Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domen Vozel | -- | 2013 | 2023-05-08 11:51:00 | | | |
| 2 | Catherine Yang | Meta information modification | 2013 | 2023-05-09 03:56:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Urbančič, J.; Vozel, D.; Battelino, S.; Bošnjak, R.; Kokošar Ulčar, B.; Matos, T.; Munda, M.; Papst, L.; Steiner, N.; Vouk, M.; et al. Atypical Skull-Base Osteomyelitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/43973 (accessed on 14 January 2026).
Urbančič J, Vozel D, Battelino S, Bošnjak R, Kokošar Ulčar B, Matos T, et al. Atypical Skull-Base Osteomyelitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/43973. Accessed January 14, 2026.
Urbančič, Jure, Domen Vozel, Saba Battelino, Roman Bošnjak, Barbara Kokošar Ulčar, Tadeja Matos, Matic Munda, Lea Papst, Nejc Steiner, Matej Vouk, et al. "Atypical Skull-Base Osteomyelitis" Encyclopedia, https://encyclopedia.pub/entry/43973 (accessed January 14, 2026).
Urbančič, J., Vozel, D., Battelino, S., Bošnjak, R., Kokošar Ulčar, B., Matos, T., Munda, M., Papst, L., Steiner, N., Vouk, M., & Zidar, N. (2023, May 08). Atypical Skull-Base Osteomyelitis. In Encyclopedia. https://encyclopedia.pub/entry/43973
Urbančič, Jure, et al. "Atypical Skull-Base Osteomyelitis." Encyclopedia. Web. 08 May, 2023.
Copy Citation
Atypical skull-base osteomyelitis is a rare but fatal disease that usually involves infection of the ethmoid, sphenoid, occipital, or temporal bones that form the skull base. Unlike typical (so-called otogenic), atypical skull-base osteomyelitis has no otogenic cause. Instead, some authors call atypical skull-base osteomyelitis sinonasal, since the infection most often originates from the nose and paranasal sinuses.
osteomyelitis
osteitis
aspergillosis
mucormycosis
clivus
sphenoid sinusitis
1. Introduction
Percival Pott first described osteomyelitis of the cranial bones in 1775 in a patient with a sub-pericranial abscess resulting from a frontal bone injury [1]. Later, it became known that the cause of such an infection was not an injury but the spread of infection from neighbouring structures, for example, paranasal sinuses. Meltzer and Kelemen first described skull-base osteomyelitis (SBO) in 1959 in patients with a burn injury and osteomyelitis of the external auditory canal [2]. Subsequently, it became known that SBO is not only the result of the progression of inflammation of the external auditory canal [3] but also inflammation of the face, nose, paranasal sinuses, oral cavity, and pharynx [4][5].
Atypical skull-base osteomyelitis (ASBO) is a rare but fatal disease and usually involves infection of the ethmoid, sphenoid, occipital, or temporal bones that make up the skull base. Unlike typical SBO, which is usually the result of advanced necrotising external otitis (so-called otogenic), ASBO does not have an otogenic cause. Some authors call ASBO sinonasal or non-otogenic SBO [4]. Other authors divide it into non-sino-rhino-otogenic and sino-rhino-otogenic, and the latter into SBO of the front (i.e., anterior), middle (i.e., central), and posterior cranial base (i.e., posterior SBO) [5].
2. Pathogenesis of Atypical Skull-Base Osteomyelitis
2.1. Causes and Routes of Disease Spread
ASBO can occur as a result of advanced or untreated infection of the deep tissues of the face, oral cavity, pharynx, or nasal and paranasal sinuses, usually the sphenoid (i.e., basisphenoid) and occipital bones (i.e., basiocciput) [4][6]. Rarely, the cause of the infection is hematogenous from a remote source, e.g., from the lung or spine [5][7].
The infection spreads along the soft tissues at the skull base, and when it invades the Haversian canals, it also begins to spread along the cancellous bone. Due to the spread of the infection, neurovascular structures are affected along their extracranial course, through their foramina at the skull base, and intracranially. Therefore, knowledge of the precise surgical anatomy of the skull base is necessary to understand the clinical picture of ASBO.
