1. Introduction
Water is a basic essential need for the sustenance of all life forms and the lack of safe and clean drinking water, especially for people living in developing nations, is a major concern. The United Nations Children’s Fund (UNICEF) and World Health Organization estimates that as of the year 2017, 435 million people used unimproved water sources and 144 million used surface water without treatment
[1]. Water intended for human consumption should be safe, palatable, and aesthetically pleasing to prevent consumers from contracting waterborne diseases such as cholera, typhoid fever, and dysentery. These diseases are predominantly due to faecal contamination of water sources and, thus, are related to sanitation conditions. There is a dire need for access to safe and clean drinking water and for cooking and hand-washing, especially in the face of the global coronavirus pandemic.
Membrane processes are widely used in the production of clean and safe water owing to their high effectiveness, no (or less) addition of chemicals, ease of scale up, and robustness
[2]. Attributes of a good membrane include a high and stable filtration flux, low filtration pressure, requires less footprint, high quality permeability, and requires minimal pre-treatment of the feed water
[3][4]. Membranes are mainly classified into two types: polymeric and inorganic (metals or ceramics) membranes. Polymeric membranes are preferred due to their high flexibility and chemical stability, and are applied in pressure-driven processes such as microfiltration (MF), ultra-filtration (UF), nano-filtration (NF), and reverse osmosis (RO)
[2]. Polymeric membranes are made from materials such as polyvinyl alcohol (PVA), poly acrylo nitrile (PAN), polyether sulfone (PES), and poly vinylidene fluoride (PVDF), among others. Inorganic membranes, on the other hand, are made of silica, zeolites, etc.
[5][6].
The transport rate of a component through a membrane is determined by driving forces based on concentration, pressure, temperature and electrical potential gradients, and the concentration and mobility of the component in the membrane matrix
[7]. The application of membranes, however, is derailed by the challenge of membrane fouling, causing loss of flux and altered rejection
[4][8][9][10]. Microfiltration (MF) and ultrafiltration (UF) membrane processes are commonly used in potable water treatment and in membrane bioreactors. This is due to low energy requirement; low costs of installation and operation; effectiveness in removal of suspended matter; and appreciable removal of microbiological contaminants. However, their application for disinfection is limited by large pore sizes, hence, not an absolute barrier to microorganisms.
The main removal mechanism in MF and UF processes is size exclusion. Therefore, these processes can theoretically achieve perfect exclusion of particles regardless of operational parameters such as influent concentration, pressure, or the skills of the operator. The separation is based on the membrane pore size and the quality of product is determined by the membrane
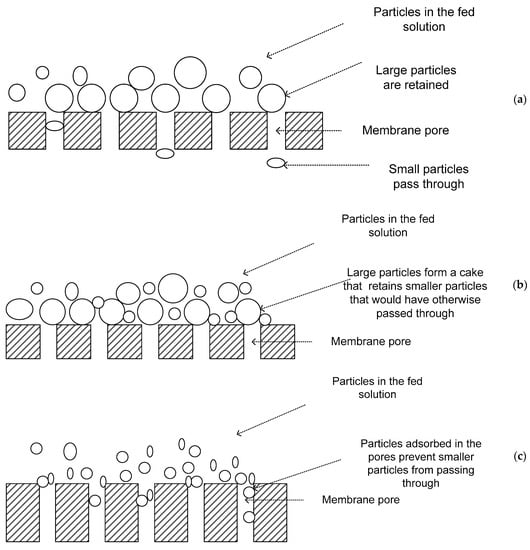
[9][10][11]. The major mechanisms of separation in MF and UF include: (a) straining, which occurs when particles are physically retained because they are larger than the pores (
Figure 1a). However, this does not mean that there is 100% retention of particles larger than the membrane pore size. The interconnecting voids in membrane filters have a distribution of sizes, including some larger and others smaller than the retention rating. Therefore, particles smaller than the retention rating may be trapped in smaller passageways and larger particles may pass through the membrane in other areas
[9]; (b) cake filtration, whereby particles that are small enough to pass through the membranes are retained by a cake of larger material that collects at the membrane surface during filtration (
Figure 1b). This cake acts as a pre-coat filtration medium, often called a dynamic membrane since its filtering capability varies with time, growing in thickness during filtration but being partially or wholly removed by cleaning
[9]; and (c) adsorption, which results when material small enough to enter pores adsorbs to the walls of the pores. If the particles and the membrane are oppositely charged, or if their zeta potentials are appropriate, the particles adhere to the membrane matrix, resulting in removal of the particles smaller than the pores of the membrane
[8][11]. This means that soluble materials may be rejected even though their physical dimensions are much smaller than the membrane retention rating (
Figure 1c). Adsorbed material can reduce the size of voids throughout the membrane. This, therefore, increases the ability of the membrane to retain smaller material by straining while increasing the chances of membrane fouling
[9].
