Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Veronica Milani | -- | 2593 | 2023-05-04 23:03:37 | | | |
| 2 | Dean Liu | Meta information modification | 2593 | 2023-05-05 04:00:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Milani, V.; Timelli, G. Chemical and Physical Properties of Solid Salt Fluxes. Encyclopedia. Available online: https://encyclopedia.pub/entry/43805 (accessed on 08 February 2026).
Milani V, Timelli G. Chemical and Physical Properties of Solid Salt Fluxes. Encyclopedia. Available at: https://encyclopedia.pub/entry/43805. Accessed February 08, 2026.
Milani, Veronica, Giulio Timelli. "Chemical and Physical Properties of Solid Salt Fluxes" Encyclopedia, https://encyclopedia.pub/entry/43805 (accessed February 08, 2026).
Milani, V., & Timelli, G. (2023, May 04). Chemical and Physical Properties of Solid Salt Fluxes. In Encyclopedia. https://encyclopedia.pub/entry/43805
Milani, Veronica and Giulio Timelli. "Chemical and Physical Properties of Solid Salt Fluxes." Encyclopedia. Web. 04 May, 2023.
Copy Citation
Solid salt fluxes are inorganic compounds that are added during the treatment of molten aluminum to improve the final quality. An understanding of the chemical composition of the flux is essential for the assessment of the physical and chemical behavior of the flux. The chemical composition of the flux can be tailored to adjust properties such as density, viscosity, reactivity, and wettability. Such properties, in turn, will impart different functions to the flux.
salt fluxes
secondary aluminum production
recycling
molten metal treatment
chlorides
fluorides
1. Chemistry of Salt Fluxes
The compounds usually found in solid fluxes can be classified into four major groups based on the main effect of the characteristics of the flux. The four major groups are chlorides, fluorides, oxidizing compounds, and solvents of aluminum oxide. They are described in the following sections.
1.1. Chlorides
Chlorides usually consist of NaCl and KCl. They mainly act as fillers, given their lower cost compared to other compounds for which chlorides act as carriers, such as fluorides. Chlorides are also used in high percentages for their fluidizing effects. When used individually, they act as passive fluxes, meaning that their reactivity with molten aluminum is negligible, and their effect on the surface tension is trivial if compared to fluorides. As aforementioned, the most common NaCl-KCl ratios are 50–50 and 70–30. Higher NaCl contents lead to a higher melting point of the flux; however, it can be economically convenient given the highest cost for KCl. Furthermore, a limited reduction of the amount of KCl in the flux does not seem to significantly affect the metal recovery [1].
Another binary mixture used as flux is MgCl2-KCl. Sodium-free fluxes are preferred by producers of Al-Mg alloys, especially when the Mg content is high, to avoid the introduction of Na in the aluminum melt. If sodium-containing fluxes are used, the higher the Mg content in the alloy, the more Na is introduced in the melt by fluxes. Furthermore, some flux additives such as NaF or Na3AlF6 can also increase the residual content of Na in the aluminum melt [2]. Nowadays, chloride fluxes such as MgCl2 and CaCl2 are also gaining more attention for the possibility of removing alkali and alkali-earth metals from molten aluminum. Purification of aluminum melt was once carried out through the injection of chlorine gas, but the practice has been abandoned due to environmental concerns for the release of chlorine gas, and it was replaced by solid chloride fluxes [3].
1.2. Fluorides
Fluorides, as previously explained, play an essential role in stripping the oxide layer and assisting the coalescence of metal droplets by acting as surfactants for wettability adjustments between salt, oxide, and molten aluminum. Common fluoride compounds include simple fluorides such as CaF2, NaF, KF, MgF2, and AlF3 and double fluoride compounds such as Na3AlF6, KalF4, K3AlF6, Na2SiF6, and K2SiF6 [4][5][6]. Fluorides are usually present in small amounts in flux mixtures, typically around 5 wt %, although their amount varies to a great extent depending on several factors, including the type of scrap, the desired metal quality, the level of inclusions in the melt, as well as the environmental regulations.
