclodextrins [46]. It has been discovered that making a fisetin-hydroxyl propyl beta-cyclodextrin (HPbCD) inclusion complex (FHIC) increases fisetin’s solubility and, consequently, its bioavailability [47].
In another study, fisetin was complexed with three types of cyclodextrin to improve solubility. The researcher found better solubility when fisetin was complexed with sulfobutylethere-b-cyclodextrin. Furthermore, the addition of 20% v/v ethanolic solution enhanced the solubilization of fisetin by 5.9 times, compared to water alone [48]. Similarly, the complexation of the fisetin and cyclosophoraose dimer improved the drug solubility by 6.5 times. The solubility of dimer was 2.4 times more compared to its b-cyclodextrin complex. The dimer used for the complexation showed higher cytotoxicity of fisetin than pure fisetin in Hela cells [42].
4.2. Self-Nanoemulsifying Drug Delivery System (SNEDDS)
SNEDDS is an isotropic anhydrous mixture of oils, surfactants (HLB>12), and cosurfactants. This system not only improves the solubility and bioavailability of the active ingredient, but also provides better stability, processing control, and reproducibility. Moreover, it offers a lower production budget with enhanced patient compliance [48]. SNEDDS performs a dual action of increasing molecule solubility and providing protection to the gastrointestinal tract. In a study, the drug was incorporated into a nanoemulsion to enhance fisetin’s therapeutic and pharmacokinetic profile. No significant difference compared to free fisetin seemed to appear upon systemic exposure in mice after intravenous administration. However, upon intraperitoneal administration, fisetin exhibited 24 times higher bioavailability than free fisetin-treated mice at lower doses [49]. Similarly, the SNEDDS consisting of castor oil, lauroglycol, Tween 80, and transcutol were made to enhance the drug solubility of fisetin. The in vitro cell line results suggested that the fisetin-loaded SNEDDS had 3.79-fold higher cellular permeation than the free drug [50].
4.3. Lipid Vesicles
Liposomes have been found to be useful in increasing the accumulation of fisetin within tumors. A study conducted in vivo on mice revealed that the bioavailability of liposomal fisetin was 47 times greater than that of free fisetin [41]. Other vesicular carrier systems that have been explored for the delivery of fisetin are ethosomes and glycerosomes. Both of these are phospholipidic vesicles with high bilayer fluidity used for dermal and transdermal drug delivery [51][52]. Glycerosomes loaded with the drug fisetin displayed added benefits, such as enhanced penetration of the drug into deeper layers of the skin due to glycerin, resulting in lipid fluidization and hydration of the skin. Hence, it is primarily used for dermal applications of fisetin [44]. In vivo studies inferred that liposomes could remain stable for 59 days, retaining their antitumor activity in different tumors and endothelial cell lines [53]. Drug-loaded binary ethosomes were applied to the skin for the treatment of skin cancer. They showed sustained release behavior and improved penetration into the skin of rhodamine B-loaded endosome formulation, which was an added advantage. In vivo studies showed increased Cskin-max and AUC0-8h (area under serum concentration–time curve 0 to 8 h) compared to conventional gel. It also showed a decrease in TNF-α and IL-α in mice pretreated with binary endosomes compared to mice exposed to UV only [54]. Furthermore, Mohapatra et al. (2011) investigated whether fisetin could be an effective fluorescent probe for lipid membranes. The fisetin was bound to the sensing lipid bilayer membrane and used as membrane expulsive target to enhance the antioxidant activity [55].
4.4. Lipid-Based Nanoparticles
Lipid-based nanocarriers, such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and nano-emulsions, are being utilized for the delivery and targeting of highly lipophilic drugs, including fisetin [56]. Kulbacka et al. (2016) reported in their study that they prepared multifunctional SLNs loaded with cyanine-type IR-780 as a photosensitizer/diagnostic agent, along with fisetin or baicalein, to explore the potential of combination therapy in cancer eradication. They stated that delivery of drugs through these carriers was precise and depicted tumor growth inhibition with lower toxicity [57]. The glycerol monostearate-, sodium deoxycholate- and sodium cholate-based SLNs loaded with fisetin improved the photophysical properties of the drug, and were photostable at room temperature. The fisetin did not show any polymorphic transformation during storage when loaded in a lipid-based nanocarrier [58]. Similarly, the fisetin-loaded nano-emulsion was prepared using miglyol 812 N, lipoid E 80, labrasol, Tween 80, and water. No significant changes were observed in the pharmacokinetic profile of the fisetin-loaded nano-emulsion after IV injection (13 mg/kg) compared to that of pure drug. However, intraperitoneal administration improved the bioavailability of the drug by 24-fold. The antitumor activity of the fisetin-loaded nanoemulsion was shown at a concentration of 36.6 mg/kg, which was far lower than that of free drug (223 mg/kg), against a Lewis lung carcinoma model in mice. The results demonstrated that solubilization of fisetin was improved by the nano-emulsion, and it depicted enhanced antitumor activity [49].
