
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Narimane Segueni | -- | 4456 | 2023-05-05 19:22:16 | | | |
| 2 | Catherine Yang | -1 word(s) | 4455 | 2023-05-06 02:43:21 | | |
Video Upload Options
Propolis is a natural hive product collected by honeybees from different plants and trees. The collected resins are then mixed with bee wax and secretions. Propolis has a long history of use in traditional and alternative medicine. Propolis possesses recognized antimicrobial and antioxidant properties. Both properties are characteristics of food preservatives. The antimicrobial and antioxidant properties of propolis were extensively studied. In addition, propolis is recognized as safe (GRAS) and can be considered as an alternative to chemical preservatives. Before its use in food products, propolis was in most cases extracted using different solvents such as ethanol, water, and glycerol. Each solvent offers advantages and disadvantages. Ethanol extracts (PEE) are reported to be the richest extracts in bioactive components. While water extracts (WPE) are the lowest extract on phenolic content. In contrast, the two extracts exhibited a strong flavor and aroma.
1. Fruits and Vegetables
| Foods | Geographical Origin | Used Extracts | References |
|---|---|---|---|
| Vegetables | |||
| Lettuce | Portugal (Bragança, 41°48′ N; 06°45′ W) |
2% Methanol extract | [4] |
| Cucumber and soybean | Brazil (Pato Branco) |
PEE | [5] |
| Celery, leek and butternut squash | Argentine (Juricich, Mendoza) |
PEE | [7][8] |
| Tomato, lettuce seeds, onion roots | Argentine (Tucumán, Santiago del Estero, Chaco) |
PEE | [6] |
| Tomato | Italy | Glycolic extract | [9] |
| Tomato | Bandung | PEE diluted with propylene glycol | [10] |
| Cherry tomato | China | PEE | [11] |
| Cherry tomato | Poland Toruń County (53.03′ N, 18.62′ E) |
PEE + pullulan | [12] |
| Mashed potatoes | Jordan (naour region) |
Nd | [13] |
| Potatoes | Argentine (semi-arid regions of Salta, Santiago del Estero and Tucumán) |
PEE | [14] |
| Chili | Brazil | PEE | [15] |
| Rice | Brazil (Minas Gerais) |
Ethanol, methylene chloride and hexane extract | [16] |
| Fruits | |||
| Sweet cherry | Turkey (Hatay province (Eastern Mediterranean region of Turkey)) |
WPE and PEE | [17] |
| Sweet cherry | China | Propolis fresh liquid | [18] |
| Citrus | China (Baoding County, Hebei Province) |
PEAE (propolis ethyl acetate extract) | [19] |
| Mandarins | China (Baoding County, Hebei Province) |
PEAE | [19] |
| Orange | Egypt | PEE | [21] |
| Orange | Iraq (Baghdad) |
Hydro-alcohol extract | [22] |
| Orange | Brazil (southern state of Paraná) |
PEE | [23] |
| Citrus reticulata Blanco | Indonesia | PEE | [24] |
| Star Ruby grapefruit | Turkey (Hatay province) |
PEE | [25] |
| Table grape, cv. Muscatel | Spain (Bonamel Organic S.L. (Alquería de Aznar, Spain)). |
PEE | [26] |
| Dragon fruit | China | PEE | [27] |
| Apple | Iraq (Baghdad) |
PEE | [28] |
| Apple | Egypt | PEE | [29] |
| Mango | Brazil | PEE | [30] |
| Mango | Saudi Arabia | PEE | [31] |
| Banana | Brazil (Bambuí, Minas Gerais) |
Hydro-alcohol extract and aqueous extract | [32] |
