Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dingzi Zhou | -- | 2213 | 2023-05-05 08:42:45 | | | |
| 2 | Beatrix Zheng | + 9 word(s) | 2222 | 2023-05-06 07:40:28 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 2222 | 2023-05-06 07:41:13 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 2222 | 2023-05-06 07:41:54 | | | | |
| 5 | Beatrix Zheng | -1 word(s) | 2221 | 2023-05-08 11:04:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhou, D.; Xie, L.; Fu, D.; Yan, L. Hyperbaric Oxygen Therapy in Treating Surgical Site Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/43828 (accessed on 07 February 2026).
Zhou D, Xie L, Fu D, Yan L. Hyperbaric Oxygen Therapy in Treating Surgical Site Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/43828. Accessed February 07, 2026.
Zhou, Dingzi, Linshen Xie, Daigang Fu, Ling Yan. "Hyperbaric Oxygen Therapy in Treating Surgical Site Infections" Encyclopedia, https://encyclopedia.pub/entry/43828 (accessed February 07, 2026).
Zhou, D., Xie, L., Fu, D., & Yan, L. (2023, May 05). Hyperbaric Oxygen Therapy in Treating Surgical Site Infections. In Encyclopedia. https://encyclopedia.pub/entry/43828
Zhou, Dingzi, et al. "Hyperbaric Oxygen Therapy in Treating Surgical Site Infections." Encyclopedia. Web. 05 May, 2023.
Copy Citation
Hyperbaric oxygen therapy (HBOT) can result in rapid healing and epithelialization of various wounds and has potential beneficial effects in the treatment of surgical site infections (SSIs) or other similar infections following cardiac, neuromuscular scoliosis, coronary artery bypass, and urogenital surgeries. Moreover, it was a safe therapeutic procedure in most cases. The mechanisms related to the antimicrobial activity of HBOT include direct bactericidal effects through the formation of reactive oxygen species (ROS), the immunomodulatory effect of HBOT that increase the antimicrobial effects of the immune system, and the synergistic effects of HBOT with antibiotics.
hyperbaric oxygen therapy
surgical site infections
treatment
1. Introduction
Surgical site infections (SSIs) are among the most prevalent postoperative complications, responsible for significant morbidity and mortality, delayed wound healing, increased length of hospital stay, unnecessary pain, and a high cost to the patient and the institution [1][2][3]. SSIs are the most common hospital-associated infections (HAI) in low- or middle-income countries, affecting up to a third of patients who had surgery, as well as being a frequent HAI in high-income countries in the European Union and the USA [4][5]. The risk of developing an SSI is multifactorial, and several intrinsic (patient) and extrinsic (e.g., procedure, facility, preoperative, and operative) risk factors have been determined for the development or incidence of SSIs [6]. The intrinsic factors include increasing age, diabetes mellitus, radiation, immunosuppression, history of skin or soft tissue infection, obesity, use of alcohol, smoking, dyspnea, low serum albumin concentration, and total bilirubin > 1.0 mg/dL [6][7][8]. The extrinsic factors comprise emergency and more complex surgery, inadequate ventilation, inappropriate sterilization of equipment, increased operating room traffic, the presence of a pre-existing infection, inadequate skin preparation or hair removal, inappropriate or no antibiotic prophylaxis, blood transfusion, the length of the operation, the duration of the surgical scrub, the maintenance of asepsis, poor-quality surgical hand scrubbing and gloving, hypothermia, and poor glycemic control [6][7][9].
Despite significant progress in the control of infectious diseases in hospitals, SSIs are still among the most prevalent postoperative problems. The prevention and management of SSIs are complex, and an integration of pre-, intra-, and post-operative measures is required to reduce the burden and complications of SSIs [5][10][11]. Antibiotic prophylaxis and treatments are wildly used in the prevention and management of SSIs following surgeries [11][12], and have a critical role in the achievement of a better clinical outcome for wound healing and SSIs [11][13]. Unfortunately, antibiotic resistance in microorganisms has continuously increased over the past decades, and antibiotic efficacy has decreased for many pathogens [14][15]. Recent data showed that more than 50% of the pathogens isolated from SSIs were bacterial pathogens that were multidrug-resistant organisms (MDROs) [16][17]. Increasing trends in antibiotic resistance potentially threaten the safety and efficacy of surgical procedures; therefore, the development of alternative treatments is essentially needed to reduce the amount of antibiotic usage and better manage SSIs [18]. Various therapy procedures using oxygen, including local oxygen therapy, supplemental inspired oxygen therapy, and hyperbaric oxygen therapy, have shown beneficial impacts on wound healing and reduced the burden of SSIs [19][20][21].
Hyperbaric oxygen therapy (HBOT) is a promising treatment modality, as either a primary or alternative therapy, for the management of some complex medical conditions [22][23][24] including non-healing wounds [25][26] as well as various hypoxic or ischemic events [27][28]. Moreover, HBOT has a potential therapeutic effect which could be used for the treatment of acute infections caused by MDROs [29][30][31].
