Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qingle Zeng | -- | 1411 | 2023-05-05 09:49:18 | | | |
| 2 | Lindsay Dong | Meta information modification | 1411 | 2023-05-06 08:14:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yin, X.; Zhang, Q.; Zeng, Q. Synthesis of Sulfoxides and Sulfinamides from β-Sulfinyl Esters. Encyclopedia. Available online: https://encyclopedia.pub/entry/43837 (accessed on 07 February 2026).
Yin X, Zhang Q, Zeng Q. Synthesis of Sulfoxides and Sulfinamides from β-Sulfinyl Esters. Encyclopedia. Available at: https://encyclopedia.pub/entry/43837. Accessed February 07, 2026.
Yin, Xianjie, Qiaoling Zhang, Qingle Zeng. "Synthesis of Sulfoxides and Sulfinamides from β-Sulfinyl Esters" Encyclopedia, https://encyclopedia.pub/entry/43837 (accessed February 07, 2026).
Yin, X., Zhang, Q., & Zeng, Q. (2023, May 05). Synthesis of Sulfoxides and Sulfinamides from β-Sulfinyl Esters. In Encyclopedia. https://encyclopedia.pub/entry/43837
Yin, Xianjie, et al. "Synthesis of Sulfoxides and Sulfinamides from β-Sulfinyl Esters." Encyclopedia. Web. 05 May, 2023.
Copy Citation
Sulfoxides and sulfinamides play important roles in the pharmaceutical industry, organic synthesis and fine chemicals. Under catalysis by transition metals, β-sulfinyl esters, as nucleophilic reagents, react with a variety of electrophilic reagents to produce sulfoxides and sulfinamides.
β-sulfinyl esters
sulfenate anions
sulfoxides
sulfinamides
1. Introduction
Sulfoxides and sulfinamides have extensive applications in the pharmaceutical industry, organic synthesis, fine chemical industry and material production. They have shown a wide range of biological activities. More than 30% of chemicals contain sulfur components [1], such as the widely used proton pump inhibitors. The drugs in the proton pump inhibitor class contain a sulfoxide pharmacophore structure, among which Esomeprazole is a chiral sulfoxide molecule (Figure 1) [2]. The well-known drug Modafinil is also a type of sulfoxide (Figure 1) [3]. Both sulfoxides and sulfinamides are also key motifs in the chiral synthesis of chiral molecules, such as chiral auxiliaries, chiral ligands and organocatalysts [4].
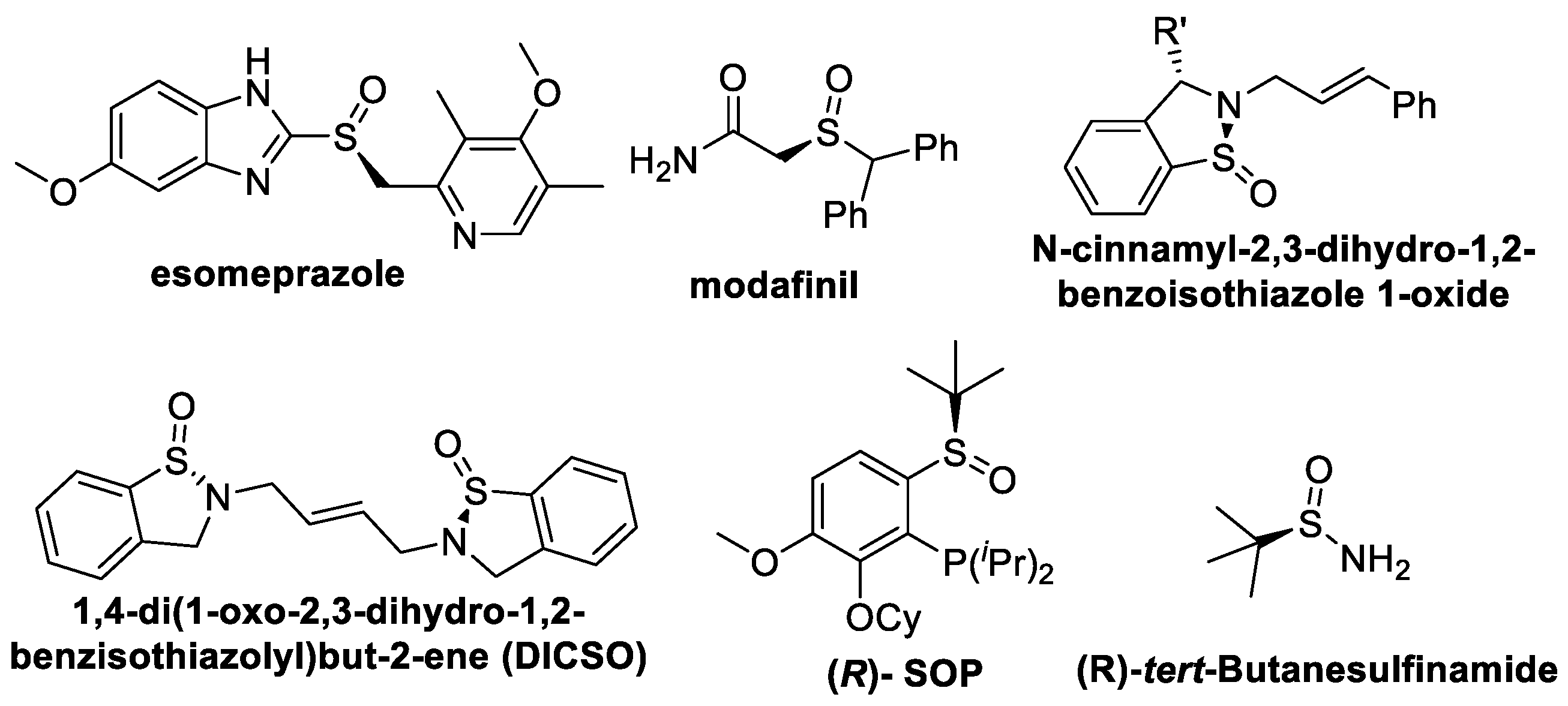
Figure 1. Some typical sulfoxides and sulfinamides.