| Banana | Saudi Arabia (Hada Al-Sham 21°48′3′′ N, 39°43′25′′ E) |
PEE | [33] |
| Banana | Thailand (Ratchaburi province) |
PEE | [34] |
| Blueberry | Poland (Torun County) |
PEE | [35] |
| Strawberry | Mexico | - | [36] |
| Fig | Mexico | - | [37] |
| Pear | Portugal (central coast and the northern region) |
PEE | [38] |
2. Beverages
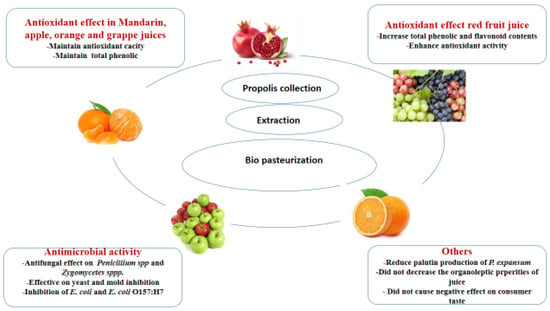
3. Dairy
4. Meat
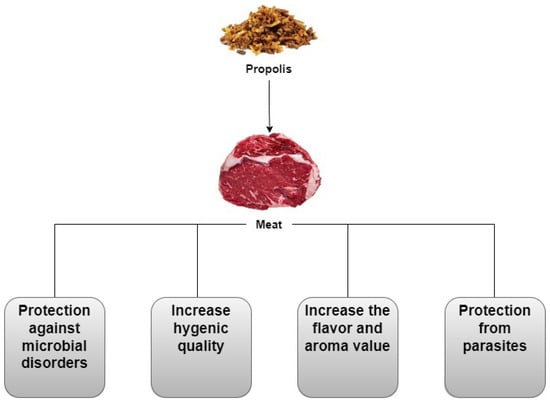
5. Chicken
6. Fish
| Food Product | Propolis Type | Treated Microbe | Origin | Chemical Composition | Reference | |
|---|---|---|---|---|---|---|
| Dairy | Buffaloes’ milk | PE | Total bacterial count, coliforms, molds, and yeasts | Plant Protection Department at the Faculty of Agriculture, Mansoura University | Phenolic compounds (11.18 ± 0.511 mg/g) Flavonoids (7.716 ± 0.587 mg/g) Antioxidant activity (70.44 ± 0.327%) |
[54] |
| Milk | PEE | L. monocytogenes, S. aureus, B. cereus | Val di Cecina (Tuscany, 50–450 m above sea level) | Flavonoids (2.3%) | [52] | |
| Yogurt | PE | B. animalis ssp. lactis, L. delbrueckii subsp. bulgaricus, L. acidophilus, S. thermophilus | Purchased from Sepe Natural Organic Products (Turkey) |
300 mg of Brazilian propolis (3.5 mg Gallic Acid and 2.1 mg Quercetin per mL product) | [55] | |
| Gorgonzola type cheese | Brazilian green PEE | S. equorum, S. saprophyticus, C. parapsilosis, S. cerevisiae, C. flavescens | Campo das Vertentes (Latitude 21 140 S. Longitude 44 590) in the state of Minas Gerais, Brazil |
ND | [73] | |
| Kareish Cheese | PEE | S. thermophilus, B. bifidium, L. bulgaricus | Plant Protection Department at the Faculty of Agriculture, Mansoura University | Phenolic Compounds (13.64 ± 0.440 mg/g), Flavonids (10.57 ± 0.605 mg/g), Antioxidant activity (67.12 ± 0.288%) | [59] | |
| Milk | PEE | L. monocytogenes | Collected in June from Megalopolis (MEG), in the region of Arcadia (central Peloponnese), Greece | Phenolic acids and derivatives (1.42 ± 0.04 mg/g), Flavones and flavonols (6.20 ± 0.32 mg/g), Flavanones and dihydroflavonols (3.56 ± 0.11 mg/g) | [74] | |
| Kashkaval Cheese | PE | Lactic acid bacteria, yeast, and fungi | Purchased from producers from three different regions in the Western part of Bulgaria—town of Simitli, Blagoevgrad district (41°53′ N 23°7′ E), town of Bankya, Sofia district (42°42′ N 23°8′ E), and village of Vladimir, Pernik district (42°26′ N 23°5′ E). |
ND | [75] | |
| Pasteurized and raw milk | Crude organic propolis | Total microbial count | New Valley, Dakhla | ND | [51] | |
| Meat | Dry meat sausage | Raw dark brown propolis | S. aureus, P. aeruginosa | Honey Mahvin Company | Phenolic content (27.08 ± 2.4 mg/g) |
[76] |
| Chicken breast meat | PE | Pseudomonas spp. | Natural propolis was gathered from four beehives. | Flavonoids (25.2%), Sesquiterpenes (9.5%), Aromatic acids (5.01%), Aliphatic hydrocarbons (4.87%), Aromatic hydrocarbons (5.80%), Fatty acids (2.24%), Alcohol (2.05%), Aldehydes (1.45%), Triterpenes (1.12%) | [60] | |
| Beef patties | PE | Mesophilic and Psychrotrophic | Commercial propolis samples 1 and 2 Non-commercial propolis; Pueblo de Alamos, Sonora, Mexico (29°07′129′N) |