4.5. Polymeric Micelles and Nanoparticles
Polymeric micelles are formed by amphiphilic block copolymers, which possess nanoscopic core/shell structures [59]. These systems are used to entrap drugs, resulting in increased anticancer activity against ovarian carcinoma by destroying the tumor mass and inhibiting further multiplication of cells [60]. Fisetin-loaded albumin nanoparticles were prepared using the desolvation method. It displayed advantages such as improved bioavailability, good entrapment, and delivery to a specific target site [61].
In another study, fisetin-loaded polymeric micelles composed of TPGS-PLA exhibited dose-dependent cytotoxicity against MCF-7 cells. The delivery of fisetin through polymeric micelles enhanced the cytotoxic effect in breast cancer cell lines, and induced 42% cell apoptosis at 48 h compared with free fisetin, which showed only 30% cell apoptosis at a similar time. Moreover, it reduced the tumor burden in mice, induced cell apoptosis, and reduced the tumor mass (which tumor) [62].
Similarly, monomethyl poly(ethylene glycol)-poly(ε-caprolactone) polymeric micelles loaded with fisetin were evaluated for anticancer efficacy against ovarian cancer, and showed induced cell apoptosis in a dose-dependent manner in SKOV3 cells. The fisetin-loaded micelles exhibited reduced tumor growth, enhanced tumor apoptosis, and angiogenesis inhibition [63].
The delivery of fisetin also shows promising results with polymeric nanoparticles. The poly(lactic acid) nanoparticle (PLA-NP)-based fisetin formulation was found to enhance fisetin solubility and therapeutic efficacy against HCT116 colon cancer cells in vitro and xenograft 4T1 breast cancer in vivo [64]. Similarly, human serum albumin-based nanoparticles (HSA-NPs) were also developed, with an aim to improve the bioavailability of fisetin. The potent antioxidant effects of fisetin-loaded HSA-NPs were confirmed by the DPPH assay, and the results demonstrated the capabilities associated with the developed system, to deliver fisetin efficiently [65].
Furthermore, polymeric nanoparticles made by poly-(ε-caprolactone) (PCL) and PLGA-PEG-COOH that were loaded with fisetin depicted the controlled release of the drug in simulated gastric as well as intestinal conditions. The nanocarriers were prepared with the aim of delivering fisetin for antioxidant as well as antihyperglycemic effects, and to observe the stability of encapsulated fisetin. The process for developing nanoparticles was efficient enough to retain the DPPH and ABTS scavenging capacity, as well as α-glucosidase inhibition activity [26]. In another study, poly(vinyl pyrrolidone) polymeric nanoparticles (PVP-NPs) processed through a supercritical antisolvent (SAS) method improved the hydrophilicity of fisetin. As a result, the anticancer efficacy, pharmacokinetics, and bioavailability of the fisetin was improved [66].6. Toxicity and Clinical Status of Fisetin
Potential chemotherapeutic drugs kill cancerous as well as healthy cells, and demonstrate undesired side effects. To minimize the side effects and improve the therapeutic outcomes of cancer therapy, plant-based nutritional supplements are currently being explored. Flavonoids offer great potential to eliminate cancer cells and provide protection to healthy cells via antioxidant properties. Recently, fisetin has been used against several types of cancers, and exhibited much fewer side effects than other chemotherapeutic agents [67]. Fisetin is an ingredient available in common plant-based foods, and has reported no adverse effects. Irrespective of the benefits bestowed by fisetin to treat breast cancer, thorough scrutiny of its toxicity is needed, as it requires a high dose to offer therapeutic benefits. Despite numerous scientific interventions performed on animals, no severe toxicological evidence has been observed, even at higher drug levels.