It is well known that sulfenate anions (RSO−) [5] are the conjugate bases of sulfenic acids (RSOH) [6], and they demonstrate their peculiar S-nucleophilicity in various organic reactions. Sulfenate anions can be depicted by two limited valence bond structures, which can effectively elucidate the ambident nature of sulfenate anions, reacting as nucleophiles either at the sulfur atom or at the oxygen atom (Scheme 1).
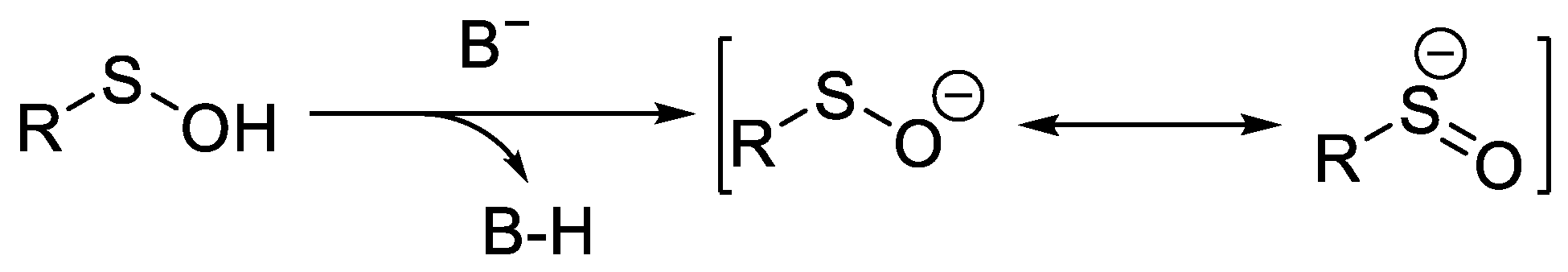
Scheme 1. Generation of sulfenate anions and their tautomerism.
Sulfenate anions are generally transient intermediates and must be generated in situ from a variety of sulfoxides (Scheme 2). One approach to obtaining sulfenate anions is via transition-metal-catalyzed transformations of benzyl (a) [7] and allyl (b) [8] and sulfoxides. However, it seems that transition-metal-free preparation methods are the better choice, for example, the pyrolyzation of tert-butyl aryl sulfoxides and then the base treatment of the resulting sulfenic acids (c) [9][10], the strong nucleophile-caused decomposition of 2-sulfinyl acrylates (d) [11], the fluoride-promoted desilylative fragmentation of 2-(trimethylsilyl)ethyl alkyl sulfoxides (e) [12] and the base-promoted retro-Michael reaction of β-sulfinyl esters (f) [13]. Among these generation methods, the preparation of sulfenate anions via the base-promoted retro-Michael reaction of β-sulfinyl esters (f) has developed most rapidly.
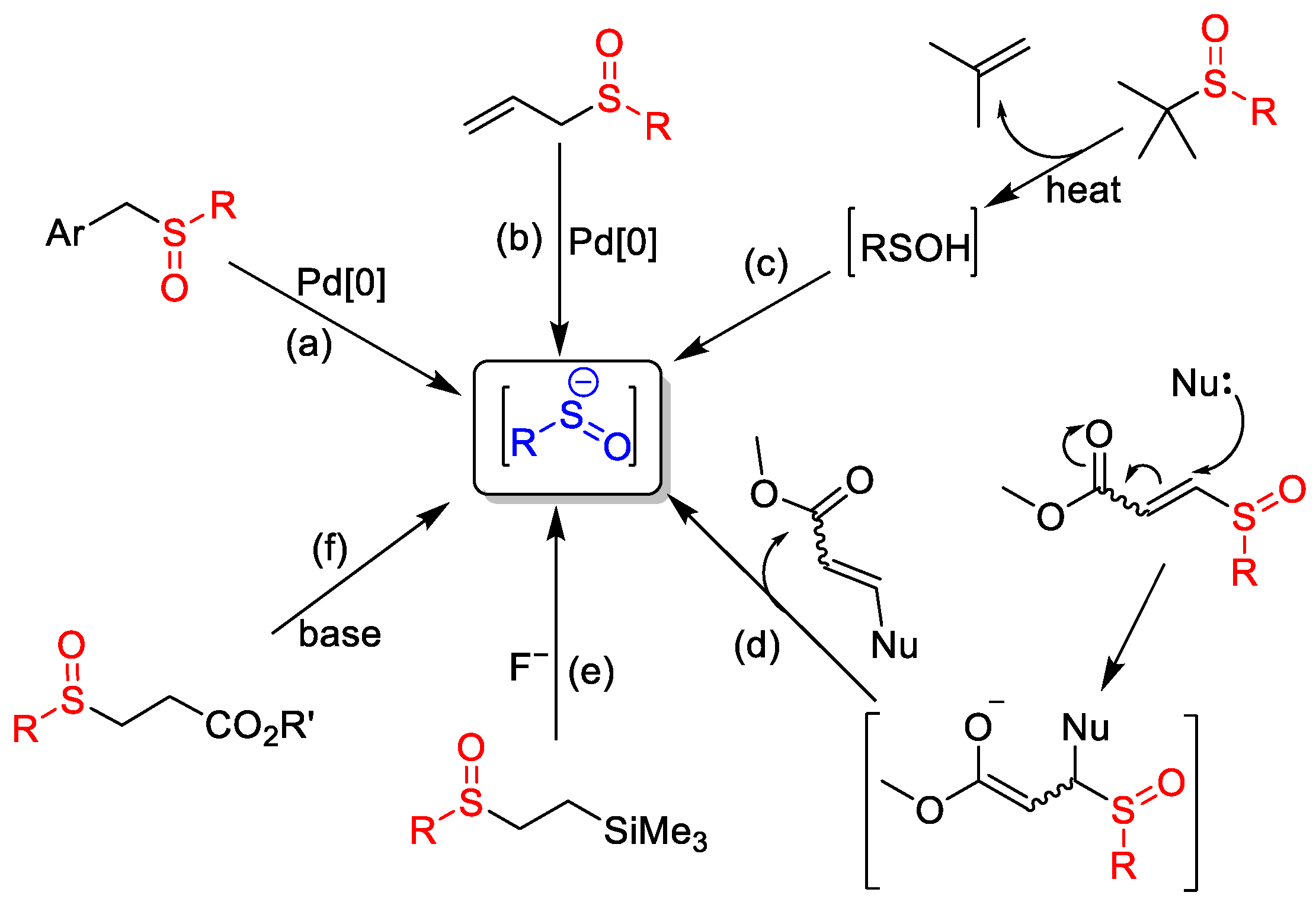
Scheme 2. Some preparation methods of sulfenate anions.