Figure 1. (a–c) Mechanisms of microfiltration and ultrafiltration.
There is a need to enhance the membrane properties to meet needs such as adequate disinfection, flux amelioration, and reduced membrane fouling. To achieve these, the incorporation of nanoparticles with unique properties in membranes is a potential option, leading to the development of advanced ceramic and polymeric membranes with enhanced filtration performance
[12]. Zwitterionic materials have also been incorporated in membranes as antifouling agents
[13]. Nanoparticles of silver and copper have received considerable interest for use in water purification, especially for disinfection and for decentralized and emergency response water treatment systems. Such systems are low cost, portable, and easy to use and maintain
[14][15]. Incorporation of nanoparticles into the membranes leads to increased surface per unit of mass, surface reactivity, and quantum-related effects
[16]. For example, by converting bulk silver into nano-size silver, its effectiveness for controlling bacteria and viruses can be increased several times, primarily because the nano silver has an extremely large surface area, resulting in increased contact with the microorganisms
[17][18]. The incorporation of nanoparticles into membranes concentrates the nanoparticles at the membrane surface where reaction occurs
[15][19]. It also makes the membranes reactive instead of simply being a physical barrier, thereby performing multiple functions such as increasing water flux, improving contaminant rejection, and reducing organic and biological fouling
[20][21][22].
For disinfection, metallic nanoparticles such as zinc, copper, gold, titanium, and silver have been explored. Among these, silver is the most widely studied oligodynamic material due to advantages such as its antimicrobial effectiveness on a range of microorganisms, low toxicity to human beings, and ease of incorporation into various substrates for disinfection applications. It is widely applied in domestic water filters to reduce biofouling, and in conjunction with copper ionization to prevent colonization by bacteria such as
Legionella spp. in plumbing hospital hot-water systems
[23]. Although silver nanoparticles are not toxic, especially at low concentration, their disadvantage is that accumulation in mammalian cells can lead to
argyria, resulting from silver overload in the tissues
[24][25]. Silver nanoparticles (AgNPs), when incorporated into a membrane, display strong inhibitory and biocidal properties against microorganisms that would otherwise colonize the membrane surface
[26]. This is achieved using silver ions either in solution or adsorbed onto nanoparticles and the nanoparticles themselves
[27].
2. Synthesis of Silver Nanoparticles
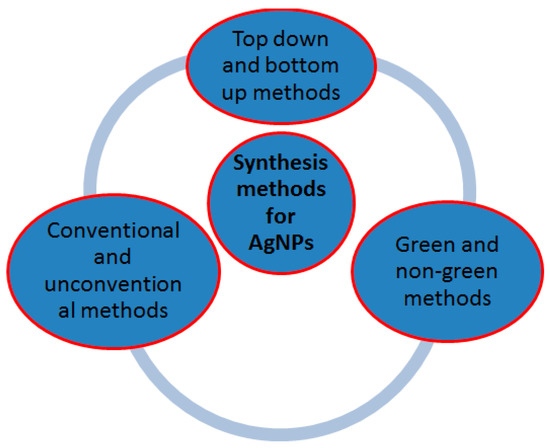
Synthesis methods of silver nanoparticles can be categorized as top–down versus bottom–up, conventional versus non-conventional, and green versus non-green (
Figure 2). In the top–down approach, a large structure is gradually reduced in dimensions, until nano-size dimensions are attained after the application of severe mechanical stresses and deformations. It includes physical methods such as milling, repeated quenching, photolithography, cutting, etching, and grinding. In the bottom–up approach, nanoparticles are constructed atom-by-atom or molecule-by-molecule. Bottom–up techniques start with silver salt precursor dissolved in a solvent that is reduced in a chemical reaction and the nanoparticles are formed through nucleation and growth. They include chemical synthesis, self-assembly, and positional assembly among others
[16][17][28][29].
Figure 2. Synthesis methods for silver nanoparticles.