Cinnamic acid, Rutin, Myricetin, Quercetin, Chrysin, Kaempferol, Apigenin, Pinocembrin, Luteolin, Acetin. | [77] | |
| Minced beef | PE | L, monocytogenes, P. aeruginosa, F. oxysporum, S. cerevisiae | National Research Center in Cairo, Egypt |
Pentacosane (0.36%), 5,5-D2-Trans-4,3-Dihydroxycylopentene (0.64%), Dodecane (1.04%), Trans-cyclohexanol,2-(methylaminomethyl) (7.30%), 4-aminocyclohepta[f]thieno[2,3-b]pyridine (66.02%), A-Neooleana-3(5), 12-diene (22.99%), 4-Methoxyamphetamine (1.66%) |
[78] | |
| Minced beef | PEE | S. aureus, B. subtilis, B. cereus, L. monocytogenes, S. typhimurium and E. coli O157:H7 | Purchased from an apiary located in the suburb of Kermanshah, in the west of Iran | Lindane (11.96%), caffeic acid (11.14%), 3-cyano-5, 6-dihydro-4-(methylthio)- 2-phenylbenzo[h] quinoline (10.31%), buxozine-c (9.01%), 3,4-bis (3-byano-2-methylphenyl)-2,5-dimethylfuran (8.45%), 12-azabicyclo [9.2.2] pentadeca-1(14), 11(15)-dien-13-one (8.43%), and naringenin (7.31%) |
[79] | |
| Beef Meatballs | WPE | S. epidermidis, E. faecalis, L. monocytogenes | ND | Antioxidant Activity (IC50 38.025 ± 0.135 µg/mL) FRAP (23.27 ± 0.26 µM of Fe + 2/g) |
[62] | |
| Chicken | Chicken breast fillet | PEE | Pseudomonas spp. | Natural propolis was gathered from four beehives | Flavonoids (25.2%), Sesquiterpenes (9.5%), Aromatic acids (5.01%), Aliphatic hydrocarbons (4.87%), Aromatic hydrocarbons (5.80%), Fatty acids (2.24%), Alcohol (2.05%), Aldehydes (1.45%) | [60] |
| Chicken fillet | PEE | S. aureus | Collected from different locations of Tehran Province in May 2016 | ND | [65] | |
| 120-days old chicks | Egyptian propolis | E. coli | Dakahlia Governorate | ND | [64] | |
| One day old chickens | PE | Salmonella spp. | ND | ND | [67] | |
| Broiler chickens of hybrid combination Ross 308 | Propolis and bee pollen | E. faecalis | ND | ND | [68] | |
| Chicken breast | PE | Yeasts and molds, S. aureus, E. coli | Supplied from the mountainous area west of Guilan province | Phenolic compounds (5.83 ± 0.505 g/ 100 g), Flavonoids (4.92 ± 0.562 g/mL), Antioxidant activity (15.83 ± 2.341%) |
[69] | |
| Fish | Nile tilapia (Oreochromis niloticus) | PEE and crude propolis | A. hydrophila | Collected in summer from Upper Egypt | ND | [80] |
| Fish | PEE | A. hydrophila, Y. ruckeri and S. iniae | Adana region | Propolis antioxidant activity value (569.68 µmol trolox/g), phenolic substance content (593.31 mg GAE/g) | [72] | |
| Rainbow trout (Oncorhynchus mykiss) |
PE | Mesophilic, psychrophilic, yeast and mold, coliform | Adana region | Propolis antioxidant activity value (569.68 µmol trolox/g), phenolic substance content (593.31 mg GAE/g) | [72] | |
| Fish | PE | L. plantarum, E. cloacae, P. luteola, P. mirabilis, and P. damselea |
Propolis was produced by Apis mellifera in January 2020, Adana, Turkey. | ND | [81] | |
| Rainbow trout (Oncorhynchus mykiss) | PE | Mesophilic and psychrotrophic bacteria | Propolis was collected from a farm at village Kocaavsar in Balikesir, Turkey. |
ND | [82] | |
| Nemipterus japonicus fillets | PEE | Mesophilic and psychrotrophic bacteria | ND | ND | [83] | |
References
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62.
