
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pooja Ratre | -- | 4224 | 2023-05-05 07:54:53 | | | |
| 2 | Jessie Wu | + 3 word(s) | 4227 | 2023-05-06 04:20:19 | | | | |
| 3 | Jessie Wu | Meta information modification | 4227 | 2023-05-06 04:21:52 | | |
Video Upload Options
Semiconductor quantum dots (QDs) were a modern form of nanostructure that demonstrated excellent qualities for diagnosis and therapy. Controlling QDs size and distribution made it simple to adjust their electrical and optical characteristics. Yet, since certain semiconductor QDs include hazardous substances such as, cadmium, arsenic, selenium, and mercury, they have several disadvantages. One such disadvantage is cytotoxicity. As a result, these QDs are neither environmentally friendly nor biodegradable. On the other hand, since their inception in 2004, carbon nanodots (CNDs) have been recognized as a strong contender to replace the extremely dangerous metallic semiconductor class of quantum dots. This is partly because the characteristics of carbon quantum dots are widely acknowledged to include their nanoscale size, roughly flat or spherical morphologies, great water solubility, broad absorption in the UV-visible light spectrum, and vibrant fluorescence. CNDs have an amorphous or nanocrystalline center, mainly sp2 carbon, graphite grid spacing, and outside oxygenic functional groups, allowing for water solubility and subsequent complexation.
1. Synthesis of Carbon Nanodots
1.1. Top–Down Approach
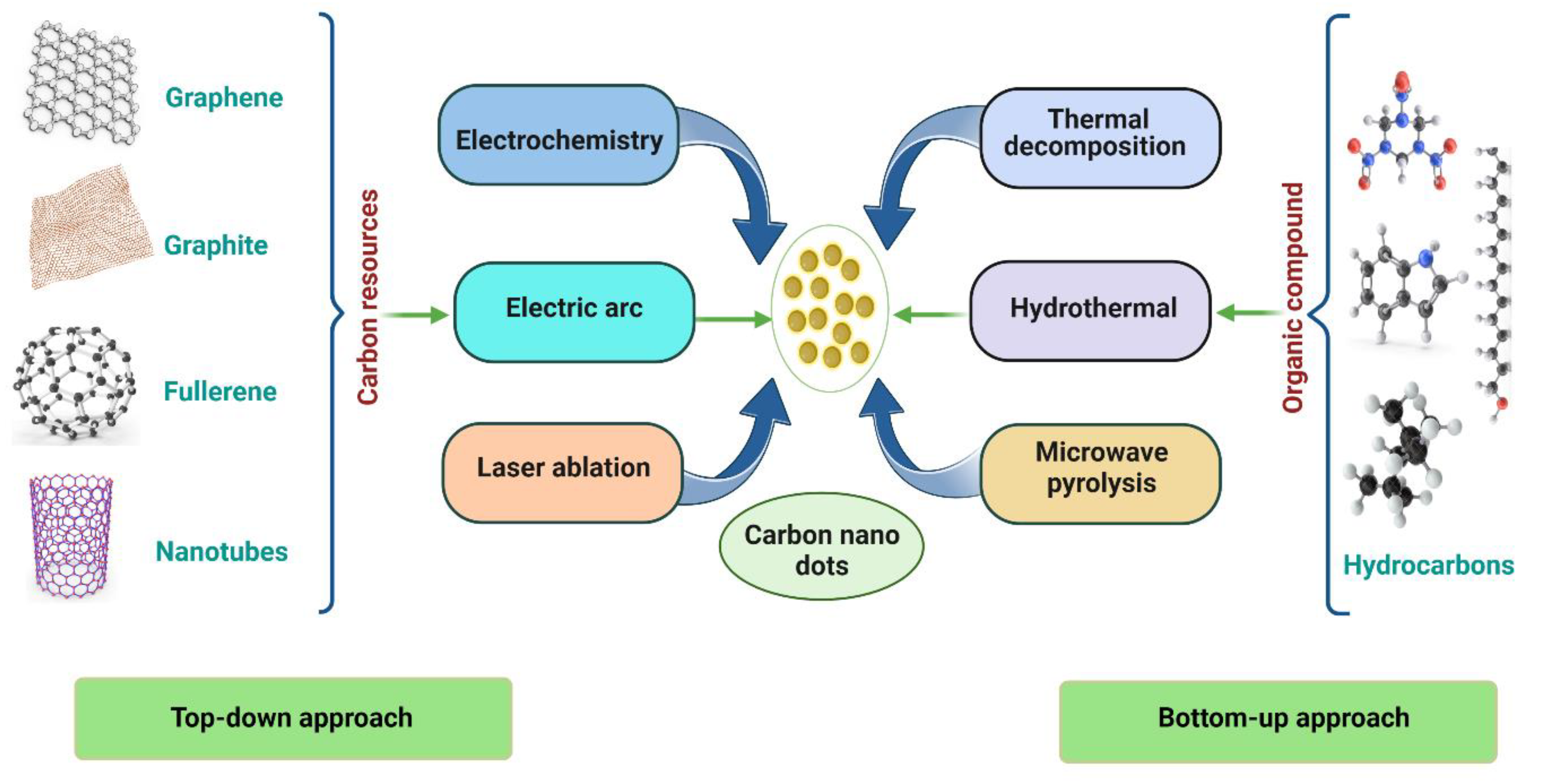
1.2. Bottom–Up Approach
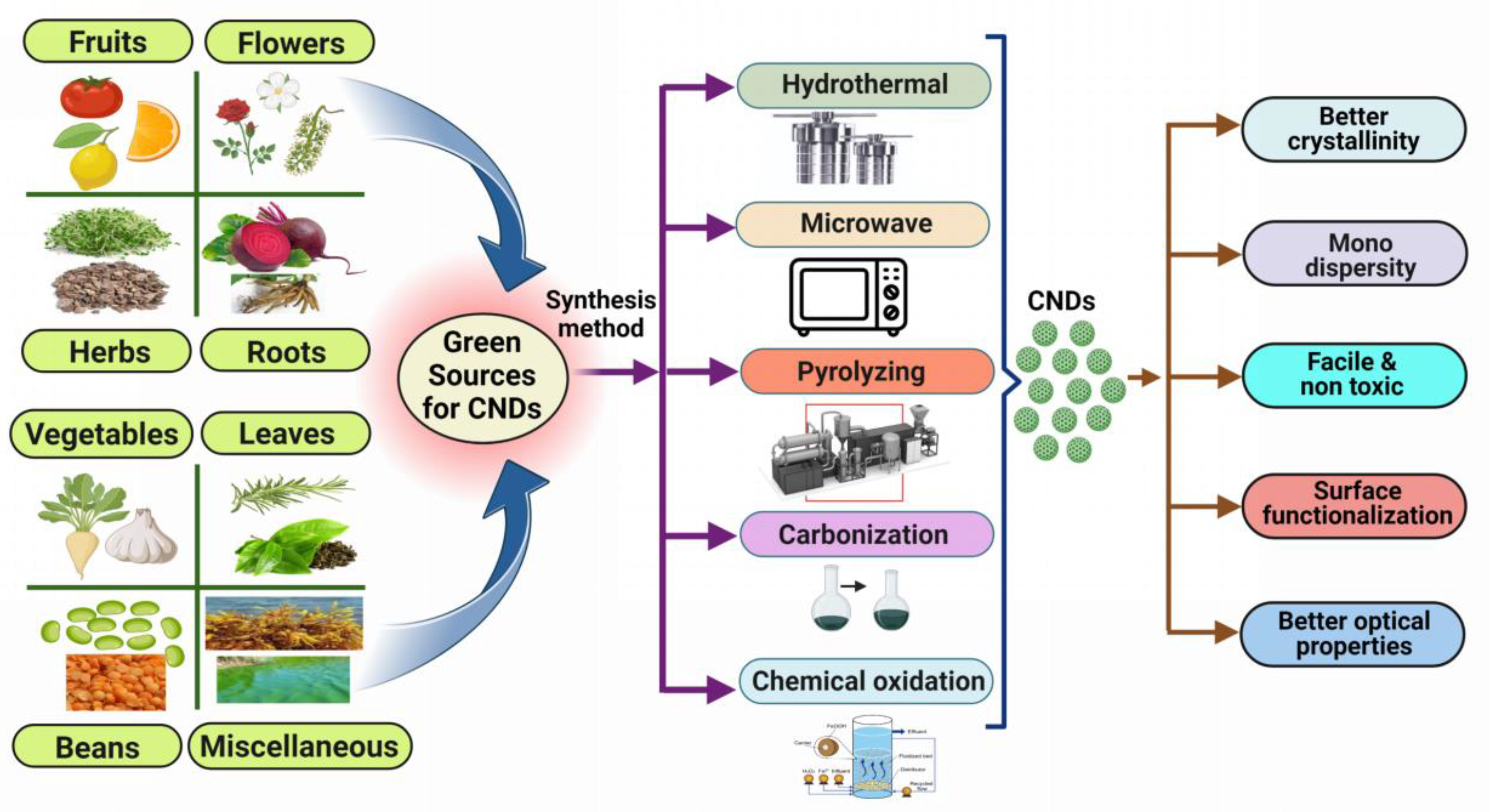
1.3. Preparation of CNDs Using Green Approach
| S. No. |
Source | Method of Synthesis | Size | Percentage Yield | Detection Limit | Inference | References |
|---|---|---|---|---|---|---|---|
| 1 | Banana peel | Microwave treatment | 5 to 15 nm | 16.0% | 1.82 × 10–17/mol | CNDs are fabricated by the microwave treatment of banana peels in a single pot for the determination of colitoxin DNA in human serum. | [11] |
| 2 | Sargassum fluitans | Hydrothermal | 2–8 nm | 18.2% | - | A hydrothermal method is used to produce CNDs from waste seaweed sargassum fluitans (S. fluitans) to detect DNA. | [12] |
| 3 | Tomato juice | Hydrothermal | 1.3–3.7 nm | 13.9% | 0.3 ng/mL | CNDs are synthesized by hydrothermal treatment of tomato juice for the sensing of carcinoembryonic antigen. | [13] |
| 4 | Limes | Pyrolyzing | 5-10 nm | - | - | The pyrolyzing process is used to synthesize CNDs for the detection of hepatitis B virus DNA. | [14] |
| 5 | Lemon juice | Carbonization | 6–9 nm | - | 0.23 mM | Carbonization of lemon juice is performed to form CNDs for the detection of l-tyrosine. | [15] |