Conventional chemical synthesis methods include the use of citrate, borohydride, organic reducers, and inverse micelles in the synthesis process. Typical reducing agents include chemical agents
[14][30][31][32], plant extracts
[32][33], biological agents
[34], or irradiation methods
[32][35][36][37][38] that provide the free electrons needed to reduce silver ions (Ag
+) and to form AgNPs
[38][39][40]. Reduction using borohydride and citrate are the most prominent. This is mainly due to the relatively high reactivity of sodium borohydride, moderate toxicity, and greater lab safety when compared to hydrogen gas and other physical methods
[32][41]. Citrate is a weaker reducing agent, and the reaction requires energy that is generally applied by heating the solution. Unconventional methods include laser ablation, radio catalysis, and vacuum evaporation of metals, among others
[17][38].
Green approaches use environmentally friendly agents such as sugars
[32][38][42][43][44] and plant extracts such as orange peels to form and stabilize AgNPs
[45][46]. However, the weakness of the green approaches is that it is more difficult to control the morphology of the produced nanosilver compared to the non-green methods
[17][32][38].
3. Incorporation of Silver Nanoparticles in Membranes
The incorporation of silver nanoparticles into membranes for water treatment is aimed at fouling mitigation, improvement in permeability quality, and flux enhancement
[47][48]. A major challenge is the dispersion of the nanoparticles in the membrane matrix. The aggregation/dispersion behaviour control is crucial
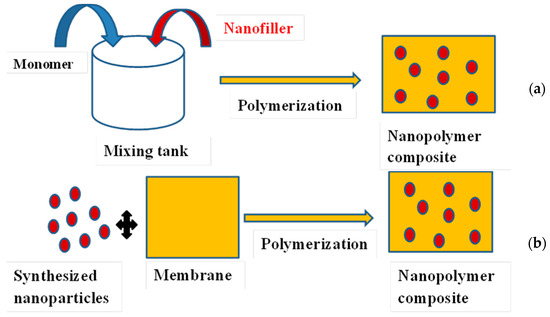
[3]. Preparation of membrane composites containing silver nanoparticles can be achieved by: (i) mechanical mixing of a polymer with the nanoparticles employing mechanisms such as convection, diffusion, and shear; (ii) in situ polymerization of a monomer in the presence of the nanoparticles and in situ reduction of metal salts or complexes in a polymer
[49]; and (iii) ex situ incorporation of pre-synthesized nanoparticles into the membrane
[50]. Therefore, in situ synthesis requires techniques to immobilize specific functional groups on the surface of the materials, which play an important role in stabilizing and anchoring AgNPs on the filtering materials, whereas ex situ synthesis methods involve submerging or brushing membranes such as conventional ceramic filters with AgNPs solution (
Figure 3). However, filters fabricated by the ex situ method sometimes lose antibacterial efficacy and clog after use
[51][52].
Figure 3. Schematic representation of in situ (a) and ex situ (b) polymerization.
The following methods have been employed to incorporate AgNPs in materials and are discussed below.
(i) Chemical reduction of silver salts; (ii) electro-spinning; (iii) physical vapour deposition; (iv) wet-phase inversion process; (v) and dipping in colloidal silver solution or brushing with colloidal silver solution.
3.1. Chemical Reduction of Silver Salts
This is the most-used approach to incorporate AgNPs into membrane matrices. It involves the entrapment of silver ions in the polymer chains followed by reduction with reducing agents (in situ synthesis). The advantages include: (i) the template role of the host macromolecular chains for the synthesis of nanoparticles helps improve their dispersion inside the polymeric matrix, and also partially prevents aggregation; (ii) it leads to reduced size of nanoparticles with a narrow size distribution and well-defined shape, all which are key parameters in the synthesis of nanomaterials
[53]. This method has been employed to attach AgNPs on cellulose membranes
[49][54], blotting paper
[14], woven fabric membranes
[55], polyurethane
[56], and hollow-fibre microfiltration membranes
[57].
To prevent NPs from aggregating, and to control the size of the final product, a stabilizing agent (capping agent) is used in the synthesis process. Agglomeration is mainly caused by excess surface energy and high thermodynamic instability of the nanoparticle surface. When solutions of silver nitrate and sodium borohydride are mixed in the absence of substances inhibiting particle growth, a fast irreversible reaction proceeds to yield a black silver metal precipitate, and the particle growth does not cease in the nanosized range. However, when the reaction is carried out in the presence of the stabilizers, the reduction process can be stopped at the stage of nanoparticle formation
[15][39][58].