2. Application of HBOT in Surgical Site Infections
Initially, 359 relevant articles were retrieved. After screening of titles and abstracts and an in-depth review of full texts, the researchers found 14 studies that used HBOT to treat different types of SSIs. The researchers categorized these studies based on the type of SSI or surgery: (1) sternal wound infection following cardiac surgery, (2) SSIs following neuromodulation or neuro-muscular surgery, and (3) SSIs following the male-to-female gender affirmation surgery (urogenital surgery).
2.1. Application of HBOT in Sternal Wound Infection Infections
In the earliest report, Petzold et al. [32] used HBOT as an adjunct to local surgical treatment to treat an established sternal infection in an immuno-suppressed patient who developed presternal fat necrosis and subsequent sternal osteomyelitis two months after an orthotopic heart transplantation. Two areas of wound dehiscence developed. Conventional measures, including local debridement, sternal wire removal, and antiseptic irrigation, were applied in this case. After these measures, one wound was completely closed, but in the next weeks another wound showed only a slight tendency for further granulation and the purulent secretions increased. At that time, the physicians decided to apply HBOT for 40 sessions, each 90 min long and under 240 kPa of O2 pressure. With HBOT treatment, rapid healing was observed and the wound closed and was completely epithelialized. In a retrospective review of 27 cases of sternal infection treated over a 2-year period, Riddick et al. [33] reported that length of hospital stays and the readmission rate were reduced in patients that received HBOT; however, the authors did not perform statistical analysis to support these findings. In another retrospective review, De Feo et al. [34] compared the effects of conservative antibiotic therapy (group A) and aggressive surgical management (early debridement, removal of wires, and closed irrigation) in combination with granulated sugar and HBOT (group B) on morbidity and mortality following post-cardiotomy deep sternal wound infection. Although this research reported that morbidity and mortality related to deep sternal wound infection were significantly lower in group B, the authors made no conclusions about the specific benefits of HBOT. In line with study [34], Siondalski et al. [35] conducted a retrospective review of 55 patients over a 5-year period. The management plan consisted of aggressive surgery in combination with 20–40 HBO treatments. The authors conclude that the combination of aggressive surgical treatment and HBOT can improve the clinical outcome of patients with sternal infection.
Barili et al. [36], in a prospective trial, assessed the effect of HBOT on organ/space sternal SSIs following cardiac surgery that required sternotomy. Of the 32 participants in their study, 14 patients received HBOT, and 18 patients who did not consent to HBOT served as controls. The duration of infection was similar in the HBOT and control groups (31.8 ± 7.6 vs. 29.3 ± 5.7 days, respectively, p = 0.357). The relapse rate of SSI was significantly lower in the HBOT group (0% vs. 33.3%, p = 0.024). Furthermore, total hospital stays (52.6 ± 9.1 vs. 73.6 ± 24.5 days, p = 0.026) and the duration of intravenous antibiotic use (47.8 ± 7.4 vs. 67.6 ± 25.1 days, p = 0.036) were both significantly shorter in the HBOT group compared with the controls. The authors concluded that HBOT is a valuable addition to the techniques available for physicians to manage and treat postoperative organ/space sternal SSIs. Furthermore, Yu et al. [37] evaluated the effect of HBOT on sternal infection and osteomyelitis following median sternotomy. They included 12 patients: six received conventional therapy (debridement and antibiotic treatment), and six others received additional HBOT plus conventional therapy. Comparisons of the data between the two study groups revealed that the length of stay in the ICU, duration of invasive and noninvasive positive pressure ventilation, and hospital mortality were all significantly lower in patients with additional HBOT as compared to patients without HBOT. In another retrospective study of 18 patients undergoing HBOT after coronary artery bypass surgery, Egito et al. [38] demonstrated that HBOT used as an adjunctive therapy for the treatment of mediastinitis patients after CABS had favorable clinical results. Litwinowicz1 et al. [39] retrospectively assessed the effects and usefulness of additional HBOT in 10 patients with deep sternal wound infection (DSWI) after cardiac surgery. After four weeks of treatment, their findings revealed that HBOT used as an adjunct therapy was effective in treating 80% of patients with DSWI, with no complications observed. A retrospective study on children [40] showed that multimodality therapy, including incision and drainage and negative pressure wound therapy combined with HBOT and appropriate antibiotics, could be very helpful for the successful management of complex DSWI in the pediatric population after congenital heart surgery.