Fisetin-related clinical trials are limited in cancer therapy. As per the NIH-clinical trials database, only two studies are listed. In one of the phase 2 studies, the researchers are investigating the effect of fisetin to improve the physical function in postmenopausal women after receiving chemotherapy for stage I–III breast cancer [68]. In another study, the efficacy, safety and tolerability of the two different senolytic therapies, which include fisetin, and dasatinib plus quercetin, are being investigated in adult survivors of childhood cancer under phase 2 [69]. However, the level of safety should also be evaluated by conducting clinical trials. The major disadvantage of fisetin is that its aqueous solubility could be addressed by converting the drug polymer complex system by synthesizing a nanocarrier system. Several studies have shown that the drug’s solubility, bioavailability and dose, along with its therapeutic efficacy, were improved without any side effects. One such clinical trial on cancer patients reported that the fisetin treatment group experienced stomach discomfort [13]. It has also been observed that fisetin lowers the blood glucose level in diabetic animals, which implies a further reduction in blood glucose levels when co-administered with antihyperglycemic drugs [70][71]. Additionally, metabolism in the liver, as both warfarin and fisetin are processed in the same way, may result in an increased effect of warfarin over time [72].
5. Patents Related to Fisetin
Fisetin patents have primarily described its various preparation techniques and effective treatments. Primarily reported methods of fisetin preparation are based on extraction from microbial sources, and conversion of fisetin from fustin [73]. Several patents have exemplified its application in the treatment of prostate cancer, senile dementia, uterine myoma, acute pancreatitis, depression, neurodegenerative diseases, gastritis and gastric ulcer, and as an antihypertensive [74]. Additionally, fisetin application for skin disorders, such as skin regeneration, anti-aging effects, prevention of hair loss, and stimulation of hair growth, have also been reported.
References
- World Health Organization. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020; International Agency for Research on Cancer: Lyon, France, 2020; pp. 13–15.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218.
- Cao, Y. Tumorigenesis as a Process of Gradual Loss of Original Cell Identity and Gain of Properties of Neural Precursor/Progenitor Cells. Cell Biosci. 2017, 7, 61.
- Lindsey, S.; Langhans, S.A. Crosstalk of Oncogenic Signaling Pathways during Epithelial-Mesenchymal Transition. Front. Oncol. 2014, 4, 358.
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015.
- Korgaonkar, N.; Yadav, K.S. Understanding the Biology and Advent of Physics of Cancer with Perspicacity in Current Treatment Therapy. Life Sci. 2019, 239, 117060.
- Sak, K. Cytotoxicity of Dietary Flavonoids on Different Human Cancer Types. Pharmacogn. Rev. 2014, 8, 122–146.
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228.
- Hodek, P.; Trefil, P.; Stiborová, M. Flavonoids-Potent and Versatile Biologically Active Compounds Interacting with Cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21.
- Jang, H.S.; Kook, S.H.; Son, Y.O.; Kim, J.G.; Jeon, Y.M.; Jang, Y.S.; Choi, K.C.; Kim, J.; Han, S.K.; Lee, K.Y.; et al. Flavonoids Purified from Rhus Verniciflua Stokes Actively Inhibit Cell Growth and Induce Apoptosis in Human Osteosarcoma Cells. Biochim. Biophys. Acta-Gen. Subj. 2005, 1726, 309–316.
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary Flavonoids: Effects on Xenobiotic and Carcinogen Metabolism. Toxicol. In Vitro 2006, 20, 187–210.
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer Effects of Fisetin on Mammary Carcinoma Cells via Regulation of the PI3K/Akt/MTOR Pathway: In Vitro and in Vivo Studies. Int. J. Mol. Med. 2018, 42, 811–820.
- Zhu, J.; Zhang, H.; Zhu, Z.; Zhang, Q.; Ma, X.; Cui, Z.; Yao, T. Effects and Mechanism of Flavonoids from Astragalus Complanatus on Breast Cancer Growth. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 965–972.
- Dinakar, Y.H.; Kumar, H.; Mudavath, S.L.; Jain, R.; Ajmeer, R.; Jain, V. Role of STAT3 in the Initiation, Progression, Proliferation and Metastasis of Breast Cancer and Strategies to Deliver JAK and STAT3 Inhibitors. Life Sci. 2022, 309, 120996.
