Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaolong Liu | -- | 1511 | 2023-05-05 04:42:50 | | | |
| 2 | Sirius Huang | + 9 word(s) | 1520 | 2023-05-06 02:54:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, G.; Zhu, X.; Liu, J.; Li, S.; Liu, X. MOS Gas Sensor for Lung Cancer Diagnosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/43816 (accessed on 08 February 2026).
Li G, Zhu X, Liu J, Li S, Liu X. MOS Gas Sensor for Lung Cancer Diagnosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/43816. Accessed February 08, 2026.
Li, Guangyao, Xitong Zhu, Junlong Liu, Shuyang Li, Xiaolong Liu. "MOS Gas Sensor for Lung Cancer Diagnosis" Encyclopedia, https://encyclopedia.pub/entry/43816 (accessed February 08, 2026).
Li, G., Zhu, X., Liu, J., Li, S., & Liu, X. (2023, May 05). MOS Gas Sensor for Lung Cancer Diagnosis. In Encyclopedia. https://encyclopedia.pub/entry/43816
Li, Guangyao, et al. "MOS Gas Sensor for Lung Cancer Diagnosis." Encyclopedia. Web. 05 May, 2023.
Copy Citation
Lung cancer is the most prevalent severe illness in both sexes and all ages and the leading cause of cancer-related deaths globally. Late-stage diagnosis is the primary cause of its high mortality rate. Therefore, the management of lung cancer needs early-stage screening. Breath analysis is a non-invasive, low-cost, and user-friendly approach to diagnosing lung cancer. Among the various types of breath sensors, metal oxide semiconductor (MOS) gas sensors are preferred due to their high gas responses, fast response times, robustness, and lower price.
MOS gas sensor
lung cancer diagnosis
sensor array
exhaled breath VOCs
1. Introduction
Metal oxide semiconductor (MOS) gas sensors are highly regarded for their high sensitivity, fast response, simple fabrication, durability, small size, and easy integration [1][2]. The origin of semiconductor gas-sensitive materials dates back to 1931 when Engelhard et al. discovered that the conductivity of Cu2O changed with water vapor adsorption. Despite this finding, it received little attention [3]. In 1962, Seiyama et al. first observed the distinct resistance of ZnO thin films in combustible gases and air at high temperatures, establishing the foundation for MOS gas sensors [2]. Since then, MOS gas sensors have evolved rapidly, with the first commercial MOS gas sensor developed in 1968. To satisfy the requirements of MOS gas sensors in complex environments, Persaud et al. proposed a method of mimicking the animal olfactory system (e-nose) in 1982 to improve the selectivity of sensors for volatile organic compounds (VOCs) by using an array of MOS materials with different characteristics to detect mixed gases concurrently [4]. In the following decades, the gas-sensitive mechanism of MOS was further explored [5][6][7][8], the gas sensitivity of a single MOS was continuously enhanced [9], and the fabrication methods of sensor arrays and analysis algorithms were constantly being improved [10][11].
2. Working Mechanism
The working mechanism of MOS gas sensors depends on the sensing material’s conductivity change when exposed to different gas environments, enabling target gas detection. Several theories, including the chemisorbed oxygen model [6], the grain boundary barrier model [7], the bulk resistance model [12], and the space-charge layer model (electron depletion layer (EDL) and hole-accumulation layer (HAL)) [5][8], can explain the MOS conductivity changes. The key to these theories is the interaction between the gas and material surfaces [13]. When the affinity energy of the gas molecules exceeds the work function of the MOS surface, electrons transfer from the MOS surface to the gas molecules, resulting in gas anions forming and being adsorbed onto the MOS surface [14]. In particular, the chemisorbed oxygen species (O2−, O−, O2−) play a crucial role in MOS surface conductivity [6]—closely related to the working temperature and MOS type—determining the MOS’s gas-sensing properties [15][16][17]. The formation process of chemisorbed oxygen species on the surface of SnO2 can be summarized by the following formulas [17] (Formulas (1)–(4)). We should note that MOS gas sensors have a broad response mode, leading to low selectivity, which can be improved by compounding them with other types of materials [18].