2.2. Application of HBOT in SSIs following Neuromodulation or Neuro-Muscular Surgery
There are two studies indicating that HBOT could be a useful additional therapy with minimal side effects for the treatment or prevention of deep SSIs in complicated spine abnormalities in high-risk neuromuscular cases [41][42]. Larsson et al. evaluated possible benefits of HBOT in the treatment of deep postoperative SSIs in six high-risk pediatric patients with neuromuscular spine deformity. Their findings indicated that all infections were resolved, and a satisfactory correction with a balanced spine and radiologically healed fusion was achieved in all cases within three months. The implants were neither removed nor changed in any of the patients as a result of infection during the study period. Wound healing and normal blood tests were achieved within 4 months [41]. Inanmaz et al. demonstrated that HBOT prophylaxis in patients undergoing neuro-muscular scoliosis surgery can reduce the incidence of SSIs and improve the wound healing process [42]. Moreover, Bartek Jr. et al. applied HBOT as adjuvant treatment for hardware-related infections in neuromodulation. The findings of these studies indicated a potential benefit of adjuvant HBOT in the treatment of hardware-related infections in neuromodulation, diminishing the need for hardware removal and treatment interruption [43].
2.3. Application of HBOT in SSIs following the Urogenital Surgery
According to the researchers' literature search, only one study by Stizzo et al. [21][44] assessed the effects of HBOT as an adjuvant treatment for SSIs after male-to-female gender affirmation surgery. In this study, 33 patients were enrolled: 15 received HBOT, while the remaining 18 patients belonged to the non-HBOT group. The results indicated that complete wound healing was not significantly different between the two groups, but the duration of antibiotic therapy, perineal drain time, bladder catheter time, and hospital stay were significantly lower in the HBOT group. The authors suggested using HBOT as an adjuvant treatment for SSIs in patients undergoing male-to-female gender affirmation surgery.
3. The Mechanisms of Action of Hyperbaric Oxygen Therapy (HBOT)
There is sufficient evidence suggesting HBOT as a useful approach in the treatment of different types of infections, either alone or as a supplement therapy, especially for deep and recalcitrant infections associated with hypoxia or induced by aerobic or anaerobic MDROs [45][46]. HBOT considerably increases the levels of O2 concentration in blood and damaged tissues, leading to several of the physiologic effects that help wound healing [47][48]. These physiologic effects include intravascular and tissue gas bubble reduction, vasoconstriction, improved oxygenation, modulation of inflammation and immune function, angiogenesis, and increased antimicrobial activity [47].
Comprehensive details about the mechanisms associated with antimicrobial activity and wound healing using HBOT are well-described in previous published reviews [49][50][51][52][53][54]. Briefly, the mechanisms related to the antimicrobial activity of HBOT could be divided into three main domains, including: (1) Direct antimicrobial or bactericidal effects by the formation of reactive oxygen species (ROS) [18]. HBOT can result in increased levels of ROS cells and eliminate the desired condition for bacterial agents that lack antioxidant defense pathways [53]. The antimicrobial activity of ROS is a dose-dependent mode of effect [49][55] and the main cellular targets of ROS are DNA, RNA, proteins, and lipids (Figure 1) [56][57]. (2) Immunomodulatory effects of HBOT that increase the antimicrobial effects of the immune system (Figure 2) [49]. HBOT has anti-inflammation effects by altering the expression of proinflammatory cytokines and other regulators of inflammation [58][59][60][61] and this anti-inflammatory effect has been reported to play an important role in reducing tissue damage and infection development [49]. HBOT also has a trigger effect on neutrophil migration to the site of infection via suppression of neutrophil beta-2 integrin (Mac-1 (CD11b/CD18)) activity [62][63][64]. As is well established, the O2 level of the environment is a critical factor for the antibacterial activity of neutrophils; therefore increased levels of O2 in the tissue environment after HBOT evidently increase the phagocytic and bactericidal activity of neutrophils [65]. (3) Additive or synergistic effects of HBOT with antibiotics. HBOT is generally applied as an adjuvant treatment in combination with antibiotic therapy in the treatment of infections; therefore, hyperoxia induced by HBOT may improve the activity of antibiotics [37][66][67]. It is suggested that the efficiency of some types of antibiotics, such as β-lactams, quinolones, and aminoglycosides, may influenced by the presence of O2 [49][68]. Experimental studies showed that HBOT used as a supplementary therapy improved the effects of tobramycin or cefazolin on Staphylococcus aureus [68][69]; vancomycin, teicoplanin, and linezolid on methicillin-resistant S. aureus [66]; and imipenem or ciprofloxacin on Pseudomonas aeruginosa [70][71].
