Lung cancer is the leading cause of cancer deaths in the world. Surgery is the most potentially curative therapeutic option for patients with early-stage non-small cell lung cancer (NSCLC). The five-year survival for these patients remains poor and variable, depending on the stage of disease at diagnosis, and the risk of recurrence following tumor resection is high. During the last 20 years, there has been a modest improvement in the therapeutic strategies for resectable NSCLC. Immune checkpoint inhibitors (ICIs), alone or in combination with chemotherapy, have become the cornerstone for the treatment of metastatic NSCLC patients. Recently, their clinical development has been shifted in the neoadjuvant and adjuvant settings where they have demonstrated remarkable efficacy, leading to improved clinical outcomes. Based on the positive results from phase III trials, ICIs have become a therapeutic option in neoadjuvant and adjuvant settings. On October 2021 the Food and Drug Administration (FDA) approved atezolizumab as an adjuvant treatment following surgery and platinum-based chemotherapy for patients with NSCLC whose tumors express PD-L1 ≥ 1%. In March 2022, nivolumab in combination with platinum-doublet chemotherapy was approved for adult patients with resectable NSCLC in the neoadjuvant setting.
1. Introduction
Lung cancer is the leading cause of cancer deaths in the world
[1]. Non-small cell lung cancer (NSCLC) accounts for 80% of cases. Surgery is the most potentially curative therapeutic option, and the treatment of choice in patients with stage I and II cancers, followed by adjuvant platinum-doublet chemotherapy in those with stage II cancers
[2]. Despite the role of adjuvant therapy in reducing the risk of recurrence by eliminating systemic, micrometastatic disease, its impact on overall survival (OS) remains modest. The LACE meta-analysis, including data from 4584 patients enrolled in five randomized trials evaluating the efficacy of postoperative platinum-based chemotherapy, showed a five-year benefit of only 5.4%. The advantage was observed in patients with stage II and III (HR = 0.83; CI, 0.73 to 0.95), but not those with stage I (HR = 0.93; CI, 0.78 to 1.10)
[2]. Multimodality approaches, including chemotherapy, radiotherapy and surgery have represented the cornerstone in patients with stage IIIA. Despite these attempts, the five-year survival of resectable NSCLC patients ranges from 67% for those with T1N0 (IA) disease to 23% for those with T1-3N2 (IIIA), and the risk of recurrence following tumor resection remains high
[3]. Data from the literature indicate that approximately 20–30% of NSCLC patients with stage I, 50% of those with stage II and 60% of those with stage IIIA die within five years
[4].
Different preoperative strategies with neoadjuvant therapies have been explored with the aims to downstage the tumor before surgery, allow the use of minimally invasive surgery, inhibit the early development of micro-metastases, thus reducing the incidence of systemic relapse, and improve patients’ survival. These approaches have been associated with limited efficacy. The phase III North American Intergroup 0139 trial evaluated the impact of surgery in 396 patients with T1-3 N2 disease who received radiotherapy up to 45 Gy, concurrent with two cycles of cisplatin and etoposide
[5]. In the absence of progression, patients were randomized between surgery and the continuation of radiotherapy to 61 Gy. The primary endpoint was OS. Results showed that surgery determines a prolongation of progression-free survival (PFS) with no OS improvement. The OS was significantly improved in the subgroup that underwent lobectomy, while worse OS was observed in those receiving pneumonectomy. A meta-analysis conducted by the NSCLC Meta-Analysis Collaborative Group involving 15 randomized clinical trials including patients with stage IB to IIIA disease compared chemotherapy with subsequent surgery versus surgery alone demonstrated an absolute 5% survival benefit at 5 years with neoadjuvant chemotherapy
[6]. These data suggest the need to develop more effective strategies to reduce the risk of recurrence and improve the survival of resectable NSCLC.
2. The Immunological Bases for the Use of ICIs in the Preoperative Setting
Preoperative treatment offers the opportunity to study
in vivo radiological and adaptive responses of tumors to systemic therapy. As a consequence, it can be potentially used to identify prognostic and predictive factors to tailor subsequent adjuvant treatment strategies
[7].
