
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giacometta Mineo | -- | 1178 | 2023-05-08 09:33:42 | | | |
| 2 | Dean Liu | Meta information modification | 1178 | 2023-05-09 02:36:07 | | | | |
| 3 | Dean Liu | Meta information modification | 1178 | 2023-05-09 02:37:53 | | |
Video Upload Options
Electrochemical energy storage devices are one of the main protagonists in the ongoing technological advances in the energy field, whereby the development of efficient, sustainable, and durable storage systems aroused a great interest in the scientific community. Batteries, electrical double layer capacitors (EDLC), and pseudocapacitors are characterized in depth in the literature as the most powerful energy storage devices for practical applications. Pseudocapacitors bridge the gap between batteries and EDLCs, thus supplying both high energy and power densities, and transition metal oxide (TMO)-based nanostructures are used for their realization. Among them, WO3 nanostructures inspired the scientific community, thanks to WO3’s excellent electrochemical stability, low cost, and abundance in nature.
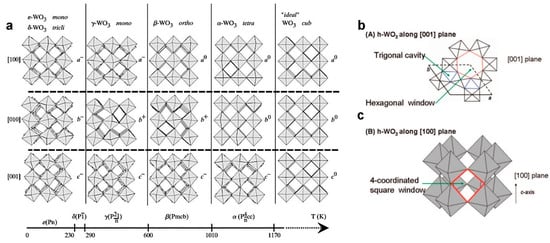
1. Crystal Structure Properties
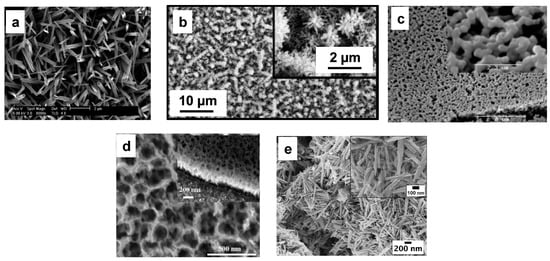
2. WO3 Nanostructure Synthesis Approaches
3. Affinity of WO3 for Energy Storage Applications
References
- Tian, J.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H Porous WO3 @ CuO Composites Derived from Polyoxometalates @ Metal Organic Frameworks for Supercapacitor. Mater. Lett. 2017, 206, 91–94.
- Panwar, N.L.; Kaushik, S.C.; Kothari, S Role of Renewable Energy Sources in Environmental Protection: A Review. Renew. Sustain. Energy Rev. 2011, 15 , 1513–1524.
- Castro-Gutiérrez, J.; Celzard, A.; Fierro, V. Energy Storage in Supercapacitors: Focus on Tannin-Derived Carbon Electrodes. . Front. Mater. 2020, 7, 217.
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater 2011, 21, 2175–2196.
- Mineo, G.; Scuderi, M.; Bruno, E.; Mirabella, S. ngineering Hexagonal/Monoclinic WO3 Phase Junctions for Improved Electrochemical Hydrogen Evolution Reaction.. ACS Appl. Energy Mater. 2022, 5, 9702–9710..
- 36. Baek, Y.; Yong, K. Controlled Growth and Characterization of Tungsten Oxide Nanowires Using Thermal Evaporation of WO3 Powder.. J. Phys. Chem. C 2007,, 111, 1213–1218.
- Shankar, N.; Yu, M.F.; Vanka, S.P.; Glumac, N.G. Synthesis of Tungsten Oxide (WO3) Nanorods Using Carbon Nanotubes as Templates by Hot Filament Chemical Vapor Deposition.. Mater. Lett. 2006, 60, 771–774.
- Yang, B.; Zhang, Y.; Drabarek, E.; Barnes, P.R.F.; Luca, V. Enhanced Photoelectrochemical Activity of Sol-Gel Tungsten Trioxide Films through Textural Control. Chem. Mater. 2007, 19, 5664–5672.
- Zheng, H.; Sadek, A.Z.; Latham, K.; Kalantar-Zadeh, K. Nanoporous WO3 from Anodized RF Sputtered Tungsten Thin Films. . Electrochem. Commun. 2009, 11, 768–771.
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 1699-1713.
- 29. Mineo, G.; Moulaee, K.; Neri, G.; Mirabella, S.; Bruno, E H2 Detection Mechanism in Chemoresistive Sensor Based on Low-Cost Synthesized WO3 Nanorods.. Sens. Actuators B Chem 2021, 348, 130704..
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Direct Growth of WO3 Nanostructures on Multi-Walled Carbon Nanotubes for High-Performance Fl Exible All-Solid-State Asymmetric Supercapacitor.. Electrochim. Acta 2019, 308, 231–242.
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge Storage in WO3 Polymorphs and Their Application as Supercapacitor Electrode Material. Results Phys 2019, 12, 2012–2020.
- Shi, F.; Li, J.; Xiao, J.; Zhao, X.; Li, H.; An, Q.; Zhai, S.; Wang, K.; Wei, L.; Tong, Y.; et al. Three-Dimensional Hierarchical Porous Lig-nin-Derived Carbon/ WO3 for High-Performance Solid-State Planar Micro-Supercapacitor. Int. J. Biol. Macromol 2021, 190, 11-18.
- Jia, J.; Liu, X.; Mi, R.; Liu, N.; Xiong, Z.; Yuan, L.; Wang, C.; Sheng, G.; Cao, L.; Zhou, X.; et al.et al. Self-Assembled Pancake-like Hexagonal Tungsten Oxide with Ordered Mesopores for Supercapacitors. J. Mater. Chem. A 2018, 6, 15330–15339..
- Dong, X.; Yang, Q.; Yuan, L.; Qi, D.; Wei, X.; Zhou, X.; Chen, S.; Cao, L.; Zeng, Y.; Jia, J.; et al.et al. Oxygen Vacancy-Rich WO3 Heter-ophase Structure : A Trade-off between Surface-Limited Pseudocapacitance and Intercalation-Limited Behaviour.. Chem. Eng. J. 2021, 425, 131431.
- Ji, S.; Chodankar, N.R.; Kim, D. . Aqueous Asymmetric Supercapacitor Based on RuO2—WO3 Electrodes. Electrochim. Acta 2019, 325, 134879.
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater 2015, 5, 1401401.
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. 2007 111 Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO 2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931.
- Lokhande, V.C.; Hussain, T.; Shelke, A.R.; Lokhande, A.C.; Ji, T. Substitutional Doping of WO3 for Ca-Ion Based Supercapacitor.. Chem. Eng. J. 2021, 424, 130557.