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11.
- Yang, W.; Wu, Z.; Huang, Z.Y.; Miao, X. Preservation of orange juice using propolis. J. Food Sci. Technol. 2017, 54, 3375–3383.
- Feás, X.; Pacheco, L.; Iglesias, A.; Estevinho, L.M. Use of propolis in the sanitization of lettuce. Int. J. Mol. Sci. 2014, 15, 12243–12257.
- Guginski-Piva, C.A.; dos Santos, I.; Wagner Júnior, A.; Heck, D.W.; Flores, M.F.; Pazolini, K. Propolis for the control of powdery mildew and the induction of phytoa-lexins in cucumber . Idesia 2015, 33, 39–47.
- Ordóñez, R.M.; Zampini, I.C.; Moreno, M.N.; Isla, M.I. Potential application of Northern Argentine propolis to control some phytopathogenic bacteria. Microbiol. Res. 2011, 166, 578–584.
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Combined effect of bioactive compounds and storage temperature on sensory quality and safety of minimally processed celery, leek and butternut squash. J. Food Saf. 2015, 35, 560–574.
- Alvarez, M.V.; Ponce, A.G.; Goyeneche, R.; Moreira, M.R. Physical treatments and propolis extract to enhance quality attributes of fresh-cut mixed vegetables. J. Food Process. Preserv. 2017, 41, e13127.
- Migliori, C.A.; Salvati, L.; Di Cesare, L.F.; Scalzo, R.L.; Parisi, M. Effects of preharvest applications of natural antimicrobial products on tomato fruit decay and quality during long-term storage. Sci. Hortic. 2017, 222, 193–202.
- Putra, R.E.; Khairannisa, S.; Kinasih, I. Effect of Application Propolis as Biocoating on the Physical and Chemical Properties of Tomatoes Stored at Room Temperature. IOP Conf. Ser. Earth Environ. Sci. 2017, 58, 012026.
- Liu, X.; An, X.; Li, Y.; Niu, Y.; Yun, J. Study on the preservation effect of propolis and beeswax composite coating agent on cherry tomatoes. Food Ferment. Technol. 2019, 55, 38–43.
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the shelf life of cherry tomatoes by pullulan coating with ethanol extract of propolis during refrigerated storage. Food Bioprocess Technol. 2020, 13, 1447–1461.
- Bahtiti, N.H. Study of preservative effect of “propolis” on the storage quality of mashed potatoes. Food Sci. Technol. 2013, 1, 17–20.
- Sampietro, D.A.; Bertini Sampietro, M.S.; Vattuone, M.A. Efficacy of Argentinean propolis extracts on control of potato soft rot caused by Erwinia carotovora subsp. J. Sci. Food Agric. 2020, 100, 4575–4582.
- Ali, A.; Chow, W.L.; Zahid, N.; Ong, M.K. Efficacy of propolis and cinnamon oil coating in controlling post-harvest anthracnose and quality of chilli (Capsicum annuum L.) during cold storage. Food Bioprocess Technol. 2014, 7, 2742–2748.
- Griffiths, G.A.; Toshitaka, U.; Fumihiko, T.; Daisuke, H. Effect of vapors from fractionated samples of propolis on microbial and oxidation damage of rice during storage. J. Food Eng. 2008, 88, 341–352.
- Candir, E.E.; Ozdemir, A.E.; Soylu, E.M.; Sahinler, N.; Gul, A. Effects of propolis on storage of sweet cherry cultivar Aksehir Napolyon. Asian J. Chem. 2009, 21, 2659–2666.