Nonetheless, too much fluoride addition can result in a degradation of flux properties because, usually, the fluidity and density increase because of fluoride additions. This results in poor coalescence and reduced metal recovery, as previously mentioned according to Stokes’ law (see Equation (2)). Additionally, an excessive increase in density may also lead to poor metal-slag separation. Thus, the amount of fluoride in the flux should be just enough to improve the wettability properties without excessively compromising the fluidity and density of the salt. Limiting the use of fluorides also implies economic and environmental benefits. As a matter of fact, not are only fluorides more expensive than chlorides, but they also cause health and environmental concerns due to dust and fumes as well as toxic and polluting compounds [7]. Most of them are classified according to EC Regulation No. 1272/2008 [8] as harmful to the environment, toxic, or both. Their amount in the salt flux should be minimized for environmental and health reasons; however, their presence is essential since fluoride-free fluxes do not yield the same efficiency for metal recovery [9].
1.3. Oxidizing Agents
Oxidizing agents promote exothermic chemical reactions, which assist the release of entrapped aluminum from the dross back into the molten metal bath. The mechanism is the improvement of fluidity due to the heat released by exothermic reactions [4]. Moreover, when added with fluorides, the heat released facilitates the chemical reactions between fluorides and inclusions [10]. Examples of oxidizing agents include nitrates such as KNO3 and NaNO3, carbonates such as CaCO3 and K2CO3 and sulfates such as K2SO4 and Na2SO4 [6].
1.4. Solvents of Aluminum Oxide
Solvents of Al2O3 can also be included in the fluxes. Cryolite is extensively used for the electrolytic conversion of Al2O3 to commercially pure aluminum metal in the Hall–Heroult process because alumina shows good solubility in cryolite melts. However, aluminum oxide has slight solubility in chloride melts with fluoride additions [11]. Some studies [12][13][14] suggested that the alumina solubility in some fluorides plays a key role in the mechanism of stripping the oxide layer, facilitating its dissolution, and improving the wettability [4]. The solvents of aluminum oxide are cryolite (Na3AlF6), borax (Na2B4O7), and potassium borate (K2B4O7) [6]. The chemical compounds used in salt fluxes are presented in Table 1.
Table 1. Chemical compounds used in salt fluxes and grouped according to the chemical type. The melting point ™ and hazardous classification are also reported for industrial relevance. Data were collected and elaborated from [4][6][15][16][17].
| Compound Type | Molecular Formula | Tm (°C) | Hazardous Classification 1 | |
|---|---|---|---|---|
| Chlorides | NaCl | 801 | × | |
| KCl | 770 | × | ||
| MgCl2 | 714 | × | ||
| CaCl2 | 782 | ✓ | ||
| LiCl | 605 | ✓ | ||
| BaCl2 | 962 | ✓ | ||
| AlCl3 | 190 | ✓ | ||
| Fluorides | Simple | KF | 858 | ✓ |
| NaF | 993 | ✓ | ||
| CaF2 | 1418 | × | ||
| LiF | 848 | ✓ | ||
| MgF2 | 1263 | ✓ | ||
| AlF3 | 1290 | × | ||
| BaF2 | 1368 | ✓ | ||
| Double | KalF4 | - | ✓ | |
| Ka3AlF6 | - | ✓ | ||
| Na2SiF6 | - | ✓ | ||
| K2SiF6 | - | ✓ | ||
| Oxidizing Compounds | Carbonates | CaCO3 | 1339 | × |
| K2CO3 | 894 | ✓ | ||
| Na2CO3 | 851 | ✓ | ||
| MgCO3 | 990 | × | ||
| Li2CO3 | 723 | ✓ | ||
| Nitrates | KNO3 | 339 | ✓ | |
| NaNO3 | 307 | ✓ | ||
| LiNO3 | 264 | ✓ | ||
| Sulphates | K2SO4 | 1069 | × | |
| Na2SO4 | 897 | × | ||
| Li2SO4 | 859 | ✓ | ||
| CaSO4 | 1450 | × | ||
| MgSO4 | 11,424 | × | ||
| Solvents of aluminum oxides | Na2B4O7 | 743 | ✓ | |
| K2B4O7 | - | ✓ | ||
| Na3AlF6 | 1010 | ✓ | ||
1 According to EC Regulation No. 1272/2008 [8].
2. Physical Properties and Reactivity of Solid Salt Fluxes
The chemical composition of salt fluxes determines their physical properties and reactivity. The key properties of fluxes include density, fluidity, melting temperature, wettability, and reactivity. These features are described in the next sections with a focus on how they are affected by the chemical composition of the fluxes.