| 6 | Lemon | Pyrolyzing | 10 nm | - | 0.0049 µM | Synthesis of CNDs from a lemon by the process of pyrolysis for the detection of doxorubicin hydrochloride in human plasma. | [16] |
| 7 | Syringa oblata lindl | Hydrothermal | 1.0–5.0 nm | 12.4%, | 0.11 μM | A hydrothermal method is used to fabricate CNDs from syringa oblata lindl for sensors and cell imaging. | [17] |
| 8 | Grapefruit | Hydrothermal | >30 nm | 20% | - | Grapefruit is used to create CNDs using a hydrothermal process for the detection of E. coli bacteria. | [18] |
| 9 | Alfalfa and garlic | Hydrothermal | 1.3–6.9 nm | 10% | 86 nM | A hydrothermal method is used to form CNDs from alfalfa and garlic as a fluorescent probe for cysteine, glutathione, and homocysteine. | [19] |
| 10 | Catharanthus roseus (white flowering plant) | Hydrothermal carbonization | - | - | - | Catharanthus roseus (white flowering plant) is hydrothermally carbonized to create CNDs to detect the Al3+ and Fe3+ ions. | [10] |
| 11 | Lemon juice | Hydrothermal | - | - | - | The one-pot facile hydrothermal approach was used to create highly luminous carbon dots (C-dots) from lemon juice. | [20] |
| 12 | Daucus carota | Hydrothermal | - | 7.60% | - | A hydrothermal method is used to produce CNDs from Daucus carota to detect mitomycin. | [21] |
| 13 | Natural polymer starch | Hydrothermal | 2.25–3.50 nm | - | - | Hydrothermal treatment of natural polymer starch is performed to produce CNDs. | [22] |
| 14 | P. acidus | Hydrothermal | 5 nm | 12.5% | - | CNDs are produced by a hydrothermal process from P. Acidus for live cell imaging. | [23] |
| 15 | Citrus peel powder | Sand bath heat-assisted method | 4.6 ± 0.28nm | - | - | The sand bath heat-assisted method is utilized to form CNDs from citrus peel powder for free radical scavenging and cell imaging. | [24] |
| 16 | Lentil | Hydrothermal | 7 ± 4 µm | 10% | 3.0 µg | A hydrothermal method is used to form CNDs from lentils for the colorimetric determination of thioridazine hydrochloride. | [25] |
| 17 | Rose flowers | Hydrothermal | 1.0–5.0 nm | - | 0.02–10 µM | CNDs are produced by a hydrothermal process from rose flowers for the determination of diazinon. | [26] |
| 18 | Saffron | Hydrothermal | >20 nm | 23.6% | 1.8 n/mol | A hydrothermal method is used to produce CNDs from saffron for the sensing of prilocaine. | [27] |
| 19 | Valerian root | Hydrothermal | >10 nm | 14% | 0.6 ng/mL | Valerian root has been used to make CNDs using a hydrothermal process for the determination of imipramine. | [28] |
| 20 | Rosemary leaves | Hydrothermal | Approx. 5 nm. | 18% | 8 ng/mL | Rosemary leaves have been used to make CNDs using a hydrothermal process for the determination of thiabendazole in juices. | [29] |
| 21 | Beetroot | Microwave | 5 & 8 nm | 6% & 5% | - | CNDs made from aqueous beetroot extract by the process of a microwave for in vivo live animal imaging applications. | [30] |