- Nde, C.; Zingue, S.; Winter, E.; Creczynski-Pasa, T.; Michel, T.; Fernandez, X.; Njamen, D.; Clyne, C. Flavonoids, Breast Cancer Chemopreventive and/or Chemotherapeutic Agents. Curr. Med. Chem. 2015, 22, 3434–3446.
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28.
- Syed, D.; Adhami, V.; Khan, M.; Mukhtar, H. Inhibition of Akt/MTOR Signaling by the Dietary Flavonoid Fisetin. Anticancer. Agents Med. Chem. 2013, 13, 995–1001.
- Ravichandran, N.; Suresh, G.; Ramesh, B.; Vijaiyan Siva, G. Fisetin, a Novel Flavonol Attenuates Benzo(a)Pyrene-Induced Lung Carcinogenesis in Swiss Albino Mice. Food Chem. Toxicol. 2011, 49, 1141–1147.
- Touil, Y.S.; Seguin, J.; Scherman, D.; Chabot, G.G. Improved Antiangiogenic and Antitumour Activity of the Combination of the Natural Flavonoid Fisetin and Cyclophosphamide in Lewis Lung Carcinoma-Bearing Mice. Cancer Chemother. Pharmacol. 2011, 68, 445–455.
- Syed, D.N.; Afaq, F.; Maddodi, N.; Johnson, J.J.; Sarfaraz, S.; Ahmad, A.; Setaluri, V.; Mukhtar, H. Inhibition of Human Melanoma Cell Growth by the Dietary Flavonoid Fisetin Is Associated with Disruption of Wnt/β-Catenin Signaling and Decreased Mitf Levels. J. Investig. Dermatol. 2011, 131, 1291–1299.
- Murtaza, I.; Adhami, V.M.; Hafeez, B.B.; Saleem, M.; Mukhtar, H. Fisetin, a Natural Flavonoid, Targets Chemoresistant Human Pancreatic Cancer AsPC-1 Cells through DR3-Mediated Inhibition of NF-ΚB. Int. J. Cancer 2009, 125, 2465–2473.
- Bhat, T.A.; Nambiar, D.; Pal, A.; Agarwal, R.; Singh, R.P. Fisetin Inhibits Various Attributes of Angiogenesis in Vitro and in Vivo-Implications for Angioprevention. Carcinogenesis 2012, 33, 385–393.
- Mohd Yusof, Y.A. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207.
- Rengarajan, T.; Yaacob, N.S. The Flavonoid Fisetin as an Anticancer Agent Targeting the Growth Signaling Pathways. Eur. J. Pharmacol. 2016, 789, 8–16.
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of Dietary Flavonoid Fisetin: Formulation and in Vitro Antioxidant and α-Glucosidase Inhibition Activities. Mater. Sci. Eng. C 2016, 68, 594–602.
- Li, J.; Cheng, Y.; Qu, W.; Sun, Y.; Wang, Z.; Wang, H.; Tian, B. Fisetin, a Dietary Flavonoid, Induces Cell Cycle Arrest and Apoptosis through Activation of P53 and Inhibition of NF-Kappa B Pathways in Bladder Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2011, 108, 84–93.
- PeiMing, Y.; HoHsing, T.; ChihWen, P.; WenShu, C.; ShuJun, C. Dietary Flavonoid Fisetin Targets Caspase-3-Deficient Human Breast Cancer MCF-7 Cells by Induction of Caspase-7-Associated Apoptosis and Inhibition of Autophagy. Int. J. Oncol. 2012, 40, 469–478.
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin Induces Apoptosis in Human Nonsmall Lung Cancer Cells via a Mitochondria-Mediated Pathway. Vitr. Cell. Dev. Biol.-Anim. 2015, 51, 300–309.
- Drug Bank. Available online: https://go.drugbank.com/Drugs/DB07795. (accessed on 5 December 2022).
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A Bioactive Phytochemical with Potential for Cancer Prevention and Pharmacotherapy. Life Sci. 2018, 194, 75–87.
- Fisetin Item No. 15246. Available online: https://www.caymanchem.com/pdfs/15246.pdf. (accessed on 5 December 2022).