Reducing agents such as sodium borohydride
[14][54][59], sodium citrate
[56], ascorbic acid, hydrazine hydrate
[60], hydroxylamine
[49], and tri-octyl-phosphine
[61] have been used to produce AgNPs from silver salts. The relatively high reactivity of borohydride and its non-toxicity makes the borohydride reduction the most commonly used technique to prepare AgNPs
[15][62][63].
Table 1 shows the common reducing agents.
Table 1. Common reducing agents and the reaction conditions, adapted from Bonsak
[62] and Rana et al.
[64].
3.2. Electro-Spinning
Electro-spinning makes use of electrostatic forces to stretch the solution or melt as it solidifies
[65]. A polymer solution or melt is placed into a syringe with a nozzle and subjected to an electric field. Under the applied electrostatic force, the polymer is ejected from the nozzle and deposited on a collector
[66]. It is a simple, low-cost, and effective technology to produce polymer nano fibres. The basic setup for electro-spinning mainly used in lab scale consists of a high voltage supply, a spinneret (a syringe filled with the polymer solution or melt connected to the high voltage supply), and a grounded or an oppositely charged collector. The ejected polymer solution (or melt) becomes highly electrified by the applied high voltage (5–40 kV), which leads to the creation of an electrically charged jet that is drawn into the direction of the collector. On its way to the target, the jet is stretched and whipped, leading to the formation of nanometer-sized fibres that are collected on the target as a nonwoven fibre web. The advantages of electro-spinning are: (i) it does not affect the chemical composition of the nanoparticles or the utilized polymer; and (ii) some nanoparticles may be embedded inside the polymeric nano fibres and others attached on the nano fibres surface according to the particle size, thereby modifying the material to meet the desired outcome
[67]. This technique has been used by Wang et al.
[65] employing cellulose acetate solution, silver nitrate, and photo-reduction using ultra violet irradiation. Other reducing agents such as hydrazinium hydroxide and heat treatment can also be employed
[68][69].
3.3. Physical Vapour Deposition
Physical vapor deposition (PVD) entails the use of vacuum deposition methods to deposit thin films by the condensation of a vaporized form of the desired film material onto various surfaces. The coating method involves purely physical processes such as high temperature vacuum evaporation with subsequent condensation. For the incorporation of AgNPs, the silver is heated to a point where it evaporates within the vacuum chamber and then allowed to condense on the polymer surface such as poly(vinylidenefluoride) (PVDF) and polyethersulfone (PES)
[70][71]. Uniform silver deposition is achieved using electron beam bombardment of silver metal.
3.4. Wet-Phase Inversion Process
Phase inversion is a process whereby a polymer is transformed in a controlled manner from a liquid to a solid state through liquid–liquid de-mixing. At a certain stage during the de-mixing process, one of the liquid phases (the high polymer concentration phase) solidifies so that a solid matrix is formed
[72]. Porous materials produced by precipitation from a homogeneous polymer solution are termed phase-inversion membranes. They incorporate both symmetrical (homogeneous) and asymmetrical structures. The production process consists of the following important steps: production of a homogeneous polymer solution; casting of the polymer film, followed by partial evaporation of the solvent from the polymer film; immersion of the polymer film in a precipitation solution to enable the solvent to be exchanged for the precipitation agent; and heat-setting in a bath solution in order to restructure any imperfections in the precipitated membrane film
[73]. This technique has been employed to produce polysulfone UF membranes
[21][74] and polyamide 6.6 membranes
[75].
3.5. Dipping in Colloidal Silver Solution or Brushing with Colloidal Silver Solution
There are three widely used methods for impregnating ceramic pot filters with colloidal silver for disinfection: dipping the filter in a silver solution; painting the filter with silver solution using a brush; and incorporating the silver in the clay mix before firing. Ceramic filters coated with colloidal silver have been investigated for potable water treatment and disinfection applications
[15][76][77][78][79][80]. The filters are mostly manufactured from locally available labor and materials such as soil, grog (previously fired clay), and water. The filter is formed using a filter press, air-dried, and fired in a flat-top kiln, at a temperature of about 900 °C over a period of 8 h. This forms the ceramic material and combusts the sawdust, flour, or rice husk in the filters, making it porous and permeable to water. After firing, the filters are cooled and impregnated with colloidal silver by painting with, or dipping in, a colloidal silver solution
[81]. Recently, the application of silver nitrate to the clay, water, and sawdust mixture prior to pressing and firing the filter ceramic filter was reported, and shown to effectively reduce costs and improve silver retention in the filter
[82].