Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rahim Hirani | -- | 3666 | 2023-05-04 17:48:28 | | | |
| 2 | Peter Tang | + 1 word(s) | 3667 | 2023-05-05 10:38:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rubin, S.C.; Abdulkadir, M.; Lewis, J.; Harutyunyan, A.; Hirani, R.; Grimes, C.L. Endometrial Receptivity Array. Encyclopedia. Available online: https://encyclopedia.pub/entry/43789 (accessed on 08 February 2026).
Rubin SC, Abdulkadir M, Lewis J, Harutyunyan A, Hirani R, Grimes CL. Endometrial Receptivity Array. Encyclopedia. Available at: https://encyclopedia.pub/entry/43789. Accessed February 08, 2026.
Rubin, Sarah C., Mawerdi Abdulkadir, Joshua Lewis, Aleksandr Harutyunyan, Rahim Hirani, Cara L. Grimes. "Endometrial Receptivity Array" Encyclopedia, https://encyclopedia.pub/entry/43789 (accessed February 08, 2026).
Rubin, S.C., Abdulkadir, M., Lewis, J., Harutyunyan, A., Hirani, R., & Grimes, C.L. (2023, May 04). Endometrial Receptivity Array. In Encyclopedia. https://encyclopedia.pub/entry/43789
Rubin, Sarah C., et al. "Endometrial Receptivity Array." Encyclopedia. Web. 04 May, 2023.
Copy Citation
Successful outcomes of in vitro fertilization (IVF) rely on both the formation of a chromosomally normal embryo and its implantation in a receptive endometrium. Pre-implantation genetic testing for aneuploidy (PGT-A) has been widely accepted as a tool to assess the viability of an embryo. In 2011, the endometrial receptivity array (ERA) was first published as a tool to determine when the endometrium is most receptive to an embryo, commonly referred to as the “window of implantation” (WOI). The ERA uses molecular arrays to assess proliferation and differentiation in the endometrium and screens for inflammatory markers. Unlike PGT-A, there has been dissent within the field concerning the efficacy of the ERA.
endometrial receptivity array
in vitro fertilization
pre-implantation testing
frozen embryo transfer
hormone replacement therapy
implantation failure
1. Introduction
In vitro fertilization relies on the success of a multi-fold process which includes both the creation of a normal chromosomal embryo and the implantation of the embryo into a receptive endometrium [1][2]. Owing to the development of precision medicine and technology in the field of reproductive endocrinology, there are tools that can be used to aid in both of these processes. As for the creation of a chromosomally normal embryo, pre-implantation genetic testing for aneuploidy (PGT-A) has been accepted within practice guidelines as the definitive tool to be used to test for whether an embryo is euploid or aneuploid [3]. While PGT-A can be used in all patients, it has been shown to offer a benefit particularly for patients experiencing recurrent implantation failure (RIF) and severe male infertility [4][5]. For the implantation of an embryo into a receptive endometrium, the ERA is a tool that was developed to determine when the endometrium is most receptive to an embryo [6].
Endometrial receptivity has been defined in the literature as the period of endometrial maturation during which the trophectoderm of the blastocyst can attach to the endometrial epithelial cells and subsequently invade the endometrial stroma and vasculature [7]. Endometrial maturation is in part accomplished by exposure to steroid hormones such as estrogen in the follicular phase, and progesterone in the luteal phase [8]. In the follicular phase, estrogen signals induce the proliferation of the endometrial lining, consequently causing an increase in progesterone receptor expression [9]. Following ovulation, progesterone further changes the endometrium to create an environment receptive to implantation and ultimately maintain an early pregnancy [8].
In addition to hormones, many molecular pathways including adhesion molecules, cytokines, and growth factors work synchronously to create a window of implantation (WOI) [10]. This WOI is a short period of optimal endometrial receptivity and typically occurs between days 20 and 24 of a normal 28-day menstrual cycle.
