Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bruno Mégarbane | -- | 1713 | 2023-05-09 02:15:18 | | | |
| 2 | Dean Liu | -1 word(s) | 1712 | 2023-05-09 02:45:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jaffal, K.; Chevillard, L.; Mégarbane, B. Rationale for ILE Use in Clinical Toxicology. Encyclopedia. Available online: https://encyclopedia.pub/entry/43996 (accessed on 07 February 2026).
Jaffal K, Chevillard L, Mégarbane B. Rationale for ILE Use in Clinical Toxicology. Encyclopedia. Available at: https://encyclopedia.pub/entry/43996. Accessed February 07, 2026.
Jaffal, Karim, Lucie Chevillard, Bruno Mégarbane. "Rationale for ILE Use in Clinical Toxicology" Encyclopedia, https://encyclopedia.pub/entry/43996 (accessed February 07, 2026).
Jaffal, K., Chevillard, L., & Mégarbane, B. (2023, May 09). Rationale for ILE Use in Clinical Toxicology. In Encyclopedia. https://encyclopedia.pub/entry/43996
Jaffal, Karim, et al. "Rationale for ILE Use in Clinical Toxicology." Encyclopedia. Web. 09 May, 2023.
Copy Citation
Biodetoxification using intravenous lipid emulsion (ILE) in acute poisoning is of growing interest. As well as for local anesthetics, ILE is currently used to reverse toxicity caused by a broad-spectrum of lipophilic drugs. Both pharmacokinetic and pharmacodynamic mechanisms have been postulated to explain its possible benefits, mainly combining a scavenging effect called “lipid sink” and cardiotonic activity.
lipid emulsion
Intralipid®
poisoning
cardiotoxicant
1. Introduction
Biodetoxification using drug scavenging agents is of growing interest, as drug overdoses represent a major health problem accounting for thousands of deaths annually worldwide, most often among the young. Poisoning is responsible for more than 3 million calls to US poison control centers annually [1]. Two thirds of these calls are in relation to the ingestion of overdosed prescription drugs, with major fatality risk if cardiovascular medications are involved.
The use of intravenous lipid emulsion (ILE) as an antidote to reverse local anesthetic-related systemic toxicity (LAST) has gained widespread support following convincing data from successful case reports and animal studies [2]. ILE was suggested as a promising agent for poisonings involving lipophilic agents, especially if unresponsive to the recommended therapies [3]. Its easy administration in emergent conditions at the bedside and its original properties, supporting its ability to alter both the pharmacokinetics (PK) and the pharmacodynamics (PD) of lipophilic toxicants synergistically with supportive care, antidotes, and decontamination and elimination enhancement techniques, opened up new perspectives for emergency physicians and intensivists to improve the management of severe poisonings. To date, ILE has been used in poisoned patients to reverse cardiac toxicity induced by a broad spectrum of drugs (Table 1) [4][5].
Table 1. Therapies provided in 2,080,917 human exposures based on the 2021 Annual Report of the National Poison Data System from America’s Poison Centers.
| Activated charcoal (single or multiple doses) | 35,819 |
| Intubation and mechanical ventilation | 19,032 |
| Vasopressors | 9833 |
| Cardiopulmonary resuscitation | 2662 |
| Hemodialysis | 2538 |
| Continuous renal replacement therapy | 874 |
| High-dose insulin/glucose | 661 |
| Lipid emulsion therapy | 403 |
| Cardioversion | 374 |
| Pacemaker | 266 |
| Extracorporeal membrane oxygenation | 127 |
Evidence for ILE use in poisoning is evolving. Several mechanisms of action have been hypothesized [6], including i: intravascular sequestration of the toxicant and its enhanced redistribution to biologically inert tissues; ii: augmentation of fatty acid utilization for adenosine triphosphate (ATP) synthesis; and iii: direct cardiotonic and ion channel effects. However, much remains to be elucidated. Uncertainties are still present regarding the optimal composition, dosing, mechanisms of action, and efficiency of ILE. Without clarification of the ILE mechanism of action, caution should remain regarding its role and indications.