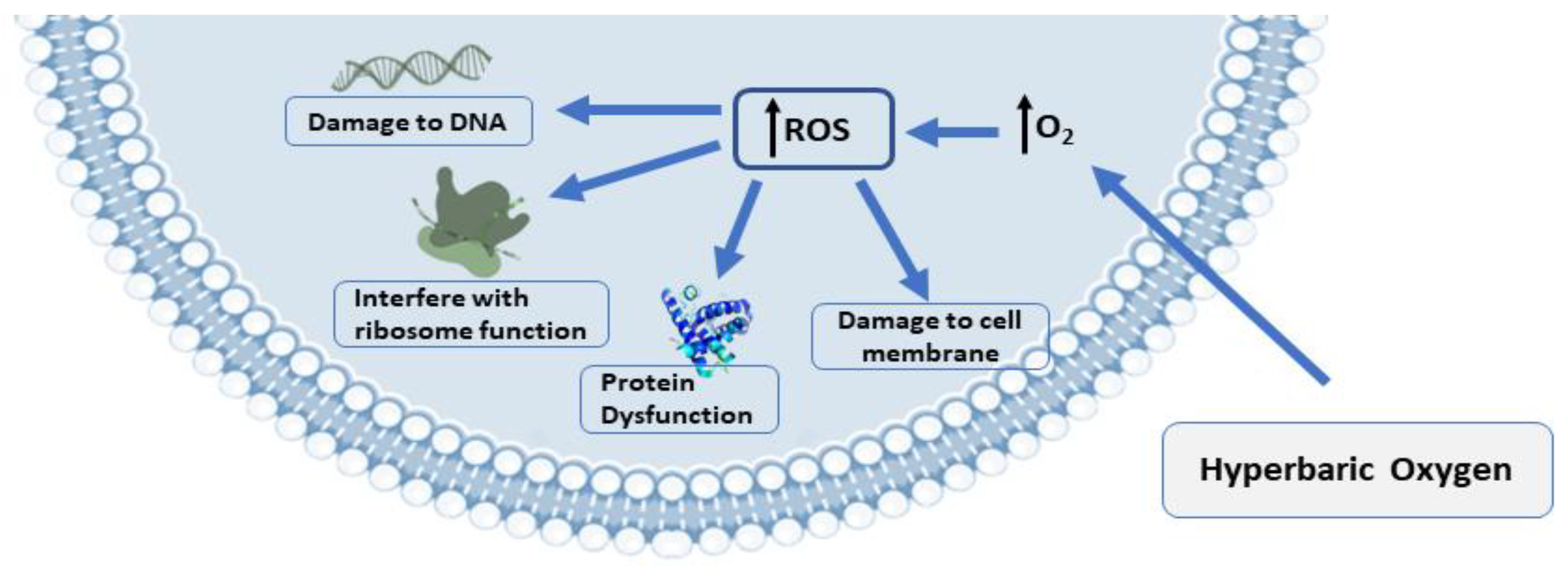
Figure 1. The presence of ROS production and biological targets in microbial growth: ROS production is the antibacterial mechanism of hyperbaric oxygen treatment (HBOT). DNA, proteins, and lipids are the targets of ROS’s damaging effects on cells. Abbreviations: ROS, reactive oxygen species.
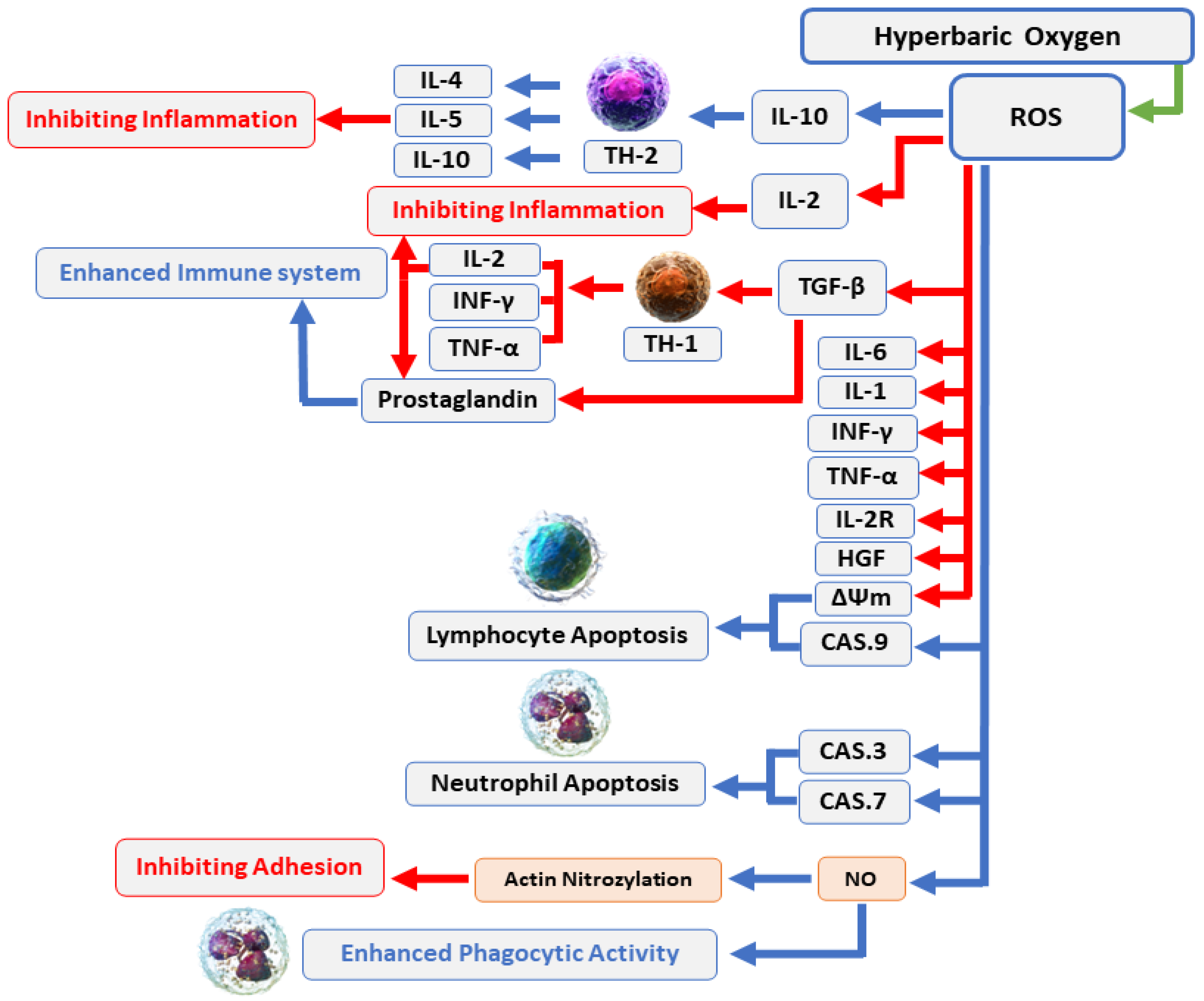
Figure 2. HBOT enhances the immune system’s antimicrobial effects: Increased O2 levels during HBOT have a variety of biological effects, including suppression of proinflammatory mediators, transitory reduction in the CD4:CD8 T cell ratio, and stimulation of lymphocyte and neutrophil death through caspase-3-, caspase-7-, and caspase-9-dependent mechanisms. In general, these effects can boost the antibacterial processes of the immune system and infection recovery. Abbreviations: ROS, reactive oxygen species; IL, interleukin; INF, interferon; TNF, tumor necrosis factor; CAS, caspase; NO, nitric oxide.
4. The Complications and Side Effects of HBOT
Alongside the beneficial clinical effects of HBOT, several side effects and complications have also been described [72][73][74][75]. The two most frequent complications are middle ear barotrauma (MEB) and claustrophobia [73][75][76]. Patients suffering from MEB have ear pain, difficulty with ear equalization, a feeling of pressure, and, in rare cases, rupture of the tympanic membrane with a conductive hearing deficit [74][76]. Sinus/paranasal, pulmonary, and dental barotrauma are other common complications [74][75]. Other rare complications associated with HBOT are related to the toxic effects of oxygen and include myopia and cataracts, decompression sickness, hyperoxic myopia,, retrolental fibroplasia, O2-induced seizures, pulmonary oxygen toxicity, pulmonary edema, blood pressure effects and hypoglycemia in diabetic patients [47][49][74][75].