The most common cause of ASBO is an advanced paranasal sinus infection. From the sphenoid sinus, which is the centre of the skull base, the infection can spread in all directions, i.e., anterior, middle, or posterior cranial fossa, orbit, and adjacent paranasal sinuses. From the ethmoid cells, the infection first spreads to the adjacent paranasal sinuses (frontal, maxillary, and sphenoid sinus) and then across the borders of the paranasal sinuses to the orbit and, above all, to the intracranial space. From the maxillary sinus, the infection (e.g., odontogenic maxillary sinusitis) spreads through the pterygopalatine and infratemporal fossa into the middle cranial fossa, orbit, sphenoid sinus, and through the ostiomeatal complex, into the ethmoid cells and frontal sinus. Finally, the infection spreads through the posterior wall of the frontal sinus to the anterior cranial fossa. The occurrence of remote intracranial infection (e.g., intracerebral abscess, epidural abscess, subdural empyema) is possible due to the venous drainage of the face and paranasal cavities into the cerebral venous sinuses.
ASBO can also be iatrogenic, for example, after endoscopic surgery of the nose and paranasal sinuses [8]. The occurrence of SBO after bilateral transnasal endoscopic sphenopalatine artery cauterisation and sphenoidotomy has been described [9]. The occurrence of clival abscess or osteomyelitis after adenoidectomy [10][11] and epipharyngeal cyst excision [12] has also been described. Thornwaldt’s cyst infection can also cause ASBO [13]. Injuries to the skull base, especially in the case of contaminated wounds (e.g., sharp or gunshot injuries), can lead to ASBO [5]. In patients after head and neck cancer radiotherapy, the tissue at the skull base is more susceptible to osteoradionecrosis and SBO [14].
In some cases, the cause of ASBO is unknown. Some authors also report otogenic ASBO without otoscopic signs of ear infection. The absence of signs is associated with regression of necrotising external otitis, but the progression of infection at the skull base spreads to the clivus [15][16].
2.2. Patients’ Predispositions
Mostly, infection usually does not progress to ASBO, as certain patient predispositions or virulence factors of the microbe are also required [10][17]. Patients with diabetes and immune suppression are particularly susceptible [18]. Diabetes causes immunodeficiency and poor blood circulation due to damage to small (i.e., microangiopathy) and large vessels (i.e., macroangiopathy), which hampers tissue regeneration. Similarly, other diseases that cause vascular damage or poor oxygen delivery, e.g., post-irradiation changes, vasculitis, cancer, diseases of bone metabolism (e.g., osteoporosis, osteopetrosis, Paget’s disease), malnutrition, anaemia, cardiovascular diseases, liver failure, kidney failure, smoking, obesity, chronic lung disease, and prolonged hospitalisation, predispose to the development of SBO [4][19]. Advanced age is an independent risk factor [5]. Risk factors for ASBO are rarely absent [4][20]. For that reason, they must be identified and controlled.
2.3. Causative Microbes of Atypical Skull-Base Osteomyelitis
ASBO is most commonly caused by Staphylococcus aureus, followed by Pseudomonas aeruginosa and atypical mycobacteria. Pseudomonas aeruginosa is less likely to cause ASBO than typical SBO. More commonly than typical SBO, ASBO results from a fungal infection, most commonly with Aspergillus spp., usually in patients with neutropenia. Less often, ASBO results from infection with Candida spp. [5], Rhizopus spp., and Mucor spp., the latter usually in patients with diabetic ketoacidosis, causing a clinical picture of acute fulminant invasive fungal rhinosinusitis [4][18][19][21]. In the literature, cases of ASBO due to infection with the Streptococcus anginosus group, Eikenella corrodens, Serratia marcescens, Enterococcus faecium, Peptostreptococcus spp., Mycobacterium tuberculosis [5][7], Klebsiella pneumoniae [14], Propionibacterium acnes [9], Nocardia spp. [22], Morganella spp. [23], and Bacteroides spp. [5] are described.
More often than in typical SBO, the results of microbiological tests are negative in ASBO, making the diagnosis even more complex [8].
3. Treatment of Atypical Skull-Base Osteomyelitis
Patients with ASBO are usually treated in the department of infectious diseases because of long-term antimicrobial therapy and poor general health. The infectious disease specialist leads the treatment and includes other specialists, including—an otorhinolaryngologist, neurosurgeon, radiologist, clinical microbiologist, and a pathologist. The mainstay of treatment for ASBO is antimicrobial therapy [18]. At the same time, managing all risk factors for developing ASBO is necessary, e.g., treating diabetes and immune deficiency [19].