2. Rationale for ILE Use in Clinical Toxicology
With functional toxicants, the most common approach to manage poisoning is to provide the support needed to maintain organ function, relying on the endogenous ability to metabolize and eliminate the toxicant. Gastrointestinal decontamination, including gastric lavage and activated charcoal administration, can minimize absorption, but these techniques are controversial and poorly effective more than 1–2 h post-ingestion [7][8]. Extracorporeal techniques such as hemodialysis and hemofiltration to enhance drug elimination are of questionable clinical pertinence for drugs with large volumes of distribution and/or high protein binding affinities. Finally, antidotes are necessary in combination with supportive care when the drug is associated with a high mortality rate or poor long-term outcome [9].
Numerous experimental and clinical studies have established that ILE can increase the blood tissue partitioning of lipophilic drugs, improve cardiac performance, and result in relevant beneficial effects such as post-conditioning, direct inotropy, and the activation of cytoprotective pathways [3][5][6]. By contrast, randomized controlled trials (RCTs), considered as the best evidence to support the benefits of ILE, are rare due to obvious ethical difficulties and the rarity of clinical indications. Therefore, to date, mainly experimental and case report data provide support for the possible efficacy of ILE in clinical toxicology in selected indications. [5]. While awaiting human data, one approach to obtain approval for therapy in acute poisoning is the « animal efficacy and human safety » rule.
2.1. A Success Story
In 1962, after the commercial release of Intralipid® (Fresenius Kabi, Bad Homburg, Germany), clinicians proposed the use of intravenous (IV) fat emulsions as drug binders or components of extracorporeal lipid dialysis. Russell et al. reported that IV cottonseed oil could shorten the duration of thiopental anesthesia [10]. Adding olive or cottonseed oil to the dialysate was shown to enhance glutethimide removal [11], and one camphor-intoxicated patient was treated successfully with lipid extracorporeal hemodialysis [12]. In the following years, devices for lipid dialysis were developed, and proposals of more specifically designed dialysates for detoxification were postulated [13][14]. Laboratory investigations demonstrated that corn oil addition to dialysates was effective at moving drugs (e.g., imipramine, amitriptyline, and glutethimide) out of the plasma and into the dialysate [15]. Krieglstein et al. were the first to demonstrate that a marketed fat emulsion (10% Lipofundin®, B. Braun, Taguig City, Philippines) could bind chlorpromazine in vitro and save rats from lethal chlorpromazine toxicity [16]. Thereafter, investigations focused on the underlying mechanistic actions of ILE.
Weinberg et al. published their original findings demonstrating that resuscitation or pretreatment with ILE resulted in the improvement of cardiac toxicity associated with bupivacaine in rats and dogs [17][18]. Following these supportive animal works, ILE was administered in human cases of cardiac arrest and neurologic toxicity attributable to LAST, with successful resuscitation after the failure of conventional resuscitation [2]. Nearly two decades later, the majority of academic societies recommend ILE as a first-line treatment of LAST [19].
2.2. ILE Constituents
ILEs are sterile nanometer-sized droplets of triglyceride oils in water stabilized by phospholipid surfactants [20]. The average particle size is between 200 and 600 nm, depending on the method and the emulsion. Phospholipids such as egg lecithin are added as emulsifiers to improve fat solubility. Emulsifiers, which are both fat- and water-soluble, surround the lipid droplet (Figure 1). Sodium hydroxide is added to adjust the pH. Currently marketed products have a pH between 6.0 and 9.0 and a ~270 mOsm/L osmolality, and are ready to infuse using peripheral vein access.
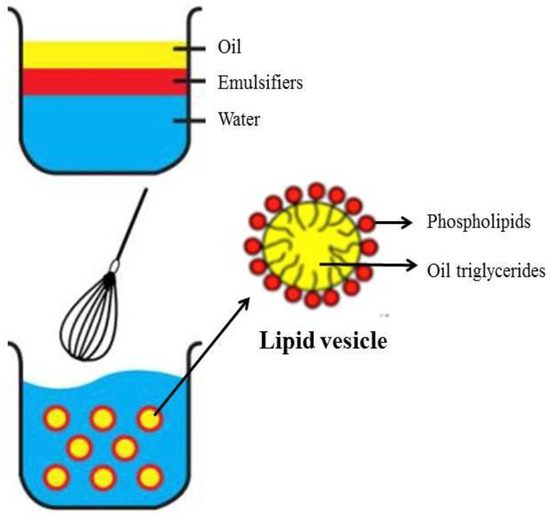
Figure 1. The making of a lipid emulsion. Droplets have a monolayer of phospholipids as emulsifier, which separates the interior lipid phase from the exterior aqueous phase.