| 22 | Eutrophic algal blooms | Chemical oxidation | Approx. 8 nm | 13% | - | Eutrophic algal blooms have been used to make CNDs using chemical oxidation for in vitro imaging. | [31] |
| 23 | Green tea leaf | Pyrolyzation | 2 nm | 14.8% | - | Synthesis of CNDs from green tea leaf by the process of pyrolysis for the sensing of gefitinib. | [32] |
| 24 | Waste tea residue | Chemical oxidation | 3.2 nm | 2.47% | Be 0.04 μg /mL | Waste tea residue has been used to make CNDs using chemical oxidation for the quantification of tetracycline. | [33] |
| 25 | Palm shell powder | Chemical oxidation | 4–10 nm | 6.8% | 0.079 µM | CNDs are synthesized by the chemical oxidation method from palm shell powder for the sensing of nitrophenol. | [34] |
| 26 | Soybeans | Ultrasonic-assisted method | 2.4 nm | 16.7% | 0.9μM | An ultrasonic-assisted method is used to produce CNDs from soybeans to detect Fe3+ ions. | [35] |
2. Carbon Nanodots in Biosensing of microRNAs
| S. No. | Carbon Nanomaterial | Source and Synthesis | Conjugation Chemistry | Biomolecule (Analyte) |
Analytical Method | Detection Limit | Inference | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Carbon nanodots (CNDs) | O-phenylene diamine, 2-amino terephthalic acid by solvothermal method | EDC-NHS | miRNA-21 | Fluorescent biosensor | 0.03 fM | CNDs are synthesized and conjugated via EDC-NHS chemistry to detect miRNA-21. | [44] |
| 2 | PEI-Carbon dots | Polyethyleneimine (PEI) by hydrothermal method | - | miRNA-21 | Fluorescence biosensor | - | The synthesized CNDs is employed to detect miRNA-21 by fluorescence biosensor. | [45] |
| 3 | CNDs/AO | Citric acid in formamide | π-π conjugation | miRNA-92a-3p | Fluorometric assay (FRET) | 0.14 nM | To detect miRNA-92a-3p, CNDs are fabricated and conjugated using π–π conjugation. | [46] |
| 4 | CNDs–DNA walker | Citric acid and urea by microwave-assisted method | EDC-NHS | miRNA-21 miRNA-155 |
Electrochemiluminescence biosensor | 33 fM for miRNA-21. 33 aM for miRNA-155 |
CNDs are created and conjugated via EDC-NHS chemistry to discover miRNA-21 and miRNA-155. | [47] |
| 5 | CNDs | Oxidized maple leaf by a pyrolytic method | EDC-NHS | miRNA-21 | Electrochemiluminescence biosensor | 21 aM | CNDs are synthesized and conjugated via EDC-NHS chemistry to detect miRNA-21 associated with breast cancer. | [43] |
| 6 | CNDs | Tiger nut milk by carbonization | - | miRNA-21 | Chemiluminescence biosensor | 0.721 fM | Synthesized CNDs are used to detect miRNA-21 associated with cardiovascular disease. | [48] |
| 7 | CNDs | Glutaraldehyde, nitro benzaldehyde by solvothermal method | - | miRNA-21 | Fluorescence sensor | 0.03 fM | An miRNA-21 associated with breast cancer is identified using a fluorescence sensor that is based on carbon dots. | [49] |