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese Intake of Flavonoids and Isoflavonoids from Foods. J. Epidemiol. 1998, 8, 168–175.
- Surnis, S.A.; Patil, P.S.; Jadhav, R.H. Extraction, Isolation and Quantification of Bioactive Compound (Fisetin) and Its Product Formulation. Int. J. Eng. Res. 2016, 5, 56–58.
- Tsurudome, N.; Minami, Y.; Kajiya, K. Fisetin, a Major Component Derived from Mulberry (Morus Australis Poir.) Leaves, Prevents Vascular Abnormal Contraction. BioFactors 2022, 48, 56–66.
- Direito, R.; Reis, C.; Roque, L.; Gonçalves, M.; Sanches-Silva, A.; Gaspar, M.M.; Pinto, R.; Rocha, J.; Sepodes, B.; Bronze, M.R.; et al. Phytosomes with Persimmon (Diospyros Kaki l.) Extract: Preparation and Preliminary Demonstration of in Vivo Tolerability. Pharmaceutics 2019, 11, 296.
- Kostanecki, S.; Lampe, V.; Tambor, J. Synthese Des Fisetins. Ber. der Dtsch. Chem. Ges. 1904, 37, 784–791.
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697.
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxidants Redox Signal. 2013, 19, 151–162.
- Jeong, D.; Choi, J.M.; Choi, Y.; Jeong, K.; Cho, E.; Jung, S. Complexation of Fisetin with Novel Cyclosophoroase Dimer to Improve Solubility and Bioavailability. Carbohydr. Polym. 2013, 97, 196–202.
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal Encapsulation of the Natural Flavonoid Fisetin Improves Bioavailability and Antitumor Efficacy. Int. J. Pharm. 2013, 444, 146–154.
- Dzakwan, M.; Ganet, E.P.; Rachmat, M.; Wikarsa, S. Nanosized and Enhancement of Solubility Fisetin. Asian J. Pharm. Res. Dev. 2019, 7, 6–10.
- Mehta, P.; Pawar, A.; Mahadik, K.; Bothiraja, C. Emerging Novel Drug Delivery Strategies for Bioactive Flavonol Fisetin in Biomedicine. Biomed. Pharmacother. 2018, 106, 1282–1291.
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and Optimization of Fisetin Loaded Glycerol Based Soft Nanovesicles by Box-Behnken Design. Int. J. Pharm. 2020, 578, 119125.
- Bothiraja, C.; Yojana, B.D.; Pawar, A.P.; Shaikh, K.S.; Thorat, U.H. Fisetin-Loaded Nanocochleates: Formulation, Characterisation, in Vitro Anticancer Testing, Bioavailability and Biodistribution Study. Expert Opin. Drug Deliv. 2014, 11, 17–29.
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33.
- Zhang, J.Q.; Jiang, K.M.; An, K.; Ren, S.H.; Xie, X.G.; Jin, Y.; Lin, J. Novel Water-Soluble Fisetin/Cyclodextrins Inclusion Complexes: Preparation, Characterization, Molecular Docking and Bioavailability. Carbohydr. Res. 2015, 418, 20–28.
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, in Vitro and in Vivo Assessment. Front. Pharmacol. 2019, 10, 459.
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion Formulation of Fisetin Improves Bioavailability and Antitumour Activity in Mice. Int. J. Pharm. 2012, 427, 452–459.
- Kumar, R.R.; Khursheed, R.; Kumar, R.R.; Awasthi, A.; Sharma, N.; Khurana, S.; Kapoor, B.; Khurana, N.; Singh, S.K.; Gowthamarajan, K.; et al. Self-Nanoemulsifying Drug Delivery System of Fisetin: Formulation, Optimization, Characterization and Cytotoxicity Assessment. J. Drug Deliv. Sci. Technol. 2019, 54, 101252.
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282.
- Gupta, P.; Mazumder, R.; Padhi, S. Glycerosomes: Advanced Liposomal Drug Delivery System. Indian J. Pharm. Sci. 2020, 82, 385–397.
- Mignet, N.; Seguin, J.; Romano, M.R.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a Liposomal Formulation of the Natural Flavonoid Fisetin. Int. J. Pharm. 2012, 423, 69–76.
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin Loaded Binary Ethosomes for Management of Skin Cancer by Dermal Application on UV Exposed Mice. Int. J. Pharm. 2019, 560, 78–91.