- Yang, Y.; Gong, Y.; Gao, Y.; Huang, J.; Xu, M.; Xiong, B. Study on the preservation effect of propolis on sweet cherry. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 032029.
- Yang, S.; Peng, L.; Cheng, Y.; Chen, F.; Pan, S. Control of citrus green and blue molds by Chinese propolis. Food Sci. Biotechnol. 2010, 19, 1303–1308.
- Abo-Elyousr, K.A.; Al-Qurashi, A.D.; Almasoudi, N.M. Evaluation of the synergy between Schwanniomyces vanrijiae and propolis in the control of Penicillium digitatum on lemons. Egypt. J. Biol. Pest Control 2021, 31, 66.
- El-Badawy, H.E.M.; Baiea, M.H.M.; Abd El-Moneim, E.A.A. Efficacy of propolis and wax coatings in improving fruit quality of ‘Washington’ navel orange under cold storage. Res. J. Agric. Biol. Sci. 2012, 8, 420–428.
- Matny, O.N. Efficacy evaluation of Iraqi propolis against gray mold of stored orange caused by Penicillium digitatum. Plant Pathol. J. 2015, 14, 153.
- Passos, F.A.R.; Mendes, F.I.Q.; da Cunha, M.C.; da Cunha, M.I.C.; de Carvalho, A.E.M.X. Propolis extract coated in Pera orange fruits: An alternative to cold storage. Afr. J. Agric. Res. 2016, 11, 2043–2049.
- Putra, R.E.; Reizandy, F.; Faizal, A.; Kinasih, I. Effication of local propolis as edible coating of tangerine cultivar garut (citrus reticulata blanco). IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012025.
- Özdemir, A.E.; Candir, E.E.; Kaplankiran, M.; Soylu, E.M.; Şahinler, N.; Gül, A. The effects of ethanol-dissolved propolis on the storage of grapefruit cv. Star Ruby. Turk. J. Agric. For. 2010, 34, 155–162.
- Pastor, C.; Sanchez-Gonzalez, L.; Marcilla, A.; Chiralt, A.; Chafer, M.; Gonzalez-Martinez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70.
- Zahid, N.; Ali, A.; Siddiqui, Y.; Maqbool, M. Efficacy of ethanolic extract of propolis in maintaining postharvest quality of dragon fruit during storage. Postharvest Biol. Technol. 2013, 79, 69–72.
- Matny, O.N.; Al-Warshan, S.H.; Ali, A.M. Antifungal evaluation of Iraqi propolis against Penicillium expansum and mycotoxin production in apple. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 399–405.
- Embaby, E.M.; Hazaa, M.M.; Kh, E.D.; Mo, A.M.; Abd-Elgalil, M.M.; Elwan, E.E. Control Apple Fruit Decay by Using ‘Ethanol Extract of Propolis’(EEP). Int. J. Adv. Med. Sci. 2020, 4, 1–11.
- Mattiuz, B.H.; Ducamp-Collin, M.N.; Mattiuz, C.F.M.; Vigneault, C.; Marques, K.M.; Sagoua, W.; Montet, D. Effect of propolis on postharvest control of anthracnose and quality parameters of ‘Kent’ mango. Sci. Hortic. 2015, 184, 160–168.
- Al-Qurashi, A.D.; Awad, M.A. Postharvest ethanolic extract of propolis treatment affects quality and biochemical changes of ‘Hindi-Besennara’ mangos during shelf life. Sci. Hortic. 2018, 233, 520–525.
- Passos, F.R.; Mendes, F.Q.; Cunha, M.C.D.; Pigozzi, M.T.; Carvalho, A.M.X.D. Propolis extract in postharvest conservation banana ‘Prata’. Rev. Bras. Frutic. 2016, 38, e931.
- Awad, M.A.; Al-Qurashi, A.D. Quality and biochemical changes of ‘Sukkari’ bananas during shelf life as affected by postharvest dipping in ethanolic extract of propolis. Philipp. Agric. Sci. 2019, 102, 132–140.