References
- Leaper, D.J.; Van Goor, H.; Reilly, J.; Petrosillo, N.; Geiss, H.K.; Torres, A.J.; Berger, A. Surgical site infection—A European perspective of incidence and economic burden. Int. Wound J. 2004, 1, 247–273.
- Leaper, D. Surgical-site infection. J. Br. Surg. 2010, 97, 1601–1602.
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization: Geneva, Switzerland, 2016.
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organization: Geneva, Switzerland, 2011.
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287.
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; Dellinger, P.E.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 2017, 224, 59–74.
- Cheadle, W.G. Risk factors for surgical site infection. Surg. Infect. 2006, 7, s7–s11.
- Florschutz, A.V.; Fagan, R.P.; Matar, W.Y.; Sawyer, R.G.; Berrios-Torres, S.I. Surgical site infection risk factors and risk stratification. J. Am. Acad. Orthop. Surg. 2015, 23, S8.
- Malone, D.L.; Genuit, T.; Tracy, J.K.; Gannon, C.; Napolitano, L.M. Surgical site infections: Reanalysis of risk factors. J. Surg. Res. 2002, 103, 89–95.
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X. Surgical site infections 2. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303.
- Chauveaux, D. Preventing surgical-site infections: Measures other than antibiotics. Orthop. Traumatol. Surg. Res. 2015, 101, S77–S83.
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F. Antimicrobial prophylaxis reduces the rate of surgical site infection in upper gastrointestinal surgery: A systematic review. Antibiotics 2022, 11, 230.
- Najjar, P.A.; Smink, D.S. Prophylactic antibiotics and prevention of surgical site infections. Surg. Clin. 2015, 95, 269–283.