| 8 | CNDs | Malic acid centrifugation | EDC-NHS | miRNAs | Fluorescence | 0.03 pM | The synthesized CNDs is conjugated via EDC NHS chemistry and used to detect miRNA. | [50] |
| 9 | CNDs | Citric acid by microwave method | π-π stacking | miRNAs | Fluorescence biosensor | 2.78 fM | CNDs were synthesized and employed to detect miRNAs by fluorescence biosensor. | [51] |
| 10 | CNDs | Tree leaves by hydrothermal method | EDC-NHS | miRNA-155 | Fluorescence biosensor FRET | 0.3 aM | CNDs were synthesized and conjugated via EDC-NHS chemistry and were used to detect miRNA-155 by fluorescence biosensor. | [52] |
| 11 | CNDs/BHQ 2 | Ethane diamine, p-benzoquinone | Maleimide-thiol | miRNA-141 | FRET | 16.5 pM | miRNA-14 is conjugated with synthesized CNDs via maleimide–thiol conjugation chemistry and detected by a fluorimetry test. | [53] |
| 12 | Carbon nanotubes (CNTs) | Hydrogen tetrachloroaurate trihydrate |
EDC-NHS | miRNA-21 | Fluorescence biosensor | 36 pM | A synthesized CNT is conjugated via EDC-NHS chemistry to detect intracellularly miRNAs-21. |
[54] |
| 13 | CNDs | Pyrolysis synthesis | Amine-amine conjugation | miRNA-21 | Ratiometric fluorescence | 1 pM | Synthesized CNDs were used to detect miRNA-21 associated with gastrointestinal cancer. | [55] |
| 14 | CNDs | Citric acid ethylene diamine/carbonization | Amine -glutaraldehyde | miRNA-155 | FRET | 0.1 aM | Fabricated CNDs are used to identify miRNA-155 present in cancer cells. | [56] |
| 15 | CNTs (MWCNT/AuNCs) | Carboxylic acid-ultrasonic cell disruption |
Thiol conjugation | miRNA-155 | FRET | 33.4 fM | CNTs are synthesized and used to detect miRNA-155. | [57] |
| 16 | CNDs | Citric acid– hydrothermal |
π-π stacking | micro-RNA | Fluorescence | A CNDs is used to detect miRNA by fluorescence method. | [58] | |
| 17 | CNTs | - | miRNA-21 | Electrochemical biosensor | 1.95 fM | miRNA-21 is detected by a carbon nanotube-based electrochemical biosensor. | [59] | |
| 18s | Carbon nanofibers/SPE | - | Amine-carboxylic acid conjugation | miRNA-34a | Electrochemical biosensor | 54 pM | The electrochemical biosensor is utilized to detect miRNA-34a using carbon nanofibers. | [60] |
| 19 | DNA-CNDs/CNTs | - | π-π stacking | miRNA-7f | Photoelectrochemical biosensor | 34 fM | A CNTs is used to detect miRNA-7f by a photoelectrochemical method. | [61] |
| 20 | Carbon nanoparticles/ssDNA probe | Graphite electrode by an electro-oxidation method | π-π stacking | miRNA let-7a | Fluorescence | 0.35 pM | Synthesized carbon nano-particles conjugated via π–π stacking are used to detect miRNA let-7a. | [62] |
References
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. . R. Soc. Open Sci. 2019, 6, 191378, 10.1098/rsos.191378.