- Sripong, K.; Srinon, T.; Ketkaew, K.; Uthairatakij, A.; Jitareerat, P. Impacts of paraffin wax and propolis on controlling crown rot disease and maintaining postharvest quality of banana. IOP Conf. Ser. Earth Environ. Sci. 2020, 515, 012036.
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020.
- Martínez-González, M.D.C.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Río-García, D.; Ramos-García, M.D.L. Effect of nanostructured chitosan/propolis coatings on the quality and antioxidant capacity of strawberries during storage. Coatings 2020, 10, 90.
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Bautista-Baños, S. Edible chitosan/propolis coatings and their effect on ripening, development of Aspergillus flavus, and sensory quality in fig fruit, during controlled storage. Plants 2021, 10, 112.
- Loebler, M.; Sánchez, C.; Muchagato Maurício, E.; Diogo, E.; Santos, M.; Vasilenko, P.; Cruz, A.S.; Mendes, B.; Gonçalves, M.; Duarte, M.P. Potential Application of Propolis Extracts to Control the Growth of Stemphylium vesicarium in “Rocha” Pear. Appl. Sci. 2020, 10, 1990.
- Aguayo, E.; Martínez-Sánchez, A.; Silveira, A.C.; Tarazona, M.P. Effects of pasteurization and storage time on watermelon juice quality enriched with L-citrulline. Acta Hortic. 2017, 1151, 267–272.
- Chang, Z.Q.; Leong, W.; Chua, L.S. Statistical approach to reveal propolis as a potential bio preservative for fruit juices. Future Foods 2021, 4, 100051.
- Silici, S.; Koc, N.; Sariguzel, F.M.; Sagdic, O. Mould inhibition in different fruit juices by propolis. Arch. Lebensm. 2005, 56, 87–90.
- Koc, A.N.; Silici, S.; Mutlu-Sariguzel, F.; Sagdic, O. Antifungal activity of propolis in four different fruit juices. Food Technol. Biotechnol. 2007, 45, 57–61.
- Sagdic, O.; Silici, S.; Yetim, H. Fate of Escherichia coli and E. coli O157: H7 in apple juice treated with propolis extract. Ann. Microbiol. 2007, 57, 345–348.
- Silici, S.; Karaman, K. Inhibitory effect of propolis on patulin production of Penicillium expansum in apple juice. J. Food Process. Preserv. 2014, 38, 1129–1134.
- Luis-Villaroya, A.; Espina, L.; García-Gonzalo, D.; Bayarri, S.; Pérez, C.; Pagán, R. Bioactive properties of a propolis-based dietary supplement and its use in combination with mild heat for apple juice preservation. Int. J. Food Microbiol. 2015, 205, 90–97.
- Kahramanoglu, I.; Usanmaz, S. Effects of propolis and black seed oil on the shelf life of freshly squeezed pomegranate juice. Food Sci. Nutr. Stud. 2017, 1, 114–121.
- Lopes, G.A.; Fidelis, P.C.; Almeida, B.M.D.; Almeida, J.J.; Ientz, G.D.A.S.; Binda, N.S.; Teixeira, A.F.; Vira-Filho, S.A.; Caligiorne, R.B.; Saude-Guimaraes, D.A.; et al. Antioxidant activity, sensory analysis and acceptability of red fruit juice supplemented with Brazilian green propolis. Food Sci. Technol. 2021, 42, e13521.
- Albaş, M.G.; Gürbüz, B.; Bölük, E.; Sözeri Atik, D.; Velioglu, M.; Palabiyik, İ. Laktik Asit Bazlı Propolis İlavesinin Taze Çilek Suyunun Raf Ömrüne Etkisi. Tekirdağ Ziraat Fakültesi Derg. 2022, 19, 788–797.
- Knight, C.H.; Dewhurst, R.J. Once daliy milking of dairy cows: Relationship between yield loss and cisternal milk storage. J. Dairy Res. 1994, 61, 441–449.
- Gaucher, I.; Mollé, D.; Gagnaire, V.; Gaucheron, F. Effects of storage temperature on physico-chemical characteristics of semi-skimmed UHT milk. Food Hydrocoll. 2008, 22, 130–143.