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443.
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 481–511.
- Fehr, J.; Hatz, C.; Soka, I.; Kibatala, P.; Urassa, H.; Battegay, M.; Jeffrey, Z.; Smith, T.; Mshinda, H.; Frei, R. Antimicrobial prophylaxis to prevent surgical site infections in a rural sub-Saharan hospital. Clin. Microbiol. Infect. 2006, 12, 1224–1227.
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437.
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019, 109, 440–447.
- de Smet, G.H.; Kroese, L.F.; Menon, A.G.; Jeekel, J.; van Pelt, A.W.; Kleinrensink, G.J.; Lange, J.F. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017, 25, 591–608.
- Greif, R.; Akça, O.; Horn, E.-P.; Kurz, A.; Sessler, D.I. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N. Engl. J. Med. 2000, 342, 161–167.
- Stizzo, M.; Manfredi, C.; Spirito, L.; Sciorio, C.; Romero Otero, J.; Martinez Salamanca, J.I.; Crocetto, F.; Verze, P.; Imbimbo, C.; Fusco, F. Hyperbaric oxygen therapy as adjuvant treatment for surgical site infections after male-to-female gender affirmation surgery: A 10-year experience. Andrology 2022, 10, 1310–1316.
- Tibbles, P.M.; Edelsberg, J.S. Hyperbaric-oxygen therapy. N. Engl. J. Med. 1996, 334, 1642–1648.
- Leach, R.; Rees, P.; Wilmshurst, P. Hyperbaric oxygen therapy. BMJ 1998, 317, 1140–1143.
- Grim, P.S.; Gottlieb, L.J.; Boddie, A.; Batson, E. Hyperbaric oxygen therapy. JAMA 1990, 263, 2216–2220.
- Velure, G.K.; Müller, B.; Hauken, M.A. Symptom burden and health-related quality of life six months after hyperbaric oxygen therapy in cancer survivors with pelvic radiation injuries. Support. Care Cancer 2022, 30, 5703–5711.
- Oscarsson, N.; Müller, B.; Rosén, A.; Lodding, P.; Mölne, J.; Giglio, D.; Hjelle, K.M.; Vaagbø, G.; Hyldegaard, O.; Vangedal, M. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): A randomised, controlled, phase 2–3 trial. Lancet Oncol. 2019, 20, 1602–1614.
- Hachmo, Y.; Hadanny, A.; Hamed, R.A.; Daniel-Kotovsky, M.; Catalogna, M.; Fishlev, G.; Lang, E.; Polak, N.; Doenyas, K.; Friedman, M. Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cells: A prospective trial. Aging 2020, 12, 22445.
- Oley, M.H.; Oley, M.C.; Durry, M.F.; Adam, R.N.; Gunawan, D.F.; Faruk, M. Fostering a faster post-operative wound healing process with hyperbaric oxygen therapy in a rare case of squamous odontogenic tumor. Int. J. Surg. Case Rep. 2022, 90, 106718.
- Kranke, P.; Bennett, M.H.; Martyn-St James, M.; Schnabel, A.; Debus, S.E.; Weibel, S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst. Rev. 2015.
- Jensen, P.; Møller, S.; Lerche, C.; Moser, C.; Bjarnsholt, T.; Ciofu, O.; Faurholt-Jepsen, D.; Høiby, N.; Kolpen, M. Improving antibiotic treatment of bacterial biofilm by hyperbaric oxygen therapy: Not just hot air. Biofilm 2019, 1, 100008.
- Oley, M.H.; Oley, M.C.; Wewengkang, L.A.W.; Kepel, B.J.; Langi, F.L.F.G.; Setiadi, T.; Aling, D.M.R.; Gunawan, D.F.; Tulong, M.T.; Faruk, M. Bactericidal effect of hyperbaric oxygen therapy in burn injuries. Ann. Med. Surg. 2022, 74, 103314.
- Petzold, T.; Feindt, P.R.; Carl, U.M.; Gams, E. Hyperbaric oxygen therapy in deep sternal wound infection after heart transplantation. Chest 1999, 115, 1455–1458.
- Riddick, M. Sternal wound infections, dehiscence, and sterna osteomyelitis: The role of hyperbaric oxygen therapy. In Hyperbaric Medicine Practice; Best Publishing Company: Flagstaff, AZ, USA, 1999; pp. 617–639.
- De Feo, M.; Gregorio, R.; Della Corte, A.; Marra, C.; Amarelli, C.; Renzulli, A.; Utili, R.; Cotrufo, M. Deep sternal wound infection: The role of early debridement surgery. Eur. J. Cardio-Thorac. Surg. 2001, 19, 811–816.