ILEs may contain varying types (soybean, coconut, olive, and fish) and concentrations of triglyceride oils (10, 20, and 30%). Droplets have similar shapes and sizes to physiological chylomicrons and are similarly metabolized. Fatty acids represent the lipid phase of ILE with neutral long-chain triglycerides (LCTs) or a mix of medium-chain triglycerides (MCTs) and LCTs.
Intralipid® is the most often studied product among ILEs. It is an emulsion containing 20% soybean oil, 2.25% glycerin, and 1.2% egg phospholipids [21]. Intralipid® is currently manufactured with lipid concentrations of 10, 20, and 30% and commonly used for parenteral nutrition, drug carrier vehicles, lipid rescue therapy, and ischemia-reperfusion injury attenuation therapy [21]. Although Intralipid® is the most often administered lipid rescue therapy, the use of other products has been reported [22][23]. The composition is of importance since it influences the scavenging properties and the side effects of ILE.
2.3. The Absorptive Properties
ILEs are useful carriers for lipophilic drugs [23]. They are used to deliver drugs such as propofol, etomidate, diazepam, and amphotericin B [24] and more recently as highly efficient and targeted delivery systems for cancer therapy [25]. Based on their absorptive properties, lipophilic drugs can diffuse across the phospholipid coating into the blood when the purpose is to deliver the drug or out of the blood when ILE is used to treat drug overdose [26][27]. The principles of Fick’s law govern drug diffusion according to the concentration gradient across the lipid droplet membrane, the surface area of the drug-containing lipid droplet, and the drug partition coefficient between the oil and aqueous phases. The amount of toxicant that can be sequestered by a given therapy is measured by its partition coefficient, which is the concentration ratio of the toxicant dissolved in the oil over the aqueous phase at equilibrium. The toxicant can also be adsorbed on the emulsion surface. Regarding the adsorption of the toxicant, the interfacial dynamics of droplet–poison binding are unknown, and phospholipid micelles likely exist in aqueous solution alongside the emulsified oil droplets and could interact with the toxicant. Of note, neither of these phenomena are understood adequately and both deserve further study. Each drug and metabolite has different degrees of plasma protein binding and partition coefficients depending on the physicochemical conditions. For example, a drop in arterial pH to 7.0 tends to reduce bupivacaine protein binding, and the resulting increase in free bupivacaine may allow lipid scavengers to play a more significant role in the drug uptake [28][29].
Of note, the octanol/water partition coefficient (LogP) used to reflect lipophilicity (Figure 2) differs from the actual drug partitioning between the serum and lipid emulsion. LogP applies only to the neutral moiety, which, at equilibrium, might be present in a very low concentration compared with the charged version. At physiological pH, several drug candidates for ILE-based detoxification are predominantly charged. The lipid solubility of charged compounds is negligible; but these drugs could potentially partition on the surface through electrostatic interactions with charged phospholipid surfactants in the emulsion. Intralipid® 20% was reported to have a zeta potential between −45 and −40 mV, indicating that the emulsion has significant surface charge [30]. Recently, the distribution coefficient (LogD) was suggested to be a better predictor of the actual equilibrium behavior of drugs [31]. LogD accounts for both the non-ionized and ionized forms of a drug in both the octanol and water phases. This ratio is more difficult to measure and depends on the pH of the system evaluated. It may also be a better predictor of drug lipophilicity and sequestration.
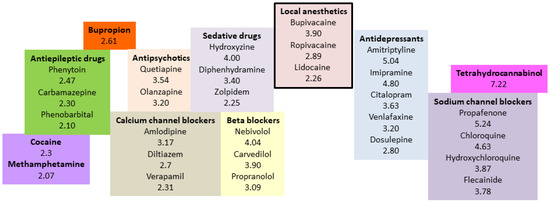
Figure 2. Partition coefficients of some selected lipophilic drugs for which the use of lipid emulsion could be considered in poisoning.