2. A Description of Endometrial Receptivity Array
While the ERA is a novel technique, the concept of endometrial receptivity is not an entirely new idea. In 1975, Noyes et al. identified morphological changes in the endometrium throughout the menstrual cycle. This laid the groundwork for the development of further tools investigating endometrial receptivity [11]. There are currently three tests for endometrial receptivity that are commercially available: 1. the endometrial function test (EFT) which consists of both a histological assessment and endometrial development, 2. the BCL6 test which evaluates inflammatory markers for endometriosis, and 3. the ERA which “dates” the endometrium [6]. While the EFT has promising clinical results, due to its time- and labor-intensive nature and the expertise necessary for assessment using it, this tool is recommended for specific cases in which further embryo creation is not possible, or in which a small number of embryos are being transferred [6][12][13]. The BCL6 test has similarly shown promising outcomes as well; however, it is specific to endometriosis, and therefore is not generalizable to all patients experiencing infertility [14][15][16]. Due to the limited context in which the EFT and BCL6 test can be applied, the remainder of this research will be dedicated to examining the ERA in detail, as the primary focus of investigation. This emphasis solely on the ERA is necessary as there is dissent in the field toward the efficacy of the ERA and when it is used—unlike the views on other endometrial receptivity tests previously mentioned. Thus, by highlighting the function of the ERA and its application in various patient populations, the researchers aim to elucidate when this tool may be best applied.
The endometrial receptivity array (ERA) was first developed in 2011 by Díaz-Gimeno et al. in an effort to define the transcriptomic signature of human endometrial receptivity [6][17]. The research fueling the development of this tool was in large part enabled by the completion of the Human Genome Project in 2001 [18][19]. The Human Genome Project laid the groundwork for microarray-based gene expression technology and transcriptomic analysis. This technology, when applied to the endometrium, has allowed further research investigating transcriptomic expression changes throughout the menstrual cycle and within the context of different gynecologic conditions [20][21]. Many studies have shown that during the menstrual cycle, and more specifically the WOI, the endometrium has a specific transcriptomic profile [21]. This transcriptomic profile is thought to be consistent with a “receptive phenotype”, meaning that the differential expression of genes during the WOI may create an endometrial environment more conducive to successful implantation [19].
In the development of the ERA, Díaz-Gimeno et al. selected genes involved in human endometrial receptivity as the basis of this tool’s analysis. To do so, they analyzed the entire human genome, highlighting the different gene expression profiles in a receptive endometrium and a pre-receptive endometrium. This was carried out by utilizing expression data for these genes collected during the group’s previous work. The genes that were shown to have an absolute >3-fold change and a false discovery rate of <0.05 in a receptive endometrium were selected. In total, 238 genes were identified as differentially expressed at the receptive phase and pre-receptive phase. Using these selected genes, they built an Agilent customized gene expression microarray (Madrid, Spain) known as the ERA. To characterize the nature of these genes, they performed a functional analysis to determine if the selected genes were over-represented in biological functions, including processes relating to the immune system, circulation, or the response to external stimulus. Some of the over-represented terms included oxidoreductase activity, receptor binding, and carbohydrate binding. Consequently, clustering sample analysis was performed, grouping samples as either “receptive” or “nonreceptive.” This clustering was carried out to detect genes with similar behavior to those in other phases of the menstrual cycle. For the endometrial transcriptomic signature, a comparison between the differential expression of the genes that were receptive and pre-receptive were compared and the genes that were receptive and proliferative were compared. The intersection of these comparisons generated a list of 134 statistically differentially expressed genes in the receptive group compared to the expression of both proliferative and pre-receptive genes [17]. To prove the translational efficiency of the ERA, the group then designed a bioinformatic test with predictive power to define the endometrium gene expression profile compatible with LH +7. The predictor showed a specificity of 0.8857 and sensitivity of 0.99758 for endometrial dating, thus demonstrating the efficacy of this tool at objectively diagnosing the endometrial receptivity status. For pathological classification, there was a specificity of 0.1571 and a sensitivity of 0.995, attributed by the authors to the difficulty in obtaining samples; nonetheless, it should be noted that none of the normal samples were labeled as pathological. While the ERA may not be a tool used primarily for pathological classification, the data makes evident that the ERA demonstrates specificity and sensitivity as a tool for endometrial receptivity.