3.1. Surgery
Surgical treatment has a role in ASBO as a complement to antimicrobial treatment. Often, it would not be possible to eradicate the disease surgically, or the procedure would be too risky. At the same time, patients are more susceptible to the surgical risks of extensive skull base surgery due to their generally poorer health status and proximity of the vital neurovascular structures (e.g., internal carotid artery, basilar artery, lower cranial nerves). The purpose of surgical treatment is tissue biopsy, decompression of vital neurovascular structures (e.g., transnasal endoscopic transodontoid decompression of the brainstem, optic nerve decompression), abscess drainage (Figure 1), drainage of the paranasal cavities, partial necrectomy, or sequestrectomy, to reduce the microbial load, to improve tissue perfusion, and consequently, for better penetration of antimicrobials. Nevertheless, indications for surgical treatment are still unclear [18][24][25].
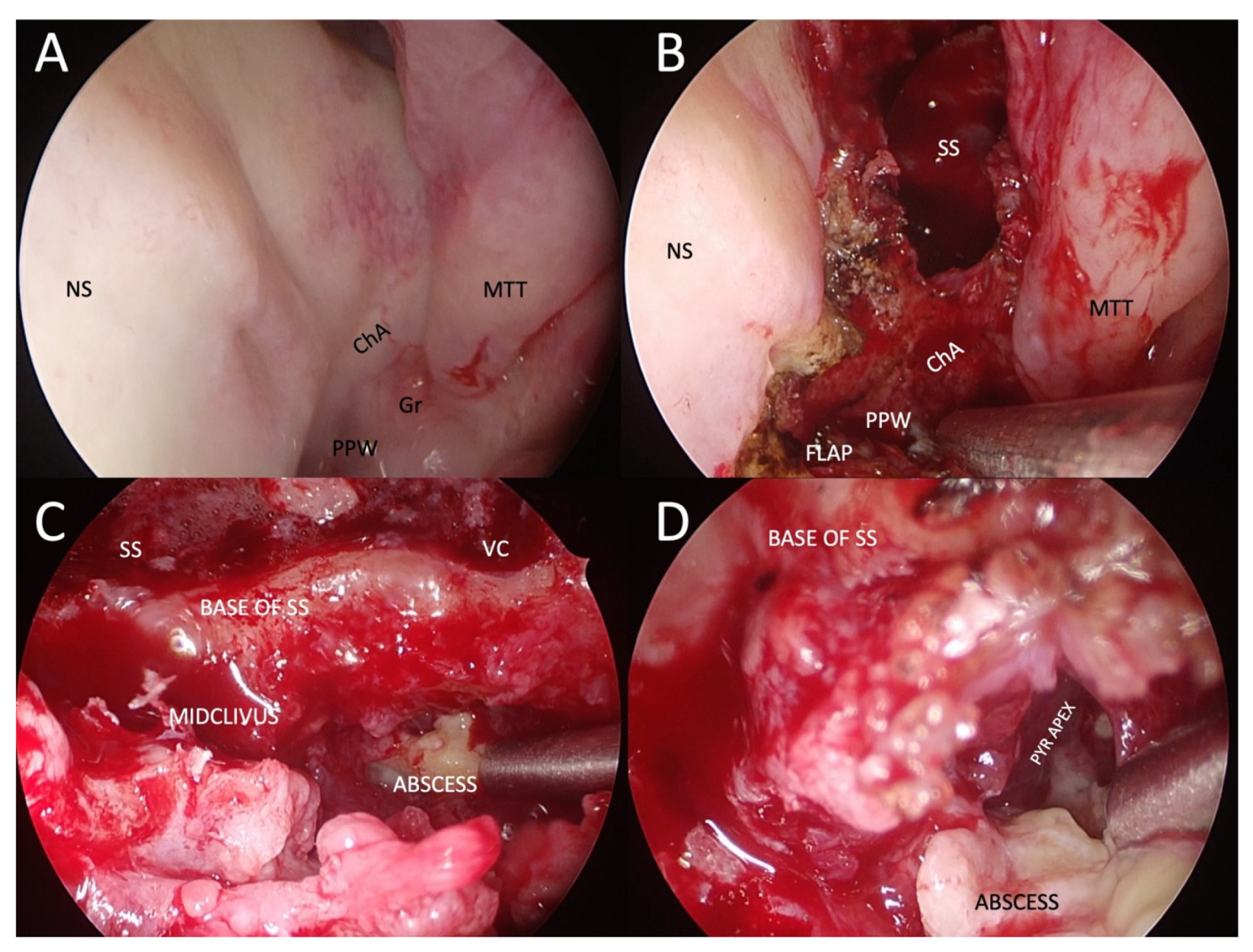
Figure 1. Example of transnasal endoscopic drainage of clival abscess in a patient with left-sided fungal atypical skull-base osteomyelitis. (A) Nasal endoscopy after decongestion, showing very subtle abnormalities, only a granulation tissue on the left side of the nasopharynx and a poorly defined choanal arch. (B) Paraseptal left-sided sphenoidotomy and elevation of nasopharyngeal flap pedicled on the inferomedial posterior nasopharyngeal wall. (C) Close-up view of the base of the left sphenoid sinus and mid-clivus after drilling to gain access to the abscess cavity. (D) Abscess cavity is drained with blunt dissection to visualise the tract under the pyramid apex. NS: nasal septum; ChA: choanal arch; MTT: middle turbinate tail; PPW: posterior pharyngeal wall; SS: sphenoid sinus; VC: an area of the Vidian canal; PYR APEX: pyramid apex.