2.1. Density
The density of the fluxes affects the slag-metal separation and the coalescence of metal droplets as described by Stokes’ law shown in Equation (2). The higher the density difference between metal and slag, the faster the droplets settle through the slag and back into the aluminum melt. The density of aluminum decreases linearly with temperature, and at 800 °C, it ranges between 2.33 and 2.39 g/cm3 [18][19]. At 800 °C, the density of an equimolar NaCl-KCl mixture is approximately 1.53 g/cm3, whereas, if the content of NaCl increases in the mixture up to 90 mol %, the density increases up to 1.55 g/cm3 [20]. Roy et al. [21] investigated the effect of some common fluoride (NaF, LiF, KF, Na3AlF6) additions up to 30 mol % on the density of an equimolar NaCl-KCl mixture at 740 °C. The results showed an increase of the density up to 1.63 g/cm3. Because the density difference between the molten aluminum and the salt mixtures is about 0.7 g/cm3, this is enough to ensure sufficient separation between the two phases [22]. Proper salt-slag separation ensures that the salt floats over the melt without joining it to avoid adhesion of salt granules or lumps which would result in a deleterious effect on the final product and to facilitate the removal of the slag at the end of the melting process.
Nonetheless, the effect of impurities and inclusions in the slag on its density cannot be neglected, as in the case of the effect on viscosity.
2.2. Viscosity
The viscosity of fluxes should also be low to assist the settling of metal droplets through the slag layer according to Stokes’ law. Few studies are available regarding the viscosity of molten NaCl-KCl with fluoride additions. Roy et al. [21] found how, after a critical amount of fluoride, the addition of LiF, NaF, and CaF2 increases the kinematic viscosity of an equimolar NaCl-KCl flux. Before reaching this critical amount, the viscosity showed a decreasing trend. Exceptions to these findings include the addition of Na3AlF6 up to 2 mol %, which results in a decrease in viscosity, and KF, which leads to a maximum value of viscosity for 5 mol % addition before declining. Tenorio et al. [23] found how the viscosity of an equimolar mixture decreases with NaF and KF additions, in contrast with the previous study. Milke et al. [24] investigated the solubility of CaF2 in NaCl-KCl at 750 °C and concluded how it decreases drastically, thus increasing the viscosity of the flux when the CaF2 concentration exceeds the solubility limit in the chlorides.
Nevertheless, the viscosity of the salt is only representative of the beginning of the process at the industrial scale. When the salt starts to collect oxides and inclusions, its viscosity increases significantly [22][25]. Xiao et al. [26] found that the presence of non-metallic particles in the slag impacts viscosity more than fluorides, especially when the volume of non-metallic particles in the slag exceeds 10 vol %. The impact of oxides in the flux during the remelting of aluminum chips with a lab-scale rotary furnace was studied by Thoraval and Friedrich [25]. The findings showed a significant decrease in the metal recovery, which was attributed to increased slag viscosity and density resulting from oxides, thus hindering the coalescence and metal-slag separation.
2.3. Melting Point
The melting point of the salt is another significant parameter, as the flux should be molten at the processing temperature. Preferably, the melting point of the salt should be close to the melting point of aluminum. If the melting point of the salt is too low, it may result in evaporation of the salt, whereas if it is too high, it may lead to excessive metal oxidation. Furthermore, an excessively high melting temperature leads to increased costs related to higher energy requirements to melt the salt. As previously noted, adding more NaCl to NaCl-KCl mixtures results in a higher melting temperature. However, certain operators, particularly in Europe, choose to prioritize cost savings by using more NaCl, which is less expensive than KCl. Flux mixtures based on KCl and MgCl2 allow obtaining even lower melting points, as the binary system presents eutectic points that melt at temperatures below 500 °C. The KCl-MgCl2 binary phase diagram is shown in Figure 1.
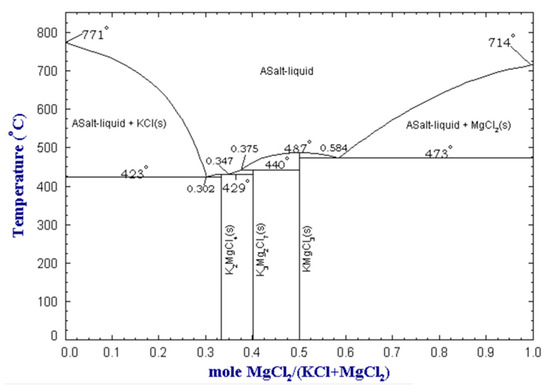
Figure 1. MgCl2-KCl binary phase diagram at atmospheric pressure, obtained from the FactSage™ FTsalt database [27].