- Singh, V.; Rawat, K.S.; Mishra, S.; Baghel, T.; Fatima, S.; John, A.A.; Kalleti, N.; Singh, D.; Nazir, A.; Rath, S.K.; et al. Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice.. J. Mater. Chem. B 2018, 6, 3366–3371, 10.1039/C8TB00503F.
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378, 10.1098/rsos.191378.
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of pristine and functionalized carbon nanotubes, graphene, and graphene nanoribbons in biomedicine. . Nanomaterials 2021, 11, 3020, 10.3390/nano11113020.
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. . Nano Today 2020, 33, 100879, 10.1016/j.nantod.2020.100879.
- Zhou, X.; Yu, G. Modified Engineering of Graphene Nanoribbons Prepared via On-Surface Synthesis.. Adv. Mater 2020, 32, 1905957, 10.1002/adma.201905957.
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy.. Green Chem. 2020, 22, 4747–4800, 10.1039/D0GC00998A.
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications.. Sustain. Mater. Technol. 2021, 29, e00304, 10.1016/j.susmat.2021.e00304.
- Yahaya Pudza, M.; Zainal Abidin, Z.; Abdul Rashid, S.; Md Yasin, F.; Noor, A.S.M.; Issa, M.A. Sustainable synthesis processes for carbon dots through response surface methodology and artificial neural network.. Processes 2019, 7, 704, 10.3390/pr7100704.
- Hashemi, N.; Mousazadeh, M.H. Green synthesis of photoluminescent carbon dots derived from red beetroot as a selective probe for Pd2+ detection. . J. Photochem. Photobiol. A Chem. 2021, 421, 113534, 10.1016/j.jphotochem.2021.113534.
- Kumar, J.V.; Kavitha, G.; Albasher, G.; Sajjad, M.; Arulmozhi, R.; Komal, M.; Nivetha, M.S.; Abirami, N. Multiplex heteroatoms doped carbon nano dots with enhanced catalytic reduction of ionic dyes and QR code security label for anti-spurious applications. . Chemosphere 2022, 307, 136003, 10.1016/j.chemosphere.2022.136003.
- Arumugham, T.; Alagumuthu, M.; Amimodu, R.G.; Munusamy, S.; Iyer, S.K. , , . A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications.. Sustain. Mater. Technol. 2020, 23, e00138, 10.1016/j.susmat.2019.e00138.
- Huang, Q.; Lin, X.; Zhu, J.-J.; Tong, Q.-X. Pd-Au@ carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum.. Biosens. Bioelectron. 2017, 94, 507–512, 10.1016/j.bios.2017.03.048.
- Godavarthi, S.; Kumar, K.M.; Vélez, E.V.; Hernandez-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, M.; Gomez, L.M. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection.. J. Photochem. Photobiol. B Biol. 2017, 172, 36–41, 10.1016/j.jphotobiol.2017.05.014.
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-free fluorimetric detection of CEA using carbon dots derived from tomato juice.. Biosens. Bioelectron. 2016, 86, 83–89, 10.1016/j.bios.2016.06.043.
- Xiang, Q.; Huang, J.; Huang, H.; Mao, W.; Ye, Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. . RSC Adv. 2018, 8, 1820–1825, 10.1039/C7RA11945C.
- Habibi, E.; Heidari, H. Renewable Surface Carbon-composite Electrode Bulk Modified with GQD-RuCl3 Nano-composite for High Sensitive Detection of l-tyrosine.. Electroanalysis 2016, 28, 2559–2564., 10.1002/elan.201600010.
- Hashemzadeh, N.; Hasanzadeh, M.; Shadjou, N.; Eivazi-Ziaei, J.; Khoubnasabjafari, M.; Jouyban, A. Graphene quantum dot modified glassy carbon electrode for the determination of doxorubicin hydrochloride in human plasma.. J. Pharm. Anal. 2016, 6, 235–241, 10.1016/j.jpha.2016.03.003.
- Diao, H.; Li, T.; Zhang, R.; Kang, Y.; Liu, W.; Cui, Y.; Wei, S.; Wang, N.; Li, L.; Wang, H.; et al. Facile and green synthesis of fluorescent carbon dots with tunable emission for sensors and cells imaging.. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 226–234, 10.1016/j.saa.2018.04.029.
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. . Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 481–493, 10.1016/j.saa.2018.06.021.
- Guo, Y.; Yang, L.; Li, W.; Wang, X.; Shang, Y.; Li, B. Carbon dots doped with nitrogen and sulfur and loaded with copper (II) as a “turn-on” fluorescent probe for cystein, glutathione and homocysteine.. Microchim. Acta 2016, 183, 1409–1416, 10.1007/s00604-016-1779-6.
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green synthesis of highly luminescent carbon quantum dots from lemon juice.. J. Nanotechnol. 2019, 2019, 2852816, 10.1155/2019/2852816.
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. . , , . Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik 2018, 158, 893–900, 10.1016/j.ijleo.2017.12.200.