3.2. Antimicrobial Treatment
Long-term pathogen-specific antimicrobial therapy remains the mainstay of treatment [18]. Early empiric therapy with broad-spectrum intravenous antibiotics should include coverage for Pseudomonas aeruginosa (e.g., antipseudomonal beta-lactam, a third-generation cephalosporin or carbapenem) and methicillin-resistant Staphylococcus aureus (e.g., vancomycin) [4][8][24]. A biopsy should be performed before initiating antimicrobial therapy to increase the microbiologic yield. Unless the patient’s history, microbiological (culture/fungal markers), or pathology results suggest fungal infection, empiric broad-spectrum antifungal therapy is not indicated. However, it should be considered if there is no clinical improvement despite appropriate empiric antibiotics [14][18]. According to the literature, the optimal treatment duration is unknown. Based on case reports and case series, the suggested length of antimicrobial therapy is 6–20 weeks [4][8][18][24], started by intravenous antimicrobial therapy for a minimum of 6 weeks [8][18]. The final duration of antimicrobial therapy with a good treatment response should be based on clinical status and serial inflammatory markers, whereby a normalisation of ESR is a good indicator of infection resolution [15][18]. Long-term monitoring with CT or MRI is generally not helpful because radiologic abnormalities of the bone may persist for weeks to months despite clinical improvement [4][18]. However, radiologic imaging should be performed in case of clinical deterioration. The variable duration of treatment is highly based on the patient’s immune status, the extent of the initial infection, the opportunity for source control procedures, the tolerability of antimicrobial therapy, and the risk of treatment failure [14]. Nevertheless, there is no evidence of an association between diabetes and the longer duration of antimicrobial therapy [18]. In patients with fungal infection (especially in immunocompromised patients), oral antifungal therapy is usually prolonged by up to 6–12 months, or even more, and depends on the patient’s underlying disease, immune status, and response to therapy.
3.3. Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy is effective in some cases of ASBO. However, the research is insufficient, so routine use is not indicated. Nevertheless, this method has a role as a complementary treatment, especially in recalcitrant ASBO [5][18]. This treatment increases the partial pressure of oxygen in tissues, reduces tissue hypoxia, improves phagocytosis, and accelerates angiogenesis and osteogenesis. An example of a hyperbaric oxygen treatment regimen is a 90-min dive at a pressure of 2.5 atmospheres, 5 days a week, for 1 month [19].
4. Prognosis of Atypical Skull-Base Osteomyelitis
ASBO is a serious life-threatening condition with the possibility of severe complications. It presents with at least one cranial nerve dysfunction in 21–48% of cases. Although neurological improvement during treatment depends on the individual case and is not universal, paresis persists in approximately 30% of patients. Correction of paresis can occur due to nerve regeneration after a cured disease, decompression of neural structures, or compensation from the contralateral side. As such, surgical intervention does not directly contribute to improving neurological deficits. Post-operative deterioration may occur at the expense of radical resection of the affected bone, which surrounds the already damaged neural structures. The disease can be complicated by the spread of the infection to the surrounding soft tissues, and in rare cases, to the brain or meninges. Disseminated infection is associated with a higher risk of sepsis and increased mortality, despite surgical intervention and aggressive antimicrobial therapy [15][18][24].