Two other new parameters, i.e., drug accommodation capacity and drug capture kinetics, were described to characterize the drug capture capability of lipid emulsions and their resulting effects on PK in overdose [32]. Drug capture prediction based on these two parameters was shown to be more accurate than LogP when considering the lipid emulsion-attributed reduction in the half-life and area under the drug concentration-time curve.
To summarize, the charged portion of ionized drugs could interact with the surface of lipid droplets through electrostatics, while the uncharged portion could interact with the oily interior and the phospholipid bilayers to maintain drug sequestration. The clinical utility of these absorptive properties (hydrophobic interior, phospholipid membrane, and surface charges) lies in increasing drug sequestration to provide recovery from toxicity. Various predictors of drug capture have been proposed, including LogP, LogD, and drug accommodation capacity. However, the best predictor, if considering their emergent availability at the bedside versus their prediction accuracy, remains debated.
References
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Rivers, L.J.; Feldman, R.; Brown, K.; Nathaniel, P.T.P.; Bronstein, A.C.; Weber, J.A. 2021 Annual report of the National Poison Data System© (NPDS) from America’s poison centers: 39th annual report. Clin. Toxicol. 2022, 60, 1381–1643.
- Hoegberg, L.C.; Bania, T.C.; Lavergne, V.; Bailey, B.; Turgeon, A.F.; Thomas, S.H.; Morris, M.; Miller-Nesbitt, A.; Mégarbane, B.; Magder, S.; et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin. Toxicol. 2016, 54, 167–193.
- Cave, G.; Harvey, M. Intravenous lipid emulsion as antidote beyond local anesthetic toxicity: A systematic review. Acad. Emerg. Med. 2009, 16, 815–824.
- Cave, G.; Harvey, M.G. Should we consider the infusion of lipid emulsion in the resuscitation of poisoned patients? Crit. Care 2014, 18, 457.
- Levine, M.; Hoffman, R.S.; Lavergne, V.; Stork, C.M.; Graudins, A.; Chuang, R.; Stellpflug, S.J.; Morris, M.; Miller-Nesbitt, A.; Gosselin, S.; et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin. Toxicol. 2016, 54, 194–221.
- Weinberg, G.L. Lipid Emulsion Infusion. Anesthesiology 2012, 117, 180–187.
- Eddleston, M.; Juszczak, E.; Buckley, N.A.; Senarathna, L.; Mohamed, F.; Dissanayake, W.; Hittarage, A.; Azher, S.; Jeganathan, K.; Jayamanne, S.; et al. Multiple-dose activated charcoal in acute self-poisoning: A randomised controlled trial. Lancet 2008, 371, 579–587.
- Benson, B.E.; Hoppu, K.; Troutman, W.G.; Bedry, R.; Erdman, A.; Höjer, J.; Mégarbane, B.; Thanacoody, R.; Caravati, E.M.; American Academy of Clinical Toxicology; et al. Position paper update: Gastric lavage for gastrointestinal decontamination. Clin. Toxicol. 2013, 51, 140–146.
- Clark, B.J.; Binswanger, I.A.; Moss, M. The intoxicated ICU patient. Crit. Care Med. 2014, 42, 1563–1564.
- Russel, R.L.; Westfall, B.A. Alleviation of barbiturate depression. Anesth. Analg. 1962, 41, 582–585.
- Shinaberger, J.H.; Sehar, L.; Clayton, L.E.; Barry, K.G.; Knowlton, M.; Goldbaum, L.R. Dialysis for intoxication with lipid soluble drugs: Enhancement of glutethimide extraction with lipid dialysate. Trans. Am. Soc. Artif. Intern. Organs 1965, 11, 173–177.
- Ginn, H.E.; Anderson, K.E.; Mercier, R.K.; Stevens, T.W.; Matter, B.J. Camphor intoxication treated by lipid dialysis. JAMA 1968, 203, 230–231.