While there are many complex mechanisms at play in defining the transcriptomic signature of endometrial receptivity, all that is required of the provider and patient from a clinical standpoint is an endometrial biopsy. The nuances of the timing of the biopsy will be described in later sections. Once the biopsy is obtained, it is sent to the laboratory for processing; the ERA begins by dissolving the biopsy specimen for molecular analysis using the differential expression of 238 genes, as described above. In dissolving the biopsy, there is no histological assessment of the patient’s endometrium; however, it is possible to perform a concurrent endometrial biopsy for a pathological review separate from the ERA. Within the scope of the ERA performed alone, RNA is extracted from the biopsy, and processed using the microarray, as described previously. The array is coupled to a computational predictor that is able to identify the receptivity status of an endometrial sample and diagnose the personalized WOI (pWOI) of a given patient.
In 2013, Díaz-Gimeno et al. further compared the accuracy and reproducibility of the ERA to standard histological methods. They found that the ERA is more accurate than histological dating, is completely reproducible, and can determine the pWOI regardless of the sample’s histologic appearance [17][22]. While studies show that WOI length, approximately two days, is largely constant amongst women, it is displaced in 20% of the population and in ¼ of women with RIF [23]. Thus, the ERA allows the identification of a personalized WOI which can then be applied to a patient’s FET plan, so that the medication and transfer timing are determined by when the endometrium is most receptive [17]. An ERA is flexible in this sense as it may be used in both hormone replacement therapy frozen embryo transfer (HRT-FET) and natural FET (nFET). In each case, minor adjustments must be made for it to be compatible with each cycle [23]. The specifics distinguishing these two cycles will be discussed further in later sections. Through the development of the ERA, it is evident that endometrial receptivity can be identified on a transcriptomic level. It is with this analysis that we can better identify each patient’s personalized WOI and harness this knowledge to optimize IVF outcomes.
3. ERA in the Context of Natural Frozen Embryo Transfer
Prior to discussing the application of the ERA in FETs, it is important to consider the development of FET and the different FET protocols available. With improvements in embryo cryopreservation technology and the rise in single embryo transfers, the rate of FETs has risen dramatically compared to that of fresh embryo transfer. The increase in single embryo transfers means that there is a higher number of quality embryos available for cryopreservation and potential future use [24]. This is in part an effort- and cost-saving measure in the case of a fresh embryo transfer failing, allowing a backup supply of frozen embryos to be available. These improvements have allowed FET success rates to rival those of fresh embryo transfer, establishing FET as an essential component of IVF treatment [25].
Paralleling the rise in FET is a growing body of evidence suggesting that pregnancy rates, as well as neonatal and perinatal outcomes, are improved when using FET rather than fresh embryo transfer [26][27]. FET has been shown to have lower rates of preterm birth and low birth weight compared to fresh cycles [28]. Additionally, there is no evidence of an increase in stillbirths or congenital malformations when using FET [25][28]. The worse outcomes associated with fresh embryo transfer are thought to be due in part to supraphysiologic estrogen and progesterone levels following the use of hCG to stimulate ovulation [29]. The rapid elevation of E2 and progesterone causes a rapid transition from a proliferative to secretory endometrium, thus “expediting” the endometrial preparedness following egg retrieval. In a fresh cycle, where embryo transfer is performed in the same cycle as egg retrieval is, this expedited progression of the endometrial lining will cause the WOI to occur earlier in the cycle; thus, the development of the embryo and endometrial receptivity will be out of sync.