Considering the relatively rare occurrence of the disease and the small set of studies, the predictive factors of the outcome of ASBO are not entirely clear. Associated diseases are generally the main prognostic factor, and worse disease outcomes have been described in elderly, male, diabetic, and immunocompromised patients, and patients with chronic ear disease [15][26]. The most important independent factor for a more favourable outcome is a multidisciplinary approach to the patient, with early and radical surgical removal of the focus of infection and adjuvant antimicrobial therapy. According to some studies, the treatment outcome is improved by the addition of hyperbaric oxygen therapy, which increases the partial pressure of oxygen and thereby reduces tissue hypoxia, and also improves phagocytosis and promotes angiogenesis and osteogenesis [19]. Clinical and radiological parameters, such as the resolution of the disease on imaging, the reduction of pain, and the improvement of cranial nerve paresis, speak in favour of a better outcome [24]. Other variables during treatment (e.g., duration of antibiotic therapy, number of repetitions of therapy) did not prove to be prognostically significant [18].
References
- Pott, P. Observations on the Nature and Consequences of Those Injuries to Which the Head Is Liable from External Violence: To Which Are Added, Some Few General Remarks on Fractures and Dislocations; Printed for L. Hawes, W. Clarke, R.; Collins: London, UK, 1768.
- Meltzer, P.E.; Kelemen, G. Pyocyaneous Osteomyelitis of the Temporal Bone, Mandible and Zygoma. Laryngoscope 1959, 69, 1300–1316.
- Sie, K.C.Y.; Glenn, M.G.; Hillel, A.H. Osteomyelitis of the Skull Base, Etiology Unknown. Otolaryngol. Neck Surg. 1991, 104, 252–256.
- Chapman, P.R.; Choudhary, G.; Singhal, A. Skull Base Osteomyelitis: A Comprehensive Imaging Review. Am. J. Neuroradiol. 2021, 42, 404–413.
- Mortazavi, M.M.; Khan, M.A.; Quadri, S.A.; Suriya, S.S.; Fahimdanesh, K.M.; Fard, S.A.; Hassanzadeh, T.; Taqi, M.A.; Grossman, H.; Tubbs, R.S. Cranial Osteomyelitis: A Comprehensive Review of Modern Therapies. World Neurosurg. 2018, 111, 142–153.
- Oka, K.; Nakano, Y.; Sazumi, Y.; Michitani, T.; Horiguchi, S.; Ocho, K.; Iwamuro, M.; Otsuka, F. Clival Osteomyelitis with Cavernous Sinus Thrombosis Due to Fusobacterium nucleatum and Campylobacter rectus Induced by Tooth Extraction. Intern. Med. 2018, 57, 3325–3328.
- Trück, J.; Thompson, A.; Dwivedi, R.; Segal, S.; Anand, G.; Kelly, D.F. Nonotogenic Skull Base Osteomyelitis in Children: Two Cases and a Review of the Literature. Pediatr. Infect. Dis. J. 2015, 34, 1025–1027.
- See, A.; Tan, T.Y.; Gan, E.C. Atypical Culture-Negative Skull Base Osteomyelitis Masquerading as Advanced Nasopharyngeal Carcinoma. Am. J. Otolaryngol. 2016, 37, 236–239.
- Shellman, Z.; Coates, M.; Kara, N. Polymicrobial Skull Base Osteomyelitis Related to Chronic Sphenoiditis and Endoscopic Sinus Surgery. Laryngoscope 2021, 131, E1086–E1087.
- Kayode-Ajala, F.; Williams, N.; Ejikeme, C.; Walji, A.; Farrer, W. A Case of Adult Clival Osteomyelitis. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221101856.
- Moreddu, E.; Treut, C.L.; Triglia, J.-M.; Nicollas, R. Abscess with Osteomyelitis of the Clivus after Adenoidectomy: An Uncommon Complication of a Common Procedure. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 81–82.
- Raskin, J.; Borrelli, M.; Wu, A.W. Skull Base Osteomyelitis After Nasopharyngeal Cyst Excision. Ear. Nose. Throat J. 2021, 100, 867S–869S.