- King, L.H.; Decherd, J.F.; Newton, J.L.; Shires, D.L.; Bradley, K.P. A clinically efficient and economical lipid dialyzer. Use in treatment of glutethimide intoxication. JAMA 1970, 211, 652–653.
- Whang, R.; Orndorff, M.; Papper, S. Lipid-electrolyte dialysis--experimental and preliminary clinical observations. Clin. Toxicol. 1970, 3, 551–560.
- Clarke, L. Dialysis of drugs in vitro. Br. J. Clin. Pharmacol. 1974, 1, 442–445.
- Krieglstein, J.; Meffert, A.; Niemeyer, D.H. Influence of emulsified fat on chlorpromazine availability in rabbit blood. Experientia 1974, 30, 924–926.
- Weinberg, G.L.; VadeBoncouer, T.; Ramaraju, G.A.; Garcia-Amaro, M.F.; Cwik, M.J. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology 1998, 88, 1071–1075.
- Weinberg, G.; Ripper, R.; Feinstein, D.L.; Hoffman, W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg. Anesth. Pain Med. 2003, 28, 198–202.
- Fettiplace, M.R.; Lis, K.; Ripper, R.; Kowal, K.; Pichurko, A.; Vitello, D.; Rubinstein, I.; Schwartz, D.; Akpa, B.S.; Weinberg, G. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J. Control. Release 2015, 198, 62–70.
- Baker, M.T.; Naguib, M. Propofol: The challenges of formulation. Anesthesiology 2005, 103, 860–876.
- Buys, M.; Scheepers, P.A.; Levin, A.I. Lipid emulsion therapy: Non-nutritive uses of lipid emulsions in anaesthesia and intensive care. S. Afr. J. Anaesth. Analg. 2015, 21, 5–11.
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New parenteral lipid emulsions for clinical use. J. Parenter. Enteral Nutr. 2006, 30, 351–367.
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable lipid emulsions-advancements, opportunities and challenges. Aaps Pharm. Sci. Tech. 2010, 11, 1526–1540.
- Tamilvanan, S. Oil-in-water lipid emulsions: Implications for parenteral and ocular delivering systems. Prog. Lipid Res. 2004, 43, 489–533.
- Shim, G.; Kim, M.-G.; Kim, D.; Park, J.Y.; Oh, Y.-K. Nanoformulation-based sequential combination cancer therapy. Adv. Drug Deliv. Rev. 2017, 115, 57–81.
- Leroux, J.-C. Injectable nanocarriers for biodetoxification. Nat. Nanotechnol. 2007, 2, 679–684.
- Qu, X.; Gou, M.; Zaidan, J.; Zhang, K.; Chen, S. Challenges and opportunities in developing nanoparticles for detoxification. Nanomedicine 2014, 9, 2437–2439.
- Coyle, D.E.; Denson, D.D.; Thompson, G.A.; Myers, J.A.; Arthur, G.R.; Bridenbaugh, P.O. The influence of lactic acid on the serum protein binding of bupivacaine: Species differences. Anesthesiology 1984, 61, 127–133.
- Ruan, W.; French, D.; Wong, A.; Drasner, K.; Wu, A.H.B. A mixed (long- and medium-chain) triglyceride lipid emulsion extracts local anesthetic from human serum in vitro more effectively than a long-chain emulsion. Anesthesiology 2012, 116, 334–339.
- Washington, C.; Koosha, F.; Davis, S.S. Physicochemical properties of parenteral fat emulsions containing 20% triglyceride; Intralipid and Ivelip. J. Clin. Pharm. Ther. 1993, 18, 123–131.
- Bhal, S.K.; Kassam, K.; Peirson, I.G.; Pearl, G.M. The rule of five revisited: Applying log D in place of log P in drug-likeness filters. Mol. Pharm. 2007, 4, 556–560.
- Li, Z.; Li, M.; Sun, H.; Yang, Z.; Huo, Q.; Bai, Y.; Mei, Y.; Li, Y.; Quan, P.; Zhang, J.; et al. Prediction of drug capturing by lipid emulsions in vivo for the treatment of a drug overdose. J. Control. Release 2022, 346, 148–157.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
703
Revisions:
2 times
(View History)
Update Date:
09 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