Although the binary systems of NaCl-KCl and KCl-MgCl2 have been extensively studied in the literature, to the author’s best knowledge, there is a lack of experimental evidence of the effect of the fluorides’ addition on the melting point of salt mixtures. Although generally, fluorides show melting points above 1000 °C, some of them show the presence of eutectic points melting below 750 °C when combined with NaCl, as in the case of NaF [27], CaF2 [24], and Na3AlF6 [28]. Even though binary systems of NaCl and fluorides may contribute to the understanding of the melting behavior, they are not representative of common flux mixtures because they are usually constituted by several compounds.
2.4. Wetting Properties
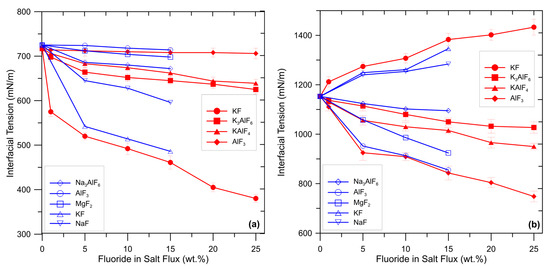
The wetting characteristics of a flux determine its ability to envelop inclusions and transfer them into the slag. It is widely recognized that the ability of flux to strip away and suspend oxides is largely influenced by the degree of wetting between (i) the liquid salt and aluminum melt and (ii) solid oxide and flux. A low value of interfacial tension between flux and oxide assists in the removal of inclusion by wetting. The interfacial tension between flux and aluminum melt should be sufficiently low to allow the spreading of the flux on the melt surface but not excessively low to avoid incomplete separation [29]. As previously explained, fluorides adjust interfacial tensions by promoting chemical reactions which produce surface-active elements (such as Na or K). Enrichment of these surface-active elements at the interface flux/aluminum is responsible for changes in the interfacial tensions. Wan et al. [29] and Shi et al. [5] investigated the effect of several fluoride additions to a commercial salt flux on the interfacial tensions between aluminum melt, oxides, and flux. The results are summarized in Figure 2.
2.5. Reactivity
The reactivity of a flux determines the ability to affect the chemical reactions, which lead to the removal of impurities from the aluminum melt. Simulation studies [30][31] based on thermodynamic calculations investigated the possibility of removing dissolved chemical impurities by considering the relevant parameters of the remelting process. The studies have shown how the salt fluxes containing AlCl3 affect the equilibrium constant of elements such as Mg, Ca, Be, Zn, Hg, Cd, Li, and Sr, allowing their removal either by chlorination into the salt flux, oxidation into the slag phase or by evaporation. However, low efficiency in the removal of such impurities and the impossibility of reducing other harmful impurities such as Cu, Si, Fe, and Mn by fluxing was revealed.
In the past, the injection of chlorine gas, i.e., chlorination, was a common strategy to remove alkali and alkali-earth metals. The tramp elements react with Cl2 forming more stable chlorides than aluminum chloride, assisted by the bubbling of the gas. Elements such as Mg, Na, Ca, Li, and K react with fluxes and form compounds which will either settle or float into the slag [32]. The toxicity of chlorine has led to its replacement with other methods, such as the use of fluxes. Nowadays, the most popular technology for the removal of inclusions and alkali from molten aluminum is through the addition of chlorine or fluorine-containing compounds to the salt flux, namely MgCl2 and AlF3, and AlCl3 [3][33]. Replacement of chlorination with solid fluxes can reduce environmental concerns without excessively compromising the efficiency of impurities’ removal; however, to replace the mixing due to bubbles, mechanical agitation is necessary [34]. Even though the use of MgCl2 is effective in the removal of alkali, it is not easily handled due to its high reactivity and tendency to absorb moisture. Thus, it is usually pre-melted in mixtures such as NaCl-KCl to obtain easier handling.
Impurities such as Zn, Si, Fe, Mn, and Cu are extremely difficult to remove from molten aluminum using fluxing [4][31]. Nonetheless, some experimental studies [35][36][37] have shown the possibility of reducing the Fe content in aluminum alloys by treating the melt with a flux mixture containing Na2B4O7. The methodology has shown promising efficiency at the lab scale, even when compared to other strategies, such as filtration and centrifugal separation. Furthermore, the addition of Na2B4O7 not only reduces the Fe content but also shapes Fe intermetallic compounds into less detrimental morphologies and promotes the formation of compounds such as AlB2, which act as grain refiners.