- Benadjaoud, Y.; Klopp-Dutote, N.; Choquet, M.; Brunel, E.; Guiheneuf, R.; Page, C. A Case of Acute Clival Osteomyelitis in a 7-Year-Old Boy Secondary to Infection of a Thornwaldt Cyst. Int. J. Pediatr. Otorhinolaryngol. 2017, 95, 87–90.
- Czech, M.M.; Hwang, P.H.; Colevas, A.D.; Fischbein, N.; Ho, D.Y. Skull Base Osteomyelitis in Patients with Head and Neck Cancer: Diagnosis, Management, and Outcomes in a Case Series of 23 Patients. Laryngoscope Investig. Otolaryngology 2022, 7, 47–59.
- Clark, M.P.A.; Pretorius, P.M.; Byren, I.; Milford, C.A. Central or Atypical Skull Base Osteomyelitis: Diagnosis and Treatment. Skull Base Off. J. North Am. Skull Base Soc. Al. 2009, 19, 247–254.
- Di Lullo, A.M.; Russo, C.; Grimaldi, G.; Capriglione, P.; Cantone, E.; del Vecchio, W.; Motta, G.; Iengo, M.; Elefante, A.; Cavaliere, M. Skull Base Fungal Osteomyelitis: A Case Report and Review of the Literature. Ear. Nose. Throat J. 2021, 100, 1089S–1094S.
- Radhakrishnan, S.; Mujeeb, H.; Radhakrishnan, C. Central Skull Base Osteomyelitis Secondary to Invasive Aspergillus Sphenoid Sinusitis Presenting with Isolated 12th Nerve Palsy. IDCases 2020, 22, e00930.
- Johnson, A.K.; Batra, P.S. Central Skull Base Osteomyelitis: An Emerging Clinical Entity. Laryngoscope 2014, 124, 1083–1087.
- Jáñez, F.Á.; Barriga, L.Q.; Iñigo, T.R.; Lora, F.R. Diagnosis of Skull Base Osteomyelitis. Radiographics 2021, 41, 156–174.
- Gómez, D.F.; Vargas-Osorio, M.P.; Hakim, F.; Tejada-Angarita, K.S. Skull Base Osteomyelitis Mimicking Skull Base Tumor in Immunocompetent Pediatric Patient: Case Report. Childs Nerv. Systems 2022, 38, 1833–1835.
- Blyth, C.C.; Gomes, L.; Sorrell, T.C.; da Cruz, M.; Sud, A.; Chen, S.C.-A. Skull-Base Osteomyelitis: Fungal vs. Bacterial Infection. Clin. Microbiol. Infect. 2011, 17, 306–311.
- Abou-Al-Shaar, H.; Mulvaney, G.G.; Alzhrani, G.; Gozal, Y.M.; Oakley, G.M.; Couldwell, W.T. Nocardial Clival Osteomyelitis Secondary to Sphenoid Sinusitis: An Atypical Skull Base Infection. Acta Neurochir. 2019, 161, 529–534.
- Schreiber, A.; Ravanelli, M.; Rampinelli, V.; Ferrari, M.; Vural, A.; Mattavelli, D.; Mataj, E.; Mazza, V.; Zorza, I.; Bonù, M.L.; et al. Skull Base Osteomyelitis: Clinical and Radiologic Analysis of a Rare and Multifaceted Pathological Entity. Neurosurg. Rev. 2021, 44, 555–569.
- Ridder, G.J.; Breunig, C.; Kaminsky, J.; Pfeiffer, J. Central Skull Base Osteomyelitis: New Insights and Implications for Diagnosis and Treatment. Eur. Arch. Otorhinolaryngol. 2015, 272, 1269–1276.
- Burns, T.C.; Mindea, S.A.; Pendharkar, A.V.; Lapustea, N.B.; Irime, I.; Nayak, J.V. Endoscopic Transnasal Approach for Urgent Decompression of the Craniocervical Junction in Acute Skull Base Osteomyelitis. J. Neurol. Surg. Rep. 2015, 76, e37–e42.
- Mejzlik, J.; Cerny, M.; Zeinerova, L.; Dedkova, J.; Kopriva, J.; Zadrobilek, K.; Adamkov, J.; Chrobok, V.; Pellantova, V. The Routes of Infection Spread in Central Skull-Base Osteomyelitis and the Diagnostic Role of CT and MRI Scans. BMC Med. Imaging 2019, 19, 60.
More
Information
Subjects:
Otorhinolaryngology; Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
09 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