Inair:O2gas→O2abs
T<150 °C:O2abs+e−→O2−abs
150 °C<T<400 °C:O2−abs+e−→2O−abs
T>400 °C:O−abs+e−→O2−abs
For n-type SnO2, the gas–surface interaction process can be explained as follows (Figure 1) [13][14]. Firstly, O2 in the air is adsorbed onto the oxygen adsorption site on the SnO2 surface when heated to a specific temperature. Subsequently, oxygen molecules capture the conduction band electrons of MOS to form chemisorbed oxygen species (O2−, O−, O2−) and simultaneously create an EDL at the MOS grain contact interface, which results in a higher barrier, increasing the resistance. When introducing a reducing gas such as ethanol, the gas reacts with the chemisorbed oxygen species and releases the electrons back into the MOS simultaneously, thus lowering the resistance. These oxygen species can desorb or be adsorbed again onto the MOS surface to create new oxygen anions. On the other hand, introducing an oxidizing gas will further deplete the sensing layer electrons and increases MOS resistance.
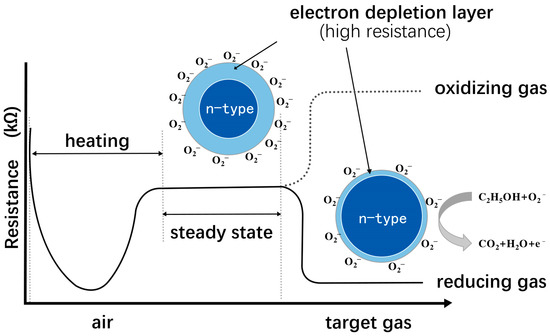
Figure 1. Schematic diagram of detection principle of n-type MOS sensitive material.
Although there is no unified theory on gas-sensing mechanisms, rational experimental designs based on existing materials can enhance their gas-sensing properties. The width of the space-charge layer, also known as the Debye length, typically ranges from 2 to 100 nm due to oxygen adsorption on the MOS surface. When the MOS grain size is close to or less than twice the Debye length, it amplifies the sensitivity considerably, demonstrating the remarkable potential of nanomaterials in gas sensors [19]. Nevertheless, excessively small particle sizes can cause nanoparticle aggregation, which will hinder the participation of internal particles in the reaction and decrease the gas-sensing properties of MOS [20]. Hence, specific nanoscale structural designs are necessary to reduce particle size while preventing nanoparticle aggregation. The heterojunction effect and synergistic effect can also impact the gas-sensing properties of MOS materials [21], which can be achieved through metal ion doping, metal particle modification, and compounding with other substances on the MOS substrate. In summary, the micro/nanostructure design and “second-phase modification” are essential methods for enhancing the gas-sensing properties of MOS materials. However, designing a new gas-sensitive material from scratch is still challenging.
3. Candidate Materials
Early comments on gas sensor design suggested that any sufficiently fine dispersed metal oxide could serve as a gas-sensitive layer for gas sensors, regardless of the practical usage requirements [22]. However, the exhaled gas of humans contains many components with low concentrations [23][24], making it necessary to identify the most appropriate candidate material among a wide range of available materials to accurately and efficiently measure the components and concentrations of exhaled breath [25]. Researchers conducted a search using keywords such as “material name”, “chemical formula”, and “gas sensor” to investigate the most researched MOS over the past few decades. For the n-type semiconductors SnO2, ZnO, TiO2, WO3, In2O3, Fe2O3, and CeO2, the number of search results was 12,663, 10,920, 5269, 4102, 3487, 2643, and 1012, respectively, while for the p-type semiconductors CuO, NiO, Co3O4, Cr2O3, and Mn3O4, they were 3003, 2649, 1604, 822, and 564, respectively. The statistics show in Figure 2 that the overall proportions of the research results for the n-type and p-type MOS gas-sensitive materials were 82.3% and 17.7%, respectively. Among the top five materials, SnO2, ZnO, TiO2, WO3, and In2O3 were n-type MOS materials, indicating that more attention has been paid to the research and development of n-type MOS gas-sensitive materials. According to Hübner et al., the inherent low sensitivity of p-type MOS materials restricts their development [26]. Furthermore, only TiO2 belongs to the bulk resistance-controlled MOS among the top five materials. The conductivity change in them involves the reaction of gas with the lattice oxygen of the material, leading to a higher reaction temperature (usually above 700 °C), slower response rate, and poorer stability, making it less suitable for the detection of VOCs in exhaled breath [27][28][29][30].