- Mohapatra, M.; Mishra, A.K. Photophysical Behavior of Fisetin in Dimyristoylphosphatidylcholine Liposome Membrane. J. Phys. Chem. B 2011, 115, 9962–9970.
- Jain, V.; Kumar, H.; Chand, P.; Jain, S.; S, P. Lipid-Based Nanocarriers as Drug Delivery System and Its Applications. In Nanopharmaceutical Advanced Delivery Systems; Wiley Online Library: New York, NY, USA, 2021; pp. 1–29.
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.P.; Wilk, K.A. Electroporation and Lipid Nanoparticles with Cyanine IR-780 and Flavonoids as Efficient Vectors to Enhanced Drug Delivery in Colon Cancer. Bioelectrochemistry 2016, 110, 19–31.
- Talele, P.; Sahu, S.; Mishra, A.K. Physicochemical Characterization of Solid Lipid Nanoparticles Comprised of Glycerol Monostearate and Bile Salts. Colloids Surf. B Biointerfaces 2018, 172, 517–525.
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39.
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric Micelles for Delivery of Poorly Soluble Drugs: Preparation and Anticancer Activity in Vitro of Paclitaxel Incorporated into Mixed Micelles Based on Poly(Ethylene Glycol)-Lipid Conjugate and Positively Charged Lipids. J. Drug Target. 2005, 13, 73–80.
- Vadaye Kheiry, E.; Parivar, K.; Baharara, J.; Fazly Bazzaz, B.S.; Iranbakhsh, A. The Osteogenesis of Bacterial Cellulose Scaffold Loaded with Fisetin. Iran. J. Basic Med. Sci. 2018, 21, 965–971.
- Wang, L.; Zhang, D.Z.; Wang, Y.X. Bioflavonoid Fisetin Loaded α-Tocopherol-Poly(Lactic Acid)-Based Polymeric Micelles for Enhanced Anticancer Efficacy in Breast Cancers. Pharm. Res. 2017, 34, 453–461.
- Xiao, X.; Zou, J.; Fang, Y.; Meng, Y.; Xiao, C.; Fu, J.; Liu, S.; Bai, P.; Yao, Y. Fisetin and Polymeric Micelles Encapsulating Fisetin Exhibit Potent Cytotoxic Effects towards Ovarian Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 91.
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and Optimization of Poly (Lactic Acid) Nanoparticles Loaded with Fisetin to Improve Anti-Cancer Therapy. Int. J. Biol. Macromol. 2019, 125, 700–710.
- Ghosh, P.; Singha Roy, A.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of Albumin Based Nanoparticles for Delivery of Fisetin and Evaluation of Its Cytotoxic Activity. Int. J. Biol. Macromol. 2016, 86, 408–417.
- Chen, L.F.; Xu, P.Y.; Fu, C.P.; Kankala, R.K.; Chen, A.Z.; Wang, S. Bin Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone) (PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322.
- Sari, E.N.; Soysal, Y. Molecular and Therapeutic Effects of Fisetin Flavonoid in Diseases. J. Basic Clin. Health Sci. 2020, 4, 190–196.
- City of Hope Medical. Treatment of Frailty With Fisetin (TROFFi) in Breast Cancer Survivors. Available online: https://clinicaltrials.gov/ct2/show/NCT05595499. (accessed on 5 December 2022).
- St. Jude Children’s Research Hospital. An Open-Label Intervention Trial to Reduce Senescence and Improve Frailty in Adult Survivors of Childhood Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04733534 (accessed on 5 December 2022).
- Maher, P. How Fisetin Reduces the Impact of Age and Disease on CNS Function. Front. Biosci.-Sch. 2015, 7S, 58–82.
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430.
- Croy, S.; Kwon, G. Polymeric Micelles for Drug Delivery. Curr. Pharm. Des. 2006, 12, 4669–4684.
- Na, N.-C.; Dong, C.-S.; Jung, M.-S. Composition Comprising Phenolic Compound for Preventing and Treating Liver Cirrhosis. WO Pantent. WO2004002471A1, 8 January 2014.
- Cole, G.M.; Fruatshy, S.A.; Maher, P.; Schubert, D. Medical Food for Cognitive Decline. 2012.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
10 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