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z.; et al. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. . Green Chem. 2018, 20, 4438–4442, 10.1039/C8GC02106F.
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Selvam, N.C.S.; Lee, Y.R. Green synthesized multiple fluorescent nitrogen-doped carbon quantum dots as an efficient label-free optical nanoprobe for in vivo live-cell imaging. . J. Photochem. Photobiol. A Chem. 2019, 372, 99–107, 10.1016/j.jphotochem.2018.12.011.
- Gudimella, K.K.; Appidi, T.; Wu, H.-F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand bath assisted green synthesis of carbon dots from citrus fruit peels for free radical scavenging and cell imaging. . Colloids Surf. B Biointerfaces 2021, 197, 111362, 10.1016/j.colsurfb.2020.111362.
- Amjadi, M.; Hallaj, T.; Mayan, M.A. Green synthesis of nitrogen-doped carbon dots from lentil and its application for colorimetric determination of thioridazine hydrochloride. . RSC Adv. 6, 2016, 104467–104473, 10.1039/C6RA22899B.
- Shekarbeygi, Z.; Farhadian, N.; Khani, S.; Moradi, S.; Shahlaei, M. The effects of rose pigments extracted by different methods on the optical properties of carbon quantum dots and its efficacy in the determination of Diazinon. . Microchem. J. , , . 2020, 158, 105232, 10.1016/j.microc.2020.105232.
- Ensafi, A.A.; Sefat, S.H.; Kazemifard, N.; Rezaei, B.; Moradi, F. A novel one-step and green synthesis of highly fluorescent carbon dots from saffron for cell imaging and sensing of prilocaine.. Sens. Actuators B Chem. 2017, 253, 451–460, 10.1016/j.snb.2017.06.163.
- Singh, V.; Rawat, K.S.; Mishra, S.; Baghel, T.; Fatima, S.; John, A.A.; Kalleti, N.; Singh, D.; Nazir, A.; Rath, S.K.; et al. Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice.. J. Mater. Chem. B 2018, 6, 3366–3371, 10.1039/C8TB00503F.
- Jackson, K.L.; Henderson, J.A.; Phillips, A.J. The halichondrins and E7389.. Chem. Rev. 2009, 109, 3044–3079, 10.1021/cr900016w.
- Hu, Z.; Jiao, X.-Y.; Xu, L. The N,S co-doped carbon dots with excellent luminescent properties from green tea leaf residue and its sensing of gefitinib. . Microchem. J. 2020, 154, 104588, 10.1016/j.microc.2019.104588.
- Gunjal, D.B.; Gurav, Y.M.; Gore, A.H.; Naik, V.M.; Waghmare, R.D.; Patil, C.S.; Sohn, D.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B.; et al. Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application.. Opt. Mater. 2019, 98, 109484, 10.1016/j.optmat.2019.109484.
- Soni, H.; Pamidimukkala, P.S. Green synthesis of N,S co-doped carbon quantum dots from triflic acid treated palm shell waste and their application in nitrophenol sensing.. Mater. Res. Bull. 2018, 108, 250–254, 10.1016/j.materresbull.2018.08.033.
- Zhao, W.-B.; Liu, K.-K.; Song, S.-Y.; Zhou, R.; Shan, C.-X. Fluorescent nano-biomass dots: Ultrasonic-assisted extraction and their application as nanoprobe for Fe3+ detection.. Nanoscale Res. Lett. 2019, 14, 130, 10.1186/s11671-019-2950-x.
- Saleem, M.; Naz, M.; Shukrullah, S.; Shujah, M.; Akhtar, M.; Ullah, S.; Ali, S. One-pot sonochemical preparation of carbon dots, influence of process parameters and potential applications: A review. Carbon Lett. 2021, 32, 39–55, 10.1007/s42823-021-00273-y.
- Zhao, Y.; Zhang, Y.; Liu, X.; Kong, H.; Wang, Y.; Qin, G.; Cao, P.; Song, X.; Yan, X.; Wang, Q.; et al. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci. Rep. 2017, 7, 4452, 10.1038/s41598-017-04073-1.
- Xiao, D.; Yuan, D.; He, H.; Lu, J. Microwave-assisted one-step green synthesis of amino-functionalized fluorescent carbon nitride dots from chitosan. . Luminescence 2013, 28, 612–615, 10.1002/bio.2486.
- Mani, V.; Balamurugan, T.; Huang, S.-T. Rapid one-pot synthesis of polydopamine encapsulated carbon anchored with au nanoparticles: Versatile electrocatalysts for chloramphenicol and folic acid sensors.. Int. J. Mol. Sci. 2020, 21, 2853, 10.3390/ijms21082853.
- Domagała, K.; Borlaf, M.; Kata, D.; Graule, T. Synthesis of copper-based multi-walled carbon nanotube composites.. Arch. Metall. Mater. 2020, 65, 157–162, 10.24425/amm.2019.131109.
- Delgado-Martín, J.; Delgado-Olidén, A.; Velasco, L. Carbon dots boost dsRNA delivery in plants and increase local and systemic siRNA production. . Int. J. Mol. Sci. 2022, 23, 5338, 10.3390/ijms23105338.