Preclinical data from syngeneic mice models of NSCLC showed that the administration of three doses of neoadjuvant nivolumab or ipilimumab + nivolumab significantly prolonged survival over three doses of adjuvant nivolumab or ipilimumab + nivolumab (
p < 0.05). The greatest number of tumor-infiltrating lymphocytes (TILs) and highest CD8+ TIL density found in resected primary tumors from mice receiving neoadjuvant ICIs represent the immunological bases for the major benefit observed with neoadjuvant strategies
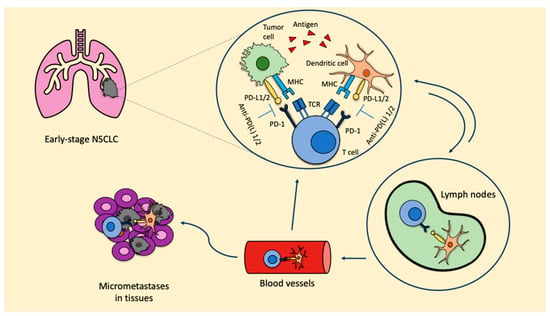
[8]. When ICIs are used in the neoadjuvant setting, due to the presence of the primary tumor, there is a higher probability of inducing tumor-specific CD8+ T cells and peripheral tumor-specific immune responses. Once activated, CD8+ T cells circulate into the blood, where they expand and infiltrate the organs. The T cell response favors the release of tumor antigens, which are recognized by the antigen-presenting cells (APC), therefore inducing the activation of prime naïve T cells. As a consequence, micrometastatic lesions are destroyed and a stable pool of CD8+ T cells remains, thus maintaining the T cells’ response and reducing the risk of recurrence (
Figure 1). These represent the bases leading to prolonged survival.
Figure 1. ICIs in the neoadjuvant setting: biological rationale. Anti-PD-L1: anti-programmed cell death-1; MHC: major histocompatibility complex; NSCLC: non-small cell lung cancer; PD-1: programmed cell death protein; PD-L1/2: programmed death ligand 1/2; TCR: T cell receptor.
The inhibition of the PD-1/PD-L1 axis allows T cells to kill tumoral cells and also induces the expansion of tumor-specific T cells in the tumor microenvironment. This expansion is mainly led by PD-L1/2 expressing dendritic cells in the tumor. Furthermore, dendritic cells that contain tumor antigens shift to lymph nodes, where they present these antigens to tumor-specific T cells, enhancing the productive stimulation of these. To this point, activated tumor-specific T cells can enter into the blood circulation and reach micrometastases in the tissues, starting a series of specific and durable antitumor immune responses. Some of these tumor-specific T cells return through blood vessels to the primary tumor where they can potentiate the antitumoral activity.
3. Efficacy of Monotherapy with PD-1 and PD-L1 Inhibitors in the Neoadjuvant Setting
In 2018, a pilot study evaluated the safety and feasibility of the anti-PD-1 agent nivolumab, administered as a neoadjuvant therapy for two cycles in 21 patients with resectable stage I-IIIA NSCLC
[9]. Secondary endpoints included radiologic and pathological responses. Feasibility was defined as the delay of surgery by no more than 37 days following nivolumab. Results demonstrated that nivolumab did not delay surgery, as the median interval between the last cycle and surgery was 18 days. Only one patient did not complete the two cycles of pre-planned therapy due to the onset of grade 3 pneumonia, which did not preclude surgery. Despite partial radiological responses being observed only in 10% of cases, and 86% developing stable disease, pathologic down-staging was registered in 40% of patients. Major pathological responses (MPR), defined as ≤10% residual viable tumor cells in the primary tumor and lymph nodes, were found in 45% of cases, while pathological complete response (pCR) in the primary tumor, defined as 0% of residual viable tumor cells, was observed in three patients. The median degree of pathological regression was—65%. In the resected tumor and in the peripheral blood, the expansion of CD8+ T cells was evidenced.