In recent years, chemists have discovered that β-sulfinyl esters can generate sulfenate anions in situ under the action of alkali, and the latter can act as a nucleophile to react with an electrophile to generate sulfoxides and sulfinamides and other sulfur-containing compounds. Moreover, chiral sulfoxides can be synthesized from sulfenate anions.
2. Synthesis of β-Sulfinyl Esters and In Situ Generation of Sulfenate Anions
β-Sulfinyl esters are generally obtained by the conjugated addition of thiophenols and acrylates to give thioether intermediates and then the further selective oxidation of the thioether intermediates. The general synthesis of β-sulfinyl esters is illustrated with 4-methylthiophenol as a specific starting reactant example (Scheme 3) [14].
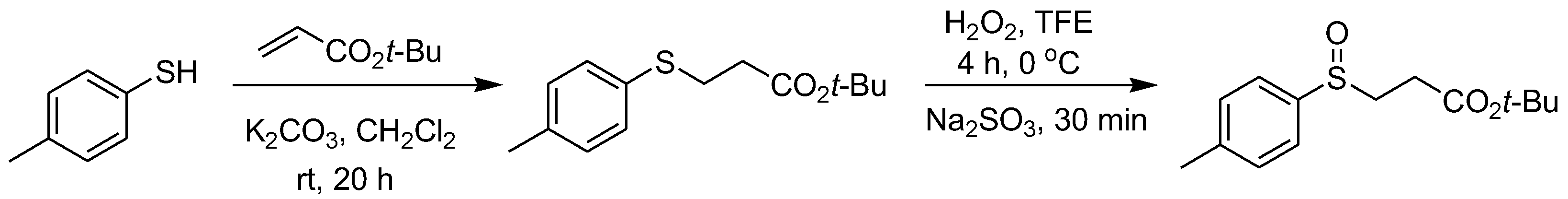
Scheme 3. Typical synthetic method of β-sulfinyl esters.
β-Sulfinyl esters are easily deprotonated by inorganic bases, followed by retro-Michael addition to generate sulfenate anions (RSO−) in situ under mild conditions (Scheme 4) [13].
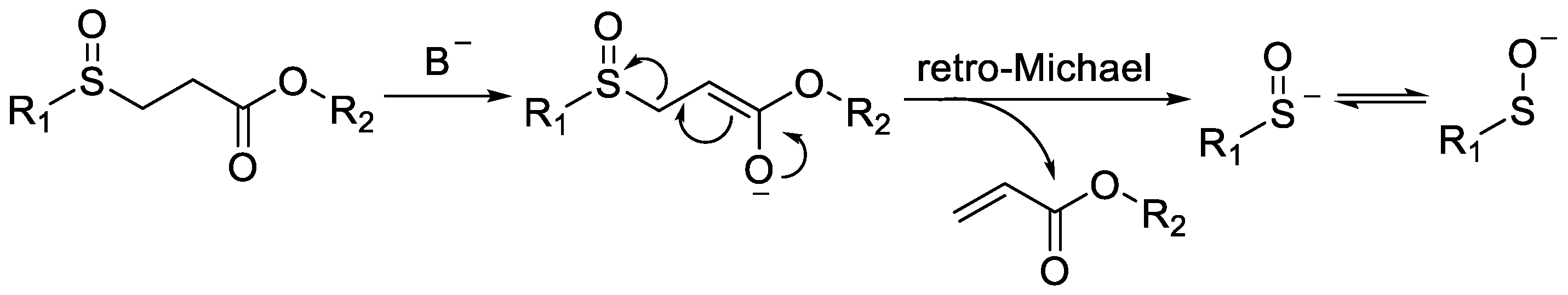
Scheme 4. Alkali-induced in situ generation of sulfenate anion by β-sulfinyl esters.
3. Synthesis of Sulfoxides from β-Sulfinyl Esters
In 2006, Maitro et al. reported a method using palladium as a catalyst to catalyze the reaction of sulfenate anions generated in situ from β-sulfinyl carboxylic acid esters as nucleophiles to synthesize allyl sulfoxides (Scheme 5) [15]. During the reaction process, the sulfenate anion interacts with the instantaneously generated η3-allylpalladium complex to generate allyl sulfoxides and regenerate the Pd(0) catalyst.