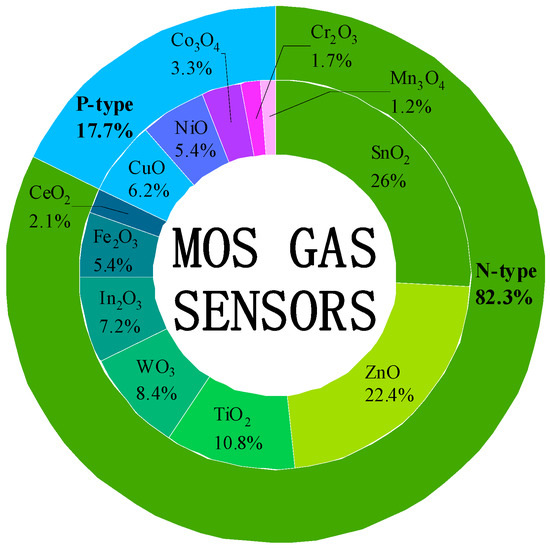
Figure 2. Publication of papers and patents on major MOS gas sensors before 15 February 2023. Here, the papers and patents were searched on the Web of Science, refined by ‘keywords = gas sensor, the chemical formula and the scientific name of the sensor material’ with all document types on 15 February 2023.
According to the statistical analysis of exhaled breath biomarkers for lung cancer VOCs and MOS gas-sensing materials (Figure 1), the n-type surface resistivity-controlled MOS materials have gained significant attention for detecting VOCs in exhaled breath for lung cancer diagnosis. Among them, SnO2 and ZnO are currently the two most widely studied MOS gas-sensing materials and receive the most attention. Therefore, the following sections will focus on the latest research on n-type MOS gas-sensing materials (especially SnO2 and ZnO), detecting exhaled breath biomarkers of lung cancer (BTEX, isoprene, hexanal, nonanal, 2-butanone, acetone, n-pentane, 1-propanol, etc.), considering the diverse types, low concentrations, and high-humidity conditions of exhaled breath analysis. The aim is to guide the development of e-nose devices with higher sensitivity, better selectivity, and more excellent stability to enable early lung cancer screening on a large scale.
4. Single MOS Gas Sensor and Performance Improvement Strategy
BTEX, comprising benzene, toluene, ethylbenzene, and xylene, has been frequently detected in the exhaled breath of lung cancer patients [31]. These compounds are metabolized similarly in lung cancer patients and are often detected together in exhaled breath samples [31][32]. However, due to the limited selectivity of MOS gas sensors for VOCs in this family, it is advisable to select one of the strongly correlated BTEX gases as a representative for detection [33]. For instance, the toluene content in the exhaled breath of lung cancer patients is approximately 2–3 times higher than that of healthy individuals, ranging from 80 to 100 ppb [34].
When evaluating the performance of MOS gas-sensing materials for detecting lung cancer exhaled breath biomarkers, several parameters should be considered beyond sensitivity and response time. In particular, the selectivity, limits of detection (LOD), and stability are significant. Selectivity refers to a gas sensor’s ability to detect the target gas in the presence of other gases, which is crucial, given that over 3000 VOCs and various inorganic gas matrices are in human exhaled breath [23][24][35]. LOD refers to the lowest target gas concentration corresponding to the gas sensor’s minimum reliable response sensitivity value. MOS sensors should exhibit high sensitivity to detect low-concentration (ppt levels) VOCs [36][37]. Stability means the ability of MOS sensors to obtain reliable results over time, which is essential for an accurate diagnosis [38].
References
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561.
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1962, 34, 1502–1503.
- Engelhard, E.; Gudden, B. Zur Frage Der Gültigkeit Des Ohmschen Gesetzes Bei Cu2O. Bemerkung Zur Arbeit “Variable Widerstände Und Ihre Hydrodynamische Analogie” von R. Auerbach. Z. Phys. 1931, 70, 701–705.
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355.
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060.
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of Major Parameters on the Sensing Mechanism of Semiconductor Metal Oxide Based Chemiresistive Gas Sensors: A Review Focused on Personalized Healthcare. Sens. Actuators B Chem. 2022, 352, 131066.
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 5, 7–19.
- Ji, H.; Zeng, W.; Li, Y. Assembly of 2D Nanosheets into Flower-like MoO3: New Insight into the Petal Thickness Affect on Gas-Sensing Properties. Mater. Res. Bull. 2019, 118, 110476.
- Wang, H.; Ma, J.; Zhang, J.; Feng, Y.; Vijjapu, M.T.; Yuvaraja, S.; Surya, S.G.; Salama, K.N.; Dong, C.; Wang, Y.; et al. Gas Sensing Materials Roadmap. J. Phys. Condens. Matter 2021, 33, 303001.
- Li, Z.; Yu, J.; Dong, D.; Yao, G.; Wei, G.; He, A.; Wu, H.; Zhu, H.; Huang, Z.; Tang, Z. E-Nose Based on a High-Integrated and Low-Power Metal Oxide Gas Sensor Array. Sens. Actuators B Chem. 2023, 380, 133289.