- Shaban, M.; Abdel-Aleem, W.; Galal, S. Organic Propolis Extract as a Natural Fortifier and Preservative for Milk. J. Food Dairy Sci. 2021, 12, 325–329.
- Pedonese, F.; Verani, G.; Torracca, B.; Turchi, B.; Felicioli, A.; Nuvoloni, R. Effect of an Italian propolis on the growth of Listeria monocytogenes, Staphylococcus aureus and Bacillus cereus in milk and whey cheese. Ital. J. Food Saf. 2019, 8, 8036.
- Michailidis, G.F.; Thamnopoulos, I.-A.I.; Fletouris, D.J.; Angelidis, A.S.J.F.S. Synergistic, bacteriostatic effect of propolis and glycerol against Listeria monocytogenes in chocolate milk under refrigerated storage. Food Sci. Technol. Int. 2021, 27, 46–55.
- El-Deeb, A. Utilization of Propolis Extract as A Natural Preservative in Raw Milk. J. Food Dairy Sci. 2017, 8, 315–321.
- Gunes-Bayir, A.; Bilgin, M.G.; Guclu, D.; Pogda, S.; Dadak, A. Preparation and evaluation of novel functional fermented dairy products containing propolis and cinnamon. J. Food Sci. Technol. 2022, 59, 2392–2401.
- Chon, J.-W.; Seo, K.-H.; Oh, H.; Jeong, D.; Song, K.-Y. Chemical and Organoleptic Properties of Some Dairy Products Supplemented with Various Concentration of Propolis: A Preliminary Study. J. Dairy Sci. Biotechnol. 2020, 38, 59–69.
- Bilici, C. Lepidium Meyenii Tozu ve Propolis Ekstraktı Ilave Edilerek Fonksiyonel Özellikleri Geliştirilmiş Yoğurt Üretilmesi. Master’s Thesis, Marmara Universitesi, Istanbul, Turkey, 2017.
- Güney, F. Bazı Propolis Özütlerinin Meyveli Yoğurtların Biyokimyasal, Fizikokimyasal ve Raf Ömrü Üzerine Etkilerinin Araştırılması. Master’s Thesis, Ordu Üniversitesi Fen Bilimleri Enstitüsü, Ordu, Turkey, 2016.
- El-Deeb, A.; Omar, S. Effect of Propolis Extract as a Natural Preservative on the Microbial Content of Kareish Cheese. J. Food Dairy Sci. 2017, 8, 295–302.
- Mehdizadeh, T.; Mojaddar Langroodi, A. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409.
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Félix, E.; Carvajal-Millán, E.; González-Córdova, A.F.; Vallejo-Galland, B.; Torres-Llanze, M.J.; Sanchez-Escalante, A. Antioxidant and antimicrobial activity of commercial propolis extract in beef patties. J. Food Sci. 2014, 79, C1499–C1504.
- Gedikoğlu, A. Antimicrobial and Antioxidant Activities of Commercialized Turkish Propolis Extract, and Application to Beef Meatballs. Turk. J. Agric.—Food Sci. Technol. 2022, 10, 2021–2029.
- Bernardi, S.; Favaro-Trindade, C.; Trindade, M.; Balieiro, J.; Cavenaghi, A.D.; Contreras-Castilo, C.J. Italian-type salami with propolis as antioxidant. Ital. J. Food Sci. 2013, 25, 433–440.
- Gutiérrez-Cortés, C.; Suarez-Mahecha, H. Antimicrobial Activity of Propolis and Its Effect on the Physicochemical and Sensorial Characteristics in Sausages. Vitae 2014, 21, 90–96.
- Jonaidi Jafari, N.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Preserv. 2018, 42, e13336.
- Mona, S.I.; Naglaa, A.A.; Ismail, H.M. Effect of propolis on the immune response and meat quality in experimentally Escherichia coli infected broilers. Assiut Vet. Med. J. 2021, 67, 101–135.
- Pochop, J.; KaÄániová, M.; Hleba, L.Å. Effects of propolis extrcats in chicken diet against Salmonella typhirium detected by real-time PCR. J. Microbiol. Biotechnol. Food Sci. 2021, 1, 113–125.