Particular attention should be dedicated to the treatment of Al-Mg alloys, especially for Mg content above 3 wt %, with fluxes containing Na, Ca, or K, as some chemical reactions may lead to the contamination of Al with Na or K. This is due to the exchange reactions of Mg with the impurities which preferentially stabilize Na, Ca, and K in the molten aluminum rather than in the flux [3][4]. Huang et al. [2], by treating an Al-Mg alloy with a NaCl-KCl flux with NaF and Na3AlF6 additions, concluded that the extent of Na contamination increases due to the increasing content of both Na in the flux and Mg in the Al alloy.
References
- Pirker, A.; Antrekowitsch, H.; Fragner, W.; Suppan, H.; Kettner, M. Optimization of the Al-recycling Process for Low Grade Scraps. BHM Berg Hüttenmänn. Mon. 2015, 160, 320–327.
- Huang, C.; Liu, Z.; Huang, J.; Liu, Q.; Li, J. Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al-Mg Alloys. Metals 2021, 11, 1591.
- Tremblay, S.; Desrosiers, L.; Levesque, D. Use of a Tertiary Salt Flux of NaCl, KCl, and MgCl2 for the Purification of Aluminium or Aluminium Alloys, and Method Thereof. U.S. Patent US 2012/0017726, 26 January 2012.
- Utigard, T.A.; Friesen, K.; Roy, R.R.; Lim, J.; Silny, A.; Dupuis, C. The properties and uses of fluxes in molten aluminum processing. JOM 1998, 50, 38–43.
- Shi, M.; Li, Y. Performance Improvement in Aluminum Alloy Treated by Salt Flux with Different Fluorides. J. Mater. Eng. Perform. 2022, 32, 3065–3072.
- Gallo, R. “I Have Inclusions! Get Me the Cheapest and Best Flux for Cleaning My Melt!”—Is This the Best Driven, Cost-Saving Approach by a Foundry? In Proceedings of the 121st Metalcasting Congress of the American Foundry Society, Milwauee, WI, USA, 25–27 April 2017.
- Cusano, G.; Gonzalo, M.R.; Farrell, F.; Remus, R.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Non-Ferrous Metals Industries, EUR 28648. 2017. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2020-01/JRC107041_NFM_bref2017.pdf (accessed on 27 February 2023).
- European Parliament and Council of European Union. Regulation (EC) 1272/200/on Classification, Labelling and Packaging of Substances and Mixtures; European Parliament and Council of European Union: Brussels, Belgium, 2008.
- Gallo, R. Development, Evaluation and Application of Granular and Powder Fluxes in Transfer Ladles, Crucible, and Reverberatory Furnace. Foundry Pract. 2002, 237, 8–16.
- Gyarmati, G.; Fegyverneki, G.; Molnar, D.; Tokár, M. The Melt Cleaning Efficiency of Different Fluxes and Their Effect on the Eutectic Modification Level of AlSi7MgCu Alloy. Livar. Vestn. 2019, 66, 70–87.
- Cherginets, V.L.; Baumer, V.N.; Galkin, S.S.; Glushkova, L.V.; Rebrova, T.P.; Shtitelman, Z.V. Solubility of Al2O3 in Some Chloride-Fluoride Melts. Inorg. Chem. 2006, 45, 7367–7371.
- Peterson, R.D. Effect of Salt Flux Additives on Aluminum Droplets Coalescence. In Second International Symposium—Recycling of Metals and Engineered Materials—TMS; TMS: Madison, AL, USA, 1990.
- Xiao, Y.; Tang, K. Solubility of alumina in molten chloride-fluoride melts. In Proceedings of the VIII International Conference on Molten Slags, Fluxes and Salts, Santiago, Chile, 18–21 January 2009.
- Tenorio, J.A.S.; Espinosa, D.C.R. Effect of salt/oxide interaction on the process of aluminum recycling. J. Light Met. 2002, 2, 89–93.
- Merck KGaA, Sigma Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 27 February 2023).
- National Library of Medicine, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 27 February 2023).
- Fisher Scientific, Fishersci. Available online: https://www.fishersci.com/us/en/home.html (accessed on 27 February 2023).