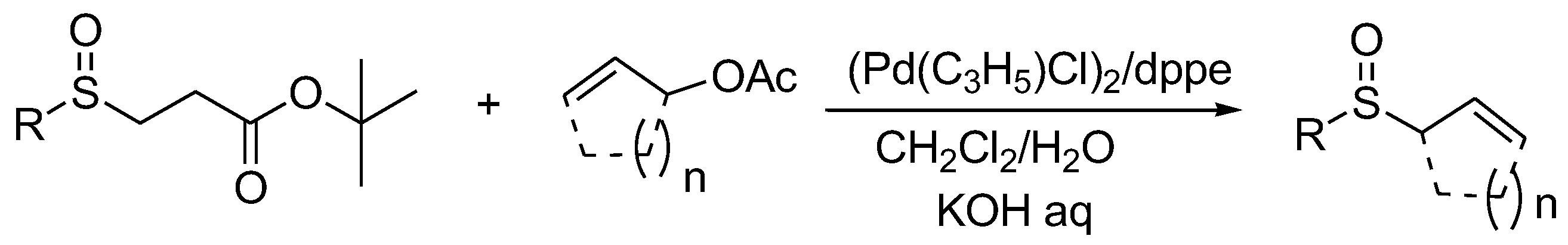
Scheme 5. Palladium-catalyzed synthesis of allyl sulfoxides.
When tert-butyl p-tolylsulfinylpropionate reacted with allyl acetate in the presence of the palladium complex, the corresponding allyl sulfoxide was obtained in an 82% yield. However, when tert-butyl p-tolylsulfinylpropionate reacted with cyclic allyl acetate, the yield of the product obtained was extremely low, possibly because steric hindrance had an impact on the reaction.
When tert-butyl 2-naphthylsulfinyl propionate was used as a raw material to react with allyl acetate compounds, only a 60% yield was obtained. Interestingly, cyclopent-2-enyl-acetate and cyclohex-2-enyl-acetate yielded 43% and 57% of the expected sulfoxides, respectively, when tert-butyl 3-isopropylsulfinylpropionate was adopted as the substrate. The reaction was carried out under biphasic conditions, which provides a simple, mild and efficient route for the synthesis of allyl sulfoxides.
More than ten years later, another new class of chiral ligands was found to perform well in the palladium-catalyzed synthesis of chiral sulfoxides. Until 2018, Zhang and co-workers reported the palladium-catalyzed asymmetric arylation of alkyl and aryl sulfenate anions with a new class of chiral ligands (Scheme 6) [16]. At first, they screened a few chiral ligands, but no satisfactory chiral ligands were suitable for this reaction. Inspired by the excellent performance of Xantphos for the racemic