- Rath, R.J.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2023, 25, 2200830.
- Wang, M.; Hou, T.; Shen, Z.; Zhao, X.; Ji, H. MOF-Derived Fe2 Why 3: Phase Control and Effects of Phase Composition on Gas Sensing Performance. Sens. Actuators B Chem. 2019, 292, 171–179.
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831.
- Sahm, T.; Gurlo, A.; Bârsan, N.; Weimar, U. Basics of Oxygen and SnO2 Interaction; Work Function Change and Conductivity Measurements. Sens. Actuators B Chem. 2006, 118, 78–83.
- Manno, D.; Micocci, G.; Rella, R.; Serra, A.; Taurino, A.; Tepore, A. Titanium Oxide Thin Films for NH3 Monitoring: Structural and Physical Characterizations. J. Appl. Phys. 1997, 82, 54–59.
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167.
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052.
- Morsy, M.; Abdel-Salam, A.I.; Mostafa, M.; Elzwawy, A. Promoting the Humidity Sensing Capabilities of Titania Nanorods/RGO Nanocomposite via de-Bundling and Maximizing Porosity and Surface Area through Lyophilization. Micro Nano Eng. 2022, 17, 100163.
- Bai, J.; Zhao, C.; Gong, H.; Wang, Q.; Huang, B.; Sun, G.; Wang, Y.; Zhou, J.; Xie, E.; Wang, F. Debye-Length Controlled Gas Sensing Performances in p-n Junctional Core–Shell Nanotubes. J. Phys. Appl. Phys. 2019, 52, 285103.
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810.
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.; Hahn, Y.-B. Improved Selectivity and Low Concentration Hydrogen Gas Sensor Application of Pd Sensitized Heterojunction N-ZnO/p-NiO Nanostructures. J. Alloys Compd. 2019, 797, 456–464.
- Moseley, P.T.; Norris, J.O.; Williams, D.E. Techniques and Mechanisms in Gas Sensing; Adam Hilger Bristol; CRC Press: Boca Raton, FL, USA, 1991; Volume 234.
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in Volatile Organic Compounds in the Breath of Normal Humans. J. Chromatogr. B. Biomed. Sci. Appl. 1999, 729, 75–88.
- Fenske, J.D.; Paulson, S.E. Human Breath Emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598.
- Jain, G.H. MOS Gas Sensors: What Determines Our Choice? In Proceedings of the 2011 Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011; pp. 66–72.
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of Humidity on CO Sensing with P-Type CuO Thick Film Gas Sensors. Sens. Actuators B Chem. 2011, 153, 347–353.
- Mohammed, R.S.; Fakhri, M.A. Titanium Dioxide–Based Sensors: A Review. AIP Conf. Proc. 2022, 2660, 020133.
- Yan, Z.; Zhang, Y.; Kang, W.; Deng, N.; Pan, Y.; Sun, W.; Ni, J.; Kang, X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials 2022, 12, 3611.
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403.
- Hazra, A.; Das, S.; Kanungo, J.; Sarkar, C.K.; Basu, S. Studies on a Resistive Gas Sensor Based on Sol–Gel Grown Nanocrystalline p-TiO2 Thin Film for Fast Hydrogen Detection. Sens. Actuators B Chem. 2013, 183, 87–95.
- Schmidt, F.; Kohlbrenner, D.; Malesevic, S.; Huang, A.; Klein, S.D.; Puhan, M.A.; Kohler, M. Mapping the Landscape of Lung Cancer Breath Analysis: A Scoping Review (ELCABA). Lung Cancer 2023, 175, 131–140.
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, Origin, and Implementation of Breath Volatile Cancer Markers. Chem. Soc. Rev. 2014, 43, 1423–1449.
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective BTEX Sensor Based on a SnO2/V2O5 Composite. Sens. Actuators B Chem. 2013, 186, 126–131.
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing Lung Cancer in Exhaled Breath Using Gold Nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673.
- Ferrus, L.; Guenard, H.; Vardon, G.; Varene, P. Respiratory Water Loss. Respir. Physiol. 1980, 39, 367–381.
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clin. Chim. Acta 2016, 459, 5–9.
- Bag, A.; Lee, N. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883.
- Jeong, S.-Y.; Kim, J.-S.; Lee, J.-H. Rational Design of Semiconductor-Based Chemiresistors and Their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