Similar findings were observed in the phase II LCMC3 trial
[10], which included 181 resectable NSCLC patients with stage IB-IIIA and selected IIIB (T3N2 or T4) who received two cycles of neoadjuvant atezolizumab. The primary endpoint was the percentage of MPR. Eighty-eight percent of the patients underwent surgery. The MPR rate was 20% and the pCR rate was 6%. The lower percentage of MPR registered in the LCMC3 trial in comparison to that observed in the study exploring nivolumab might be related to the large number of patients with stage III being included (51% in the LCMC3 study vs. 33%). The PD-L1 levels were significantly correlated with pathologic response (
p < 0.001). Among the enrolled patients, ten carried EGFR-activating mutations and six carried EML4-ALK translocations. No radiological response or MPR was registered. Lesser pathologic responses were identified in patients harboring STK11 mutations compared with wild-type. Encouraging 3-year disease-free survival (DFS) and OS of 72% and 80%, respectively, were reported. A significant expansion of peripheral blood-activated CD8+ T cells was observed in patients obtaining tumor regression.
The phase II IFCT-IONESCO study was designed to evaluate the feasibility of the anti-PD-L1 agent durvalumab as a neoadjuvant therapy in 46 resectable patients with stage IB-IIIA NSCLC
[11]. The primary endpoint was complete surgical resection, while secondary endpoints included the interval between the first administration of durvalumab and therapy, the tumor response, the rate of MPR, DFS, OS and safety. Eighty-nine percent of the patients underwent complete resection, with a time interval between the beginning of therapy and surgery of 37 days. The MPR was 19%. No correlation was observed between PD-L1 expression and the percentage of MPR. Different from the other trials, the mortality rate at 90 days following surgery was high, with 9% of deaths due to postoperative complications. However, three out of four patients who died had cardiovascular comorbidities.
The phase II NEOMUN study explored the safety of two cycles of another monoclonal antibody targeting PD-1, pembrolizumab, when administered as a neoadjuvant treatment in resectable NSCLC patients with stage IIA-IIIA cancers
[12]. Co-primary endpoints were the percentage of adverse events (AEs), the overall response rate (ORR) according to RECIST and iRECIST criteria, the percentage of pathological responses and the functional PET activity. Secondary endpoints included DFS and OS. Preliminary results showed MPR in 27% of patients, and treatment was associated with a good tolerability profile
[13].
All these data indicate that treatment with ICIs in the neoadjuvant setting is associated with a high percentage of resection rates, a good safety profile and a significant probability of prolonging patients’ survival. Data from the literature indicate that intratumoral immune cell infiltration, as a consequence of the treatment with ICIs, is associated with pseudo-progression in approximately 0.6–5.8% of patients with NSCLC
[14][15]. Nodal immune flare, registered in 13–19% of patients, refers to the presence, at the histological evaluation, of non-caseating granulomas and the absence of tumor cells within those lymph nodes radiologically suspected for tumor progression
[16]. In the neoadjuvant setting, the identification of the nodal immune flare phenomenon is particularly critical in order to differentiate between true progression and pseudo-progression, to not preclude curative surgery and to avoid an inappropriately large radiation field. Furthermore, in 7% of cases receiving neoadjuvant ICIs and no evidence of radiological nodal progression, non-caseating granulomas have been documented
[16]. Macrophages, dendritic cells, cytotoxic cells, Th1 cells and exhausted CD8+ T cells have been described in the lymph nodes presenting non-caseating granulomas. Moreover, the upregulation of genes associated with the activation of the immune system, including the interferon gamma (IFN-γ) pathway, and the downregulation of those associated with immune suppression, such as transforming growth factor beta (TGF-β) and SMAD2/3, have been identified. These data suggest the need to perform a pathological evaluation in the case of suspicious nodal immune flare before defining the therapeutic strategy in patients receiving neoadjuvant treatment with ICIs.