transformations of racemic sulfoxides, the authors used their PC-Phos ligand [17] with the same xanthene skeleton as Xantphos as a chiral ligand. After screening a series of chiral PC-Phos phosphine ligands, the PC-Phos bearing the bulky 1-adamantyl sulfinamide was found to be the best chiral ligand for the palladium-catalyzed synthesis of chiral diorganyl sulfoxides.
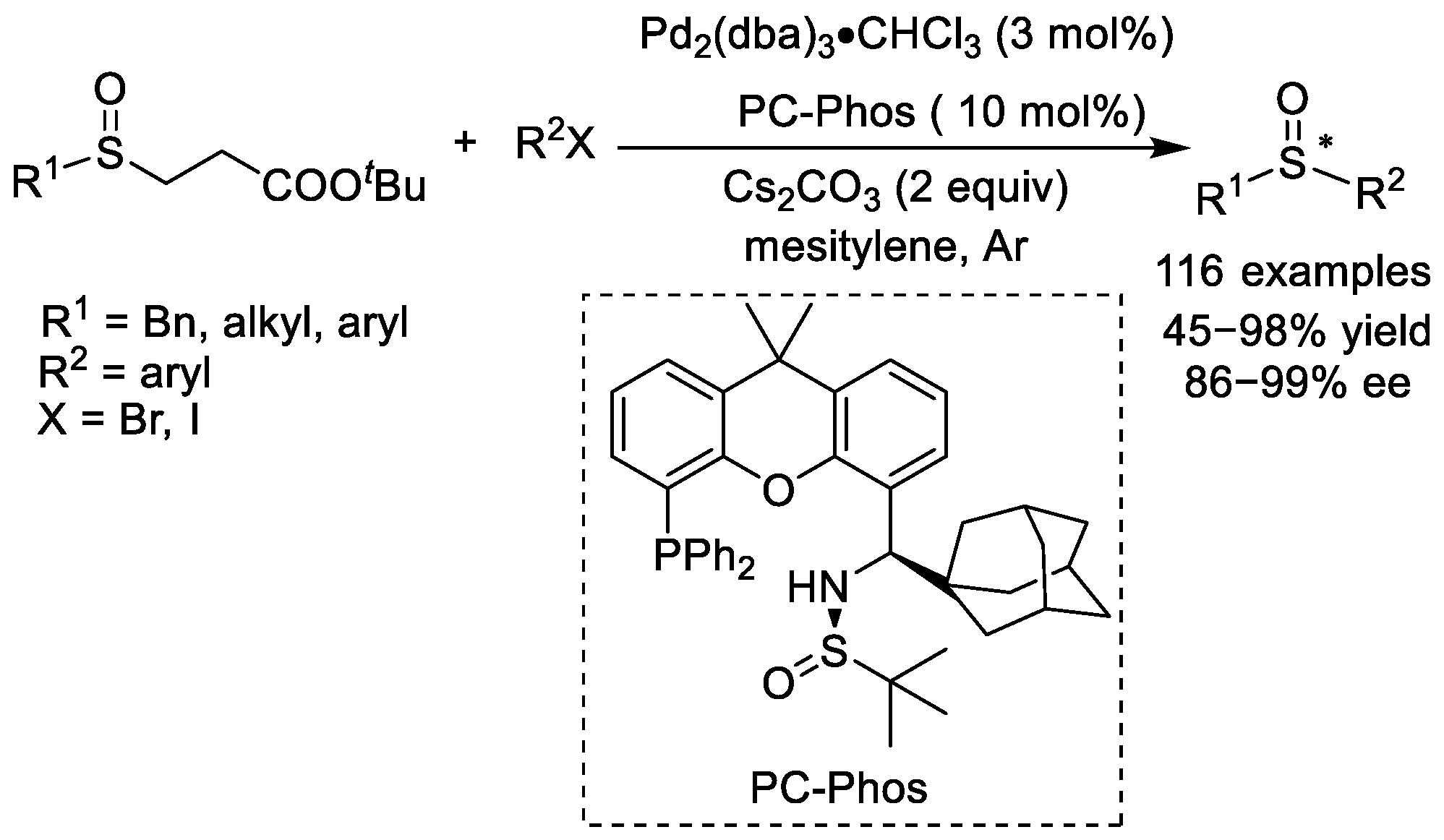
Scheme 6. Palladium-catalyzed asymmetric synthesis of chiral sulfoxides from alkyl and aryl sulfenate anions with chiral PC-Phos ligand. Note: * indicates that this atom (here is sulfur) has chirality.
In 2011, Gelat et al. described the chiral phase-transfer-catalyst-mediated asymmetric alkylation of β-sulfinyl esters with alkyl halides, which provided a new path for the synthesis of chiral sulfoxides (Scheme 7) [17]. When various alkyl halides were used to react with the corresponding sulfenate anions generated in situ from the corresponding β-sulfinyl esters, the desired sulfoxides were effectively obtained with certain enantioselectivities.
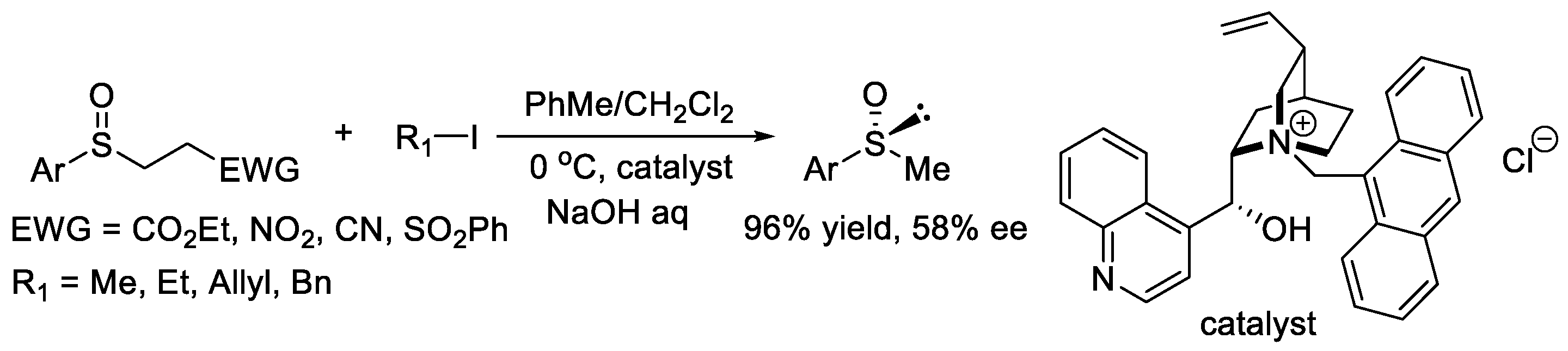
Scheme 7. Synthesis of chiral sulfoxides via reaction of β-sulfinyl esters with alkyl iodides.
In 2014, the Tan group developed structurally novel chiral pentanidium salts with halogenated benzyl groups as phase-transfer catalysts (PTCs) for the enantioselective alkylation of sulfenate anions [18], which gave excellent results compared to commercially available PTCs (Scheme 8).