- Goryacheva, O.; Mishra, P.; Goryacheva, I.Y. Luminescent quantum dots for miRNA detection.. Talanta 2018, 179, 456–465, 10.1016/j.talanta.2017.11.011.
- Zhang, Y.; Li, N.; Ma, W.; Yang, M.; Hou, C.; Luo, X.; Huo, D. Ultrasensitive detection of microRNA-21 by using specific interaction of antimonene with RNA as electrochemical biosensor. Bioelectrochemistry 2021, 142, 107890, 10.1016/j.bioelechem.2021.107890.
- Mohammadi, S.; Salimi, A.; Hoseinkhani, Z.; Ghasemi, F.; Mansouri, K. Carbon dots hybrid for dual fluorescent detection of microRNA-21 integrated bioimaging of MCF-7 using a microfluidic platform. J. Nanobiotechnology 2022, 20, 73, 10.1186/s12951-022-01274-3.
- He, M.; Shang, N.; Zheng, B.; Yue, G.; Han, X.; Hu, X. Ultrasensitive fluorescence detection of microRNA through DNA-induced assembly of carbon dots on gold nanoparticles with no signal amplification strategy. Microchim. Acta 2022, 189, 217, 10.1007/s00604-022-05309-2.
- Sun, Z.; Tong, Y.; Zhou, X.; Li, J.; Zhao, L.; Li, H.; Wang, C.; Du, L.; Jiang, Y. , , . Ratiometric Fluorescent Biosensor Based on Forster Resonance Energy Transfer between Carbon Dots and Acridine Orange for miRNA Analysis.. ACS Omega 2021, 6, 34150–34159, 10.1021/acsomega.1c05901.
- Wang, L.; Zhao, K.-R.; Liu, Z.-J.; Zhang, Y.-B.; Liu, P.-F.; Ye, S.-Y.; Zhang, Y.-W.; Liang, G.-X. An “on-off” signal-switchable electrochemiluminescence biosensor for ultrasensitive detection of dual microRNAs based on DNAzyme-powered DNA walker. Sens. Actuators B Chem. 2021, 348, 130660, 10.1016/j.snb.2021.130660.
- Gutiérrez-Gálvez, L.; García-Mendiola, T.; Gutiérrez-Sánchez, C.; Guerrero-Esteban, T.; García-Diego, C.; Buendía, I.; García-Bermejo, M.L.; Pariente, F.; Lorenzo, E. Carbon nanodot–based electrogenerated chemiluminescence biosensor for miRNA-21 detection.. Microchim. Acta 2021, 188, 398, 10.1007/s00604-021-05038-y.
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells.. Talanta 2021, 224, 121895, 10.1016/j.talanta.2020.121895.
- Chen, J.; Yan, J.; Feng, Q.; Miao, X.; Dou, B.; Wang, P. Label-free and enzyme-free fluorescence detection of microRNA based on sulfydryl-functionalized carbon dots via target-initiated hemin/G-quadruplex-catalyzed oxidation.. Biosens. Bioelectron. 2021, 176, 112955, 10.1016/j.bios.2020.112955.
- Liu, G.; Chai, H.; Tang, Y.; Miao, P. Bright carbon nanodots for miRNA diagnostics coupled with concatenated hybridization chain reaction.. Chem. Commun. 2020, 56, 1175–1178, 10.1039/C9CC08753B.
- Hamd-Ghadareh, S.; Hamah-Ameen, B.A.; Salimi, A.; Fathi, F.; Soleimani, F. Ratiometric enhanced fluorometric determination and imaging of intracellular microRNA-155 by using carbon dots, gold nanoparticles and rhodamine B for signal amplification. Microchim. Acta 2019, 186, 469, 10.1007/s00604-019-3446-1.
- Cheng, Y.Y.; Xie, Y.F.; Li, C.M.; Li, Y.F.; Huang, C.Z. Förster resonance energy transfer-based soft nanoballs for specific and amplified detection of microRNAs.. Anal. Chem. 2019, 91, 11023–11029, 10.1021/acs.analchem.9b01281.
- Liu, Y.; Jiang, L.; Fan, X.; Liu, P.; Xu, S.; Luo, X. Intracellular fluorometric determination of microRNA-21 by using a switch-on nanoprobe composed of carbon nanotubes and gold nanoclusters. Microchim. Acta 2019, 186, 1–6, 10.1007/s00604-019-3573-8.
- Wang, Z.; Xue, Z.; Hao, X.; Miao, C.; Zhang, J.; Zheng, Y.; Zheng, Z.; Lin, X.; Weng, S. Ratiometric fluorescence sensor based on carbon dots as internal reference signal and T7 exonuclease-assisted signal amplification strategy for microRNA-21 detection.. Anal. Chim. Acta 2020, 1103, 212–219, 10.1016/j.aca.2019.12.068.
- Mohammadi, S.; Salimi, A. Fluorometric determination of microRNA-155 in cancer cells based on carbon dots and MnO2 nanosheets as a donor-acceptor pair.. Microchim. Acta 2018, 185, 372, 10.1007/s00604-018-2868-5.