- Kročko, M.; Lavová, M.; Bezeková, J.; Čanigová, M.; Gábor, M.; Ducková, V.; Trakovická, A. Antibiotic resistance of Enterococcus faecalis isolated from gastrointestinal tract of broiler chickens after propolis and bee pollen addition. Anim. Sci. Biotechnol. 2012, 45, 58–62.
- Mahdavi-Roshan, M.; Gheibi, S.; Pourfarzad, A. Effect of propolis extract as a natural preservative on quality and shelf life of marinated chicken breast (chicken Kebab). LWT 2022, 155, 112942.
- Hatha, M.; Vivekanandhan, A.; Joice, G.J. Antibiotic resistance pattern of motile aeromonads from farm raised fresh water fish. Int. J. Food Microbiol. 2005, 98, 131–134.
- Petersen, A.; Andersen, J.S.; Kaewmak, T.; Somsiri, T.; Dalsgaard, A. Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl. Environ. Microbiol. 2002, 68, 6036–6042.
- Tukmechi, R. Propolis Ekstraktı ile Zenginleştirilmiş Yenilebilir Filmlerin Alabalık Filetosu Kalitesi Üzerine Etkileri. Master’s Thesis, Niğde Ömer Halisdemir Üniversitesi/Fen Bilimleri Enstitüsü, Niğde, Turkey, 2019.
- Correa, F.T.; De Souza, A.C.; De Souza Júnior, E.A.; Isidoro, S.R.; Piccoli, R.H.; Dias, D.R.; De Abreu, L.R. Effect of Brazilian green propolis on microorganism contaminants of surface of Gorgonzola-type cheese. J. Food Sci. Technol. 2019, 56, 1978–1987.
- Thamnopoulos, I.A.I.; Michailidis, G.F.; Fletouris, D.J.; Badeka, A.; Kontominas, M.G.; Angelidis, A.S. Inhibitory activity of propolis against Listeria monocytogenes in milk stored under refrigeration. Food Microbiol. 2018, 73, 168–176.
- Tumbarski, Y.D.; Todorova, M.M.; Topuzova, M.G.; Georgieva, P.I.; Ganeva, Z.A.; Mihov, R.B.; Yanakieva, V.B. Antifungal activity of carboxymethyl cellulose edible films enriched with propolis extracts and their role in improvement of the storage life of Kashkaval cheese. Curr. Res. Nutr. Food Sci. 2021, 9, 487.
- Safaei, M.; Roosta Azad, R. Preparation and characterization of poly-lactic acid based films containing propolis ethanolic extract to be used in dry meat sausage packaging. J. Food Sci. Technol. 2020, 57, 1242–1250.
- Mahecha, H.S.; Toquica, Á.J.; Moreno, A.C.D. Physico-chemical evaluation of Cachama fillets (Piaractus brachypomus) preserved with propolis during storage. Rev. Fac. Nac. Agron. 2014, 67, 7229–7236.
- El-Demery, M.; Elsebaie, E.; Zidan, N.; Essa, R. Efficiency of Propolis and Turmeric Powders as Natural Preservatives in Minced Beef. J. Food Dairy Sci. 2016, 7, 45–50.
- Shahbazi, Y.; Shavisi, N. A novel active food packaging film for shelf-life extension of minced beef meat. J. Food Saf. 2018, 38, e12569.
- Abd-El-Rhman, A.M.M. Antagonism of Aeromonas hydrophila by propolis and its effect on the performance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2009, 27, 454–459.
- Kuley, E.; Kuscu, M.M.; Durmus, M.; Ucar, Y. Inhibitory activity of Co-microencapsulation of cell free supernatant from Lactobacillus plantarum with propolis extracts towards fish spoilage bacteria. LWT 2021, 146, 111433.
- Fuat Gulhan, M.; Duran, A.; Selamoglu Talas, Z.; Kakoolaki, S.; Mansouri, S.M. Effects of Propolis on microbiologic and biochemical parameters of Rainbow trout (Oncorhynchus mykiss) after exposure to the pesticide. Iran. J. Fish. Sci. 2012, 11, 490–503.
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102.