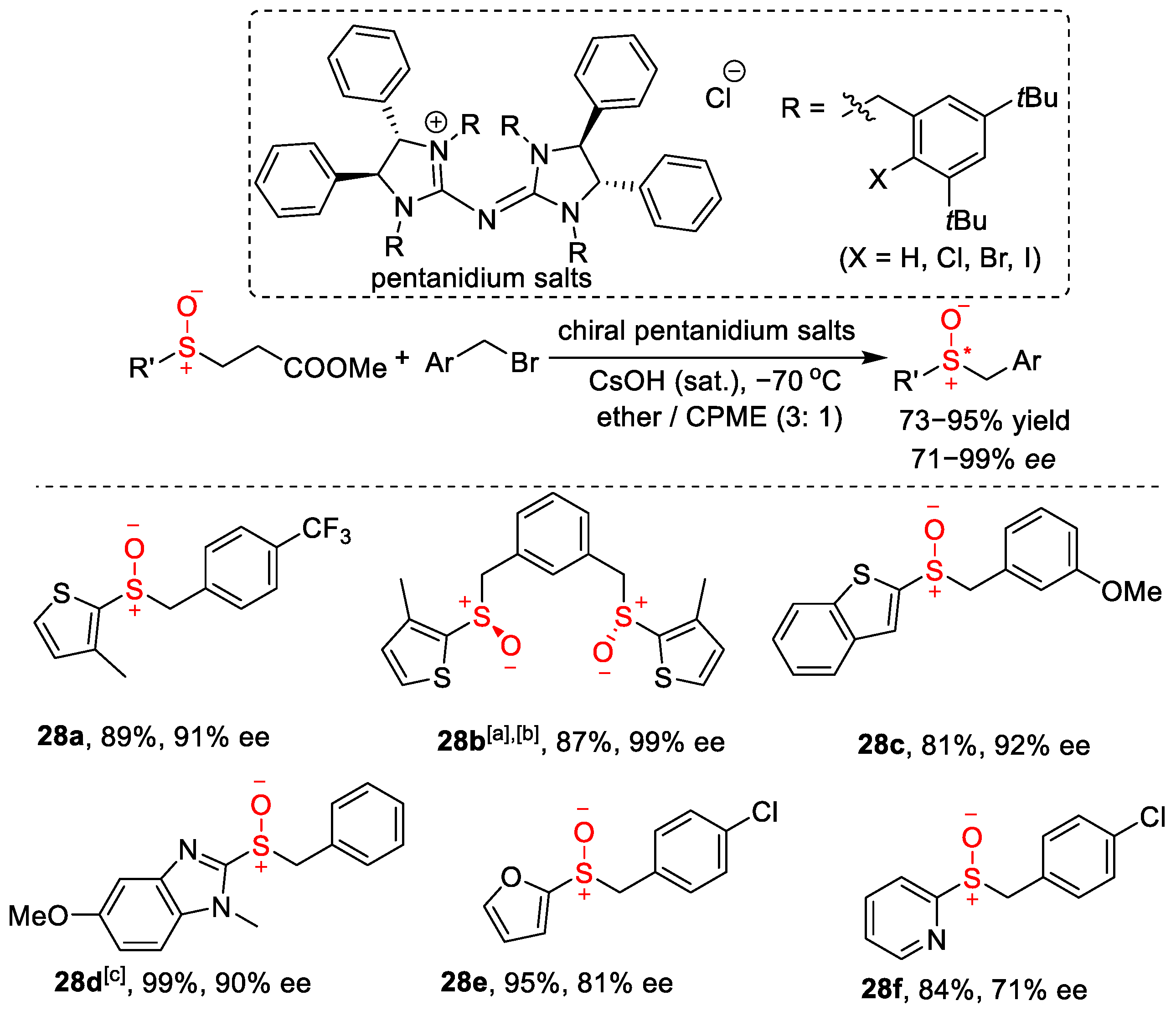
Scheme 8. Chiral pentanidium salts with halogenated benzyl groups as phase-transfer catalysts in asymmetric benzylation of (hetero)aryl sulfenate salts.
In 2007, Colobert et al. introduced the palladium-catalyzed heteroarylation of sulfenate anions [19], which could effectively synthesize heteroaryl sulfoxides. Afterward, Abarca and his collaborators also reported a related synthetic method [20]. Triazolopyridinyl sulfoxide, pyridinyl sulfoxide and thiophene sulfoxide could all be obtained at high yields under mild conditions from the reaction of the corresponding heteroaryl bromides with sulfenate anions (Scheme 9).
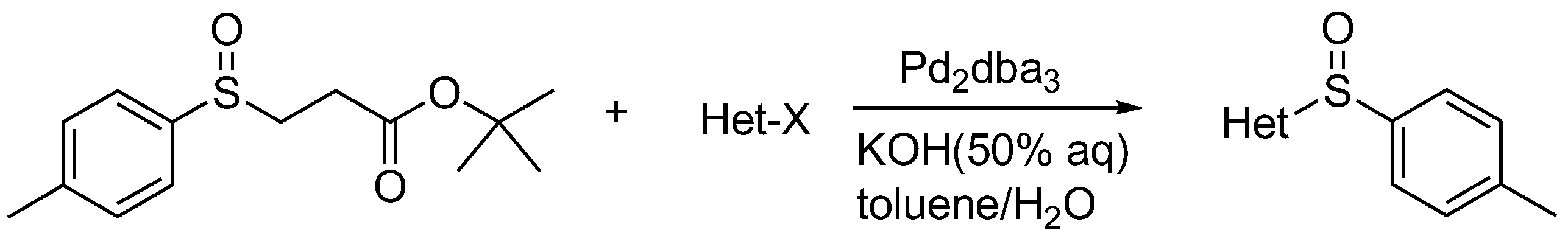
Scheme 9. Heteroarylation of β-sulfinyl esters to synthesize heteroaryl sulfoxides.
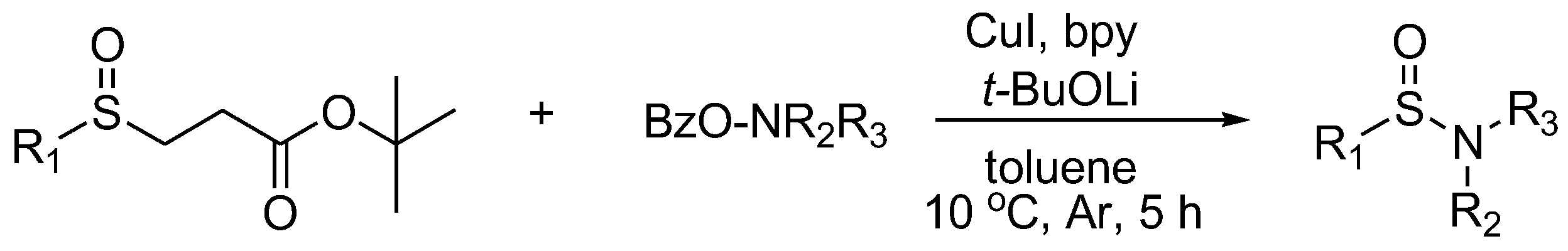
4. Synthesis of Sulfinamides from β-Sulfinyl Esters
In 2018, Dai’s group developed a method to construct sulfinamides via the copper-catalyzed electrophilic amination of sulfenate anions with N-benzoyloxyamines as an amination reagent (Scheme 10) [21]. This process was characterized by the capture of in situ generated sulfenate anions from β-sulfinyl esters under mild conditions.