- Siondalski, P.; Keita, L.; Sićko, Z.; Zelechowski, P.; Jaworski, Ł.; Rogowski, J. Surgical treatment and adjunct hyperbaric therapy to improve healing of wound infection complications after sterno-mediastinitis. Pneumonol. Alergol. Pol. 2003, 71, 12–16.
- Barili, F.; Polvani, G.; Topkara, V.K.; Dainese, L.; Cheema, F.H.; Roberto, M.; Naliato, M.; Parolari, A.; Alamanni, F.; Biglioli, P. Role of hyperbaric oxygen therapy in the treatment of postoperative organ/space sternal surgical site infections. World J. Surge. 2007, 31, 1702–1706.
- Yu, W.-K.; Chen, Y.-W.; Shie, H.-G.; Lien, T.-C.; Kao, H.-K.; Wang, J.-H. Hyperbaric oxygen therapy as an adjunctive treatment for sternal infection and osteomyelitis after sternotomy and cardiothoracic surgery. J. Cardiothorac. Surg. 2011, 6, 141.
- Egito, J.G.T.d.; Abboud, C.S.; Oliveira, A.P.V.d.; Máximo, C.A.G.; Montenegro, C.M.; Amato, V.L.; Bammann, R.; Farsky, P.S. Clinical evolution of mediastinitis in patients undergoing adjuvant hyperbaric oxygen therapy after coronary artery bypass surgery. Einstein 2013, 11, 345–349.
- Litwinowicz, R.; Bryndza, M.; Chrapusta, A.; Kobielska, E.; Kapelak, B.; Grudzień, G. Hyperbaric oxygen therapy as additional treatment in deep sternal wound infections-a single center’s experience. Kardiochir. Torakochir. Pol. 2016, 13, 198–202.
- Copeland, H.; Newcombe, J.; Yamin, F.; Bhajri, K.; Mille, V.A.; Hasaniya, N.; Bailey, L.; Razzouk, A.J. Role of negative pressure wound care and hyperbaric oxygen therapy for sternal wound infections after pediatric cardiac surgery. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 440–445.
- Larsson, A.; Uusijärvi, J.; Lind, F.; Gustavsson, B.; Saraste, H. Hyperbaric oxygen in the treatment of postoperative infections in paediatric patients with neuromuscular spine deformity. Eur. Spine J. 2011, 20, 2217–2222.
- Inanmaz, M.E.; Kose, K.C.; Isik, C.; Atmaca, H.; Basar, H. Can hyperbaric oxygen be used to prevent deep infections in neuro-muscular scoliosis surgery? BMC Surg. 2014, 14, 85.
- Bartek, J., Jr.; Skyrman, S.; Nekludov, M.; Mathiesen, T.; Lind, F.; Schechtmann, G. Hyperbaric oxygen therapy as adjuvant treatment for hardware-related infections in neuromodulation. Stereotact. Funct. Neurosurg. 2018, 96, 100–107.
- Kaide, C.G.; Khandelwal, S. Hyperbaric oxygen: Applications in infectious disease. Emerg. Med. Clin. N. Am. 2008, 26, 571–595.
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989.
- Lam, G.; Fontaine, R.; Ross, F.L.; Chiu, E.S. Hyperbaric oxygen therapy: Exploring the clinical evidence. Adv. Ski. Wound Care 2017, 30, 181–190.
- Edwards, M.L. Hyperbaric oxygen therapy. Part 2: Application in disease. J. Vet. Emerg. Crit. Care 2010, 20, 289–297.
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567.
- Gill, A.Á.; Bell, C.N. Hyperbaric oxygen: Its uses, mechanisms of action and outcomes. QJM 2004, 97, 385–395.
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J. A general overview on the hyperbaric oxygen therapy: Applications, mechanisms and translational opportunities. Medicina 2021, 57, 864.
- Shinomiya, N. Molecular mechanisms of hyperbaric oxygen therapy. In Hyperbaric Oxygenation Therapy; Springer: Singapore, 2020; pp. 3–20.
- Çimşit, M.; Uzun, G.; Yıldız, Ş. Hyperbaric oxygen therapy as an anti-infective agent. Expert Rev. Anti-Infect. Ther. 2009, 7, 1015–1026.
- Mills, C. The role of hyperbaric oxygen therapy in the treatment of sternal wound infection. Eur. J. Cardio-Thorac. Surg. 2006, 30, 153–159.
- Dryden, M.; Cooke, J.; Salib, R.; Holding, R.; Pender, S.L.; Brooks, J. Hot topics in reactive oxygen therapy: Antimicrobial and immunological mechanisms, safety and clinical applications. J. Glob. Antimicrob. Resist. 2017, 8, 194–198.
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062.
- Cabiscol Català, E.; Tamarit Sumalla, J.; Ros Salvador, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8.
- Granowitz, E.; Skulsky, E.; Benson, R.; Wright, J.; Garb, J.; Cohen, E.; Smithline, E.; Brown, R. Exposure to increased pressure or hyperbaric oxygen suppresses interferon-gamma secretion in whole blood cultures of healthy humans. Undersea Hyperb. Med. 2002, 29, 216–225.