- Ma, H.; Xue, N.; Li, Z.; Xing, K.; Miao, X. Ultrasensitive detection of miRNA-155 using multi-walled carbon nanotube-gold nanocomposites as a novel fluorescence quenching platform. Sens. Actuators B Chem. 2018, 266, 221–227, 10.1016/j.snb.2018.03.071.
- Khakbaz, F.; Mahani, M. Micro-RNA detection based on fluorescence resonance energy transfer of DNA-carbon quantum dots probes.. Anal. Biochem. 2017, 523, 32–38, 10.1016/j.ab.2017.01.025.
- Liu, L.; Song, C.; Zhang, Z.; Yang, J.; Zhou, L.; Zhang, X.; Xie, G. Ultrasensitive electrochemical detection of microRNA-21 combining layered nanostructure of oxidized single-walled carbon nanotubes and nanodiamonds by hybridization chain reaction. . Biosens. Bioelectron. 2015, 70, 351–357, 10.1016/j.bios.2015.03.051.
- Pinheiro, J.P.; van Leeuwen, H.P. Scanned stripping chronopotentiometry of metal complexes: Lability diagnosis and stability computation.. J. Electroanal. Chem. 2004, 570, 69–75, 10.1016/j.jelechem.2004.03.016.
- Cao, H.; Liu, S.; Tu, W.; Bao, J.; Dai, Z. A carbon nanotube/quantum dot based photoelectrochemical biosensing platform for the direct detection of microRNAs. Chem. Commun. 2014, 50, 13315–13318, 10.1039/C4CC06214K.
- Wang, L.; Cheng, Y.; Wang, H.; Li, Z. A homogeneous fluorescence sensing platform with water-soluble carbon nanoparticles for detection of microRNA and nuclease activity. Analyst 2012, 137, 3667–3672, 10.1039/C2AN35396B.
- Jiang, C.; Meng, F.; Mao, D.; Tang, Y.; Miao, P. Tetrahedral DNA Nanoconjugates for Simultaneous Measurement of Telomerase Activity and miRNA.. ChemBioChem 2021, 22, 1302–1306, 10.1002/cbic.202000784.
- Yan, X.; Song, Y.; Zhu, C.; Song, J.; Du, D.; Su, X.; Lin, Y. Graphene quantum dot–MnO2 nanosheet based optical sensing platform: A sensitive fluorescence “turn off–on” nanosensor for glutathione detection and intracellular imaging. . ACS Appl. Mater. Interfaces 2016, 8, 21990–21996, 10.1021/acsami.6b05465.
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes—Hitting the biosensing “target”.. Sensors 2021, 21, 5201, 10.3390/s21155201.
- Liu, Y.; Li, R.; Liang, F.; Deng, C.; Seidi, F.; Xiao, H. Fluorescent paper-based analytical devices for ultra-sensitive dual-type RNA detections and accurate gastric cancer screening.. Biosens. Bioelectron. 2022, 197, 113781, 10.1016/j.bios.2021.113781.
- Shandilya, R.; Bhargava, A.; Ratre, P.; Kumari, R.; Tiwari, R.; Chauhan, P.; Mishra, P.K. Graphene Quantum-Dot-Based Nanophotonic Approach for Targeted Detection of Long Noncoding RNAs in Circulation.. ACS Omega 2022, 7, 26601–26609, 10.1021/acsomega.2c02802.
- Xia, Y.; Wang, L.; Li, J.; Chen, X.; Lan, J.; Yan, A.; Lei, Y.; Yang, S.; Yang, H.; Chen, J.; et al. A ratiometric fluorescent bioprobe based on carbon dots and acridone derivate for signal amplification detection exosomal microRNA. Anal. Chem. 2018, 90, 8969–8976, 10.1021/acs.analchem.8b01143.
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H.; et al. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. . Int. J. Biol. Macromol. 2020, 162, 1100–1108, 10.1016/j.ijbiomac.2020.06.239.
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. . Chem. Soc. Rev. 2015, 44, 4792–4834, 10.1039/C4CS00532E.
- Guo, Y.-Z.; Liu, J.-L.; Chen, Y.-F.; Chai, Y.-Q.; Li, Z.-H.; Yuan, R. Boron and Nitrogen-Codoped Carbon Dots as Highly Efficient Electrochemiluminescence Emitters for Ultrasensitive Detection of Hepatitis B Virus DNA.. Anal. Chem. 2022, 94, 7601–7608, 10.1021/acs.analchem.2c00763.
- Song, S.; Li, N.; Bai, L.; Gai, P.; Li, F. Photo-assisted robust anti-interference self-powered biosensing of microRNA based on Pt-S bonds and the inorganic–organic hybridization strategy. Anal. Chem. 2022, 94, 1654–1660, 10.1021/acs.analchem.1c04135.
- Xu, Q.; Ma, F.; Huang, S.-q.; Tang, B.; Zhang, C.-y. Nucleic acid amplification-free bioluminescent detection of MicroRNAs with high sensitivity and accuracy based on controlled target degradation.. Anal. Chem. 2017, 89, 7077–7083, 10.1021/acs.analchem.7b00892.