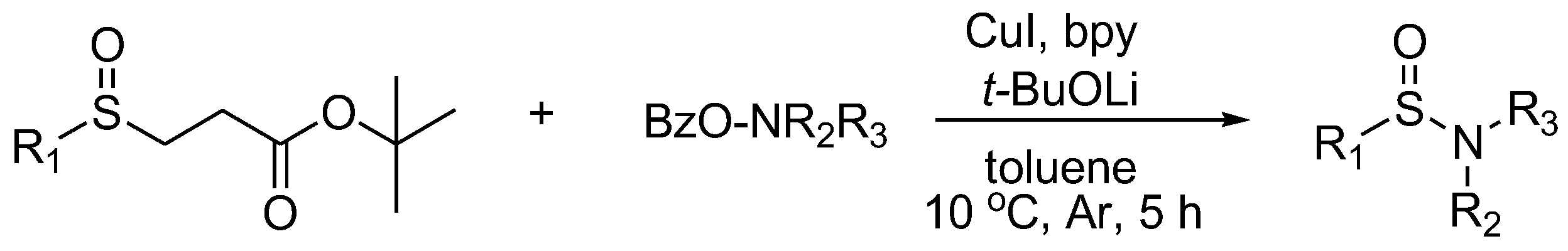
Scheme 10. Reaction of β-sulfinyl esters with amination reagents to synthesize sulfinamides.
When using β-sulfinyl esters with different structures, it was found that regardless of whether there was an electron-absorbing substituent or an electron-donating substituent on the aromatic ring, the corresponding negative sulfinyl ion could be effectively released. Methyl-substituted β-sulfinyl esters on the aromatic ring could give the target products with excellent yields, while the yields of ortho-methyl-substituted β-sulfinyl esters were significantly reduced, which suggests that steric hindrance had a negative effect on the reaction. The 2-naphthyl sulfenate anion precursor was also suitable for this reaction, efficiently providing the required sulfinamide.
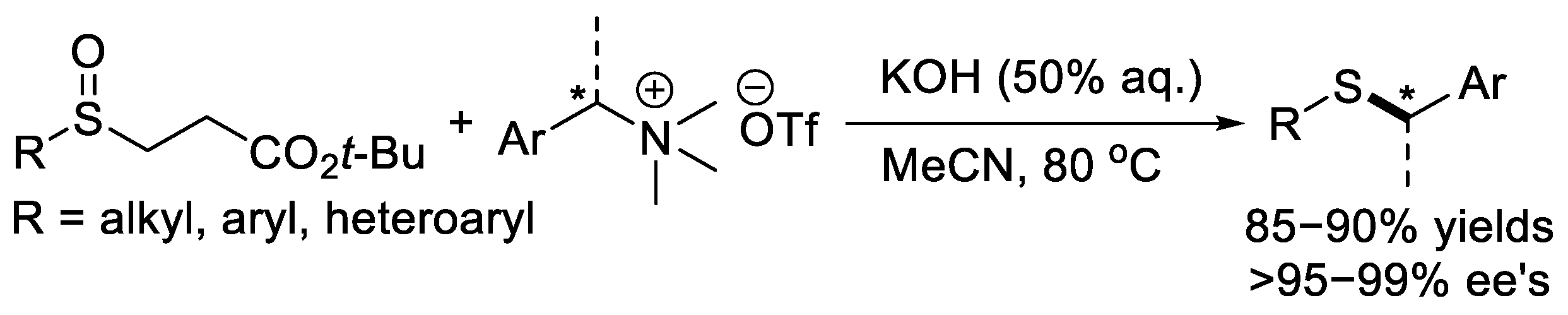
5. Synthesis of Chiral Thioethers via Deoxidative Coupling Reaction of β-Sulfinyl Esters
Our research group recently discovered an unusual deoxidative coupling reaction of β-sulfinyl esters with benzylic trimethylammonium salts promoted by the strong base KOH (Scheme 11) [22], and various thioethers were produced. Enantiomerically enriched chiral quaternary ammonium salts synthesized from chiral amines afforded highly enantiomerically pure chiral benzyl thioethers (>95–99% ee). It seems that this reaction occurs via an SN2-type nucleophilic substitution process, since the chiral benzyl thioethers obtained had configurations opposite to those of the enantiomerically enriched amines.

Scheme 11. Reaction of β-sulfinyl esters with amination reagents to synthesize sulfinamides.
6. Conclusions
β-Sulfinyl esters prepared via the in situ formation of sulfenate anions under the action of alkali as a nucleophile, under catalysis by transition metals, can react with a variety of electrophiles. Among them, the most reported is the formation of sulfoxides, some of which were chiral sulfoxides synthesized with high enantiomeric purity. This has important practical significance, because a variety of important chiral drugs have structures of chiral sulfoxide moieties. Therefore, this synthetic method could provide another way to synthesize chiral drugs.