- Weisz, G.; Lavy, A.; Adir, Y.; Melamed, Y.; Rubin, D.; Eidelman, S.; Pollack, S. Modification of in vivo and in vitro TNF-α, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J. Clin. Immunol. 1997, 17, 154–159.
- Spiegelberg, L.; Swagemakers, S.; Van Ijcken, W.F.; Oole, E.; Wolvius, E.B.; Essers, J.; Braks, J.A. Gene expression analysis reveals inhibition of radiation-induced TGFβ-signaling by hyperbaric oxygen therapy in mouse salivary glands. Mol. Med. 2014, 20, 257–269.
- Weber, S.U.; Koch, A.; Kankeleit, J.; Schewe, J.-C.; Siekmann, U.; Stüber, F.; Hoeft, A.; Schröder, S. Hyperbaric oxygen induces apoptosis via a mitochondrial mechanism. Apoptosis 2009, 14, 97–107.
- Hopf, H.W.; Holm, J. Hyperoxia and infection. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 553–569.
- Kalns, J.; Lane, J.; Delgado, A.; Scruggs, J.; Ayala, E.; Gutierrez, E.; Warren, D.; Niemeyer, D.; Wolf, E.G.; Bowden, R.A. Hyperbaric oxygen exposure temporarily reduces Mac-1 mediated functions of human neutrophils. Immunol. Lett. 2002, 83, 125–131.
- Buras, J.A.; Stahl, G.L.; Svoboda, K.K.; Reenstra, W.R. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: The role of NOS. Am. J. Physiol.-Cell Physiol. 2000, 278, C292–C302.
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792.
- Turhan, V.; Sacar, S.; Uzun, G.; Sacar, M.; Yildiz, S.; Ceran, N.; Gorur, R.; Oncul, O. Hyperbaric oxygen as adjunctive therapy in experimental mediastinitis. J. Surg. Res. 2009, 155, 111–115.
- Jallali, N.; Withey, S.; Butler, P. Hyperbaric oxygen as adjuvant therapy in the management of necrotizing fasciitis. Am. J. Surg. 2005, 189, 462–466.
- Lerche, C.; Christophersen, L.; Kolpen, M.; Nielsen, P.; Trøstrup, H.; Thomsen, K.; Hyldegaard, O.; Bundgaard, H.; Jensen, P.; Høiby, N. Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 2017, 50, 406–412.
- Mendel, V.; Reichert, B.; Simanowski, H.; Scholz, H.-C. Therapy with hyperbaric oxygen and cefazolin for experimental osteomyelitis due to Staphylococcus aureus in rats. Undersea Hyperb. Med. 1999, 26, 169.
- Lima, F.L.; Joazeiro, P.P.; Lancellotti, M.; De Hollanda, L.M.; de Araújo Lima, B.; Linares, E.; Augusto, O.; Brocchi, M.; Giorgio, S. Effects of hyperbaric oxygen on Pseudomonas aeruginosa susceptibility to imipenem and macrophages. Future Microbiol. 2015, 10, 179–189.
- Kolpen, M.; Mousavi, N.; Sams, T.; Bjarnsholt, T.; Ciofu, O.; Moser, C.; Kühl, M.; Høiby, N.; Jensen, P.Ø. Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int. J. Antimicrob. Agents 2016, 47, 163–167.
- Plafki, C.; Peters, P.; Almeling, M.; Welslau, W.; Busch, R. Complications and side effects of hyperbaric oxygen therapy. Aviat. Space Environ. Med. 2000, 71, 119–124.
- Camporesi, E.M. Side effects of hyperbaric oxygen therapy. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2014, 41, 253–257.
- Heyboer III, M.; Sharma, D.; Santiago, W.; McCulloch, N. Hyperbaric oxygen therapy: Side effects defined and quantified. Adv. Wound Care 2017, 6, 210–224.
- Zhang, Y.-Y.; Zhou, Y.-J.; Jia, Y.-Y.; Wang, T.-T.; Meng, D.-H. Adverse effects of hyperbaric oxygen therapy: A systematic review and meta-analysis. Res. Sq. 2023. preprint.
- Nasole, E.; Zanon, V.; Marcolin, P.; Bosco, G. Middle ear barotrauma during hyperbaric oxygen therapy; a review of occurrences in 5962 patients. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2019, 46, 101–106.
- Edinguele, W.F.O.P.; Barberon, B.; Poussard, J.; Thomas, E.; Reynier, J.C.; Coulange, M. Middle-ear barotrauma after hyperbaric oxygen therapy: A five-year retrospective analysis on 2610 patients. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2020, 47, 217–228.
More
Information
Subjects:
Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
5 times
(View History)
Update Date:
08 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