- Assael, M.J.; Kakosimos, K.; Banish, R.M.; Brillo, J.; Egry, I.; Brooks, R.; Quested, P.N.; Mills, K.C.; Nagashima, A.; Sato, Y.; et al. Reference Data for the Density and Viscosity of Liquid Aluminum and Liquid Iron. J. Phys. Chem. Ref. Data 2006, 35, 285–300.
- Assael, M.J.; Mihailidou, E.K. Reference Correlation for the Density and Viscosity of Eutectic Liquid Alloys Al+Si, Pb+Bi, and Pb+Sn. J. Phys. Chem. Ref. Data 2012, 41, 3.
- Janz, G.; Tomkins, R.P.T.; Allen, C.B.; Downey, J.R.; Garner, G.L.; Krebs, U.; Singer, S.K. Molten Salts: Volume 4, Part 2, Chlorides and Mixtures. J. Phys. Chem. Ref. Data 1975, 4, 871–1178.
- Roy, R.R.; Ye, J.; Sahai, Y. Viscosity and density of molten salts based on equimolar NaCl-KCl. Mater. Trans. JIM 1997, 38, 6–566.
- Schmitz, C. Handbook of Aluminium Recycling, 2nd ed.; Vulkan-Verlag GmbH: Essen, Germany, 2014.
- Tenorio, J.A.S.; Carboni, M.C.; Espinosa, D.C.R. Recycling of aluminum—Effect of fluoride additions on the salt viscosity and on the alumina dissolution. J. Light Met. 2001, 1, 195–198.
- Milke, E.; Friedrich, B.; Sydykov, A.; Arnold, A. Solubility of CaF2 in NaCl-KCl salt flux for Al-recycling and its effect on Al-loss. In Proceedings of the European Metallurgical Conference, Dusseldorf, Germany, 18–21 September 2005.
- Thoraval, M.; Friedrich, B. Metal entrapment in slag during the aluminium recycling process in tilting rotary furnace. In Proceedings of the European Metallurgical Conference, Dusseldorf, Germany, 3–5 June 2015.
- Xiao, Y.; Reuter, M.A.; Boin, U. Aluminium Recycling and Environmental Issues of Salt Slag Treatment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 40, 1861–1875.
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228.
- Holm, J.L. Phase investigations of the system Na3AlF6—NaCl. Thermochim. Acta 1988, 133, 283–286.
- Wan, B.; Li, W.; Liu, F.; Lu, T.; Jin, S.; Wang, K.; Yi, A.; Tian, J.; Chen, W. Determination of fluoride component in the multifunctional refining flux used for recycling aluminum scrap. J. Mater. Res. Technol. 2020, 9, 3447–3459.
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic Analysis of Contamination by Alloying Elements in Aluminum Recycling. Environ. Sci. Technol. 2010, 44, 5594–5600.
- Hiraki, T.; Miki, T.; Nakajima, K.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic Analysis for the Refining Ability of Salt Flux for Aluminum Recycling. Materials 2014, 7, 5543–5553.
- Zhang, L.; Lv, X.; Torgerson, A.T.; Long, M. Removal of Impurity Elements from Molten Aluminum: A Review. Miner. Process. Extr. Metall. Rev. 2011, 32, 150–228.
- Velasco, E.; Nino, J. Recycling of aluminium scrap for secondary Al-Si alloys. Waste Manag. Res. 2011, 29, 686–693.
- Bujalski, W.; Kimata, M.; Nayan, N.; Song, J.L.; Jolly, M.R.; Nienow, A.W. Mixing Studies Related to the Cleaning of Molten Aluminium. Chem. Eng. Technol. 2004, 27, 310–314.
- Gao, J.W.; Shu, D.; Wang, J.; Sun, B.D. Effects of Na2B4O7 on the elimination of iron from aluminum melt. Scr. Mater. 2007, 57, 197–200.
- Liu, X.; Liu, T.; Liu, Z.; Xie, H.; Liu, G.; Wu, Y.; Li, J. Effects of B-containing composite flux on the microstructures and mechanical properties of ADC 12 alloys. Mater. Res. Express 2019, 6, 096576.
- Rathinasuriyan, C.; Bharath, A.; Sridhar, K. Reducing iron content from aluminium molten bath through filter bag, centrifugal separation and flux refining method. Mater. Today Proc. 2002, 62, 1026–1032.
More
Information
Subjects:
Engineering, Industrial; Chemistry, Inorganic & Nuclear; Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Revisions:
2 times
(View History)
Update Date:
05 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