It is foreseeable that negative sulfinyl ions generated in situ by β-sulfinyl esters can react with carbocation species to synthesize chiral sulfoxides in the presence of chiral catalysts. This means that with a suitable chiral catalyst, sulfenate anions can also react with a variety of electrophiles, such as positively charged nitrogen, oxygen, phosphorus and sulfur species, to form various chiral sulfinyl-containing chiral compounds.
References
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5.
- Shin, J.M.; Cho, Y.M.; Sachs, G. Chemistry of Covalent Inhibition of the Gastric (H+, K+)-ATPase by Proton Pump Inhibitors. J. Am. Chem. Soc. 2004, 126, 7800–7811.
- McClellan, K.J.; Spencer, C.M. Modafinil A Review of its Pharmacology and Clinical Efficacy in the Management of Narcolepsy. CNS Drugs 1998, 9, 311–324.
- Robak, M.T.; Herbage, M.A.; Ellman, J.A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740.
- Riddell, A.B.; Smith, M.R.A.; Schwan, A.L. The generation and reactions of sulfenate anions. An update. J. Sulfur Chem. 2022, 43, 540–592.
- Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Giannetto, P. Recent Advances and Perspectives in the Chemistry of Sulfenic Acids. Curr. Org. Chem. 2007, 11, 1034–1052. Available online: https://www.eurekaselect.com/article/4614 (accessed on 1 August 2007).
- Jia, T.; Zhang, M.; McCollom, S.P.; Bellomo, A.; Montel, S.; Mao, J.; Dreher, S.D.; Welch, C.J.; Regalado, E.L.; Williamson, R.T.; et al. Palladium-Catalyzed Enantioselective Arylation of Aryl Sulfenate Anions: A Combined Experimental and Computational Study. J. Am. Chem. Soc. 2017, 139, 8337–8345.
- Aversa, M.C.; Bonaccorsi, P.; Madec, D.; Prestat, G.; Poli, G. The Fabulous Destiny of Sulfenic Acids. In Innovative Catalysis in Organic Synthesis; Andersson, P.G., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2012; pp. 47–76.
- Zhang, M.; Jia, T.; Sagamanova, I.K.; Pericás, M.A.; Walsh, P.J. tert-Butyl Phenyl Sulfoxide: A Traceless Sulfenate Anion Precatalyst. Org. Lett. 2015, 17, 1164–1167.
- Gelat, F.; Lohier, J.F.; Gaumont, A.C.; Perrio, S. tert-Butyl Sulfoxides: Key Precursors for Palladium-Catalyzed Arylation of Sulfenate Salts. Adv. Synth. Catal. 2015, 357, 2011–2016.
- Singh, S.P.; O’Donnell, J.S.; Schwan, A.L. Nucleophilic attack of 2-sulfinyl acrylates: A mild and general approach to sulfenic acid anions. Org. Biomol. Chem. 2010, 8, 1712–1717.
- Foucoin, F.; Caupene, C.; Lohier, J.F.; de Oliveira Santos, J.S.; Perrio, S.; Metzner, P. 2-(Trimethylsilyl)ethyl Sulfoxides as a Convenient Source of Sulfenate Anions. Synthesis 2007, 9, 1315–1324.
- Caupene, C.; Boudou, C.; Perrio, S.; Metzner, P. Remarkably Mild and Simple Preparation of Sulfenate Anions from β-Sulfinylesters: A New Route to Enantioenriched Sulfoxides. J. Org. Chem. 2005, 70, 2812–2815.
- Yue, H.L.; Klussmann, M. Acid-Catalyzed Oxidative Addition of Thiols to Olefins and Alkynes for a One-Pot Entry to Sulfoxides. Synlett 2016, 27, 2505–2509.
- Maitro, G.; Prestat, G.; Madec, D.; Poli, G. Preparation of Allyl Sulfoxides by Palladium-Catalyzed Allylic Alkylation of sulfenate Anions. J. Org. Chem. 2006, 71, 7449–7454.
- Wang, L.; Chen, M.; Zhang, P.; Li, W.; Zhang, J. Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General sulfenate Anions: Scope and Synthetic Applications. J. Am. Chem. Soc. 2018, 140, 3467–3473.
- Gelat, F.; Jayashankaran, J.; Lohier, J.F.; Gaumont, A.C.; Perrio, S. Organocatalytic Asymmetric Synthesis of Sulfoxides from Sulfenic Acid Anions Mediated by a Cinchona-Derived Phase Transfer Reagent. Org. Lett. 2011, 13, 3170–3173.
- Zong, L.; Ban, X.; Kee, C.W.; Tan, C.H. Catalytic Enantioselective Alkylation of sulfenate Anions to Chiral Heterocyclic Sulfoxides Using Halogenated Pentanidium Salts. Angew. Chem. Int. Ed. 2014, 53, 11849–11853.
- Colobert, F.; Garrido, R.B.; Leroux, F.R.; Ballesteros, R.; Abarca, B. Efficient palladium catalyzed synthesis of heteroaromatic sulfoxides. Tetrahedron Lett. 2007, 48, 6896–6899.
- Abarca, B.; Ballesteros, R.; Garrido, R.B.; Colobert, F.; Leroux, F.R. Triazolopyridines. Part 26, The preparation of novel triazolo pyridine sulfoxides. Tetrahedron 2008, 64, 3794–3801.
- Dai, Q.; Zhang, J. Direct Synthesis of Sulfinamides by the Copper-Catalyzed Electrophilic Amidation of sulfenate Anions. Adv. Synth. Catal. 2018, 360, 1123–1127.
- Zhang, Q.; Feng, H.; Zeng, Q. Unusual Deoxidative Coupling Reaction of β-Sulfinyl Esters with Benzylic Trimethylammonium Salts. J. Org. Chem. 2021, 86, 7806–7812.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