Endometrial receptivity is not an all-or-none phenomenon, but implantation outside of the optimal window may lead to an increased incidence of placental abnormalities, influencing the outcomes of the pregnancy [30]. By using FET, the embryos are frozen for use in subsequent cycles, thus avoiding supraphysiologic hormone levels and an asynchronous implantation window, leading to better outcomes than those seen in fresh transfers. Furthermore, using FET whether it be HRT-FET or nFET allows us to maintain a higher degree of control over the uterine lining as we are able to plan and prepare from the beginning of the cycle [31]. While this may include the administration of medications in some cases, the fine-tuning this provides in preparing the endometrium makes the use of the ERA possible. An ERA is only able to be used in the context of a FET, as will be further explicated in the following sections.
HRT-FET and nFET are the two main available protocols for FET. nFET relies on the body’s endogenous hormones to drive the menstrual cycle, followed by oocyte collection, fertilization, and FET. nFET may be considered for women who have regular menstrual cycles as it involves fewer medications and therefore has a lower cost [32]. nFET can be further divided into two subtypes: modified-nFET and true-nFET.
True nFET typically begins with transvaginal ultrasonography (TVUS) on days 2–3 of menses. This is carried out to rule out the presence of any cysts or a persistent corpus luteum from a previous cycle, either of which may interfere with the current cycle [33]. Between cycle days 7–10, TVUS monitoring continues to measure the size of the leading follicle. Blood work is performed to monitor when the LH surge begins and for ovulation shortly after [34]. Embryo transfer is scheduled for 6 days post-LH surge (LH +6). After the embryo has been transferred, luteal phase support (LPS) in the form of exogenous progesterone may be given for 8–10 weeks to support the endometrium. This is carried out to provide progesterone in place of the corpus luteum until the placenta can begin producing adequate amounts on its own, but is not always necessary [35].
Modified nFET follows the same general protocol, however, once the leading follicle has reached a diameter of 16–20 mm, hCG is given to induce ovulation. In a modified nFET cycle, embryo transfer is typically scheduled 7 days post-hCG administration (hCG +7) [33]. The benefit of the modified nFET cycle is that by artificially inducing ovulation, there is less difficulty in detecting it than there is in true nFET. This is beneficial to both the patient and provider, as it often requires less monitoring, has a decreased likelihood of missing ovulation causing a need to cancel the cycle, and allows a certain degree of planning the timing of embryo transfer. Additionally, the exogenous hCG will induce progesterone production to support the endometrium acting as its own form of LPS. The hCG trigger carries the risk of causing an inappropriately elevated progesterone level and as a result, an asynchronous WOI. To minimize this, the lowest necessary dose of hCG should be given [33].
The ERA can be used in a modified nFET cycle in an attempt to better guide the timing of embryo transfer to best match the WOI. The ERA cannot be used with true nFET as even slight cycle–cycle variations in the detection or time of ovulation can be enough to render an ERA report inaccurate. Administration of hCG is necessary in order to time embryo transfer to when the endometrium is most receptive. In an ERA, an endometrial biopsy is performed in a separate cycle, prior to embryo transfer [36] (Figure 1). The biopsy is taken at either LH +7 or hCG +7, which are considered to be equal under the ERA protocol, and sent to Igenomix (Miami, FL), the medical laboratory that produces, distributes, and analyzes the ERA [37]. A manual describing how to analyze the ERA report is included on the Igenomix website for provider use. The details highlighted in the manual are described as follows: the results of the ERA will come back as receptive or nonreceptive. If receptive, then the time in the cycle that the biopsy was performed represents the optimal WOI and embryo transfer should be performed at the same time (±3 h) in the subsequent cycle. Nonreceptive results are given as pre-receptive or post-receptive and may give a predicted WOI [38]. If the result is post-receptive, or if it is prereceptive by 2 or more days, a second biopsy and ERA may be needed in order to validate the predicted WOI [39]. The biopsy will need to be performed at LH +5 or LH +6 if the result is post-receptive or LH +9 if the result is pre-receptive [23]. The ERA has been shown to be highly consistent between menstrual cycles, so the optimal WOI measured in the previous cycles should remain stable in subsequent cycles. This consistency makes the ERA more reliable than histologic analysis [22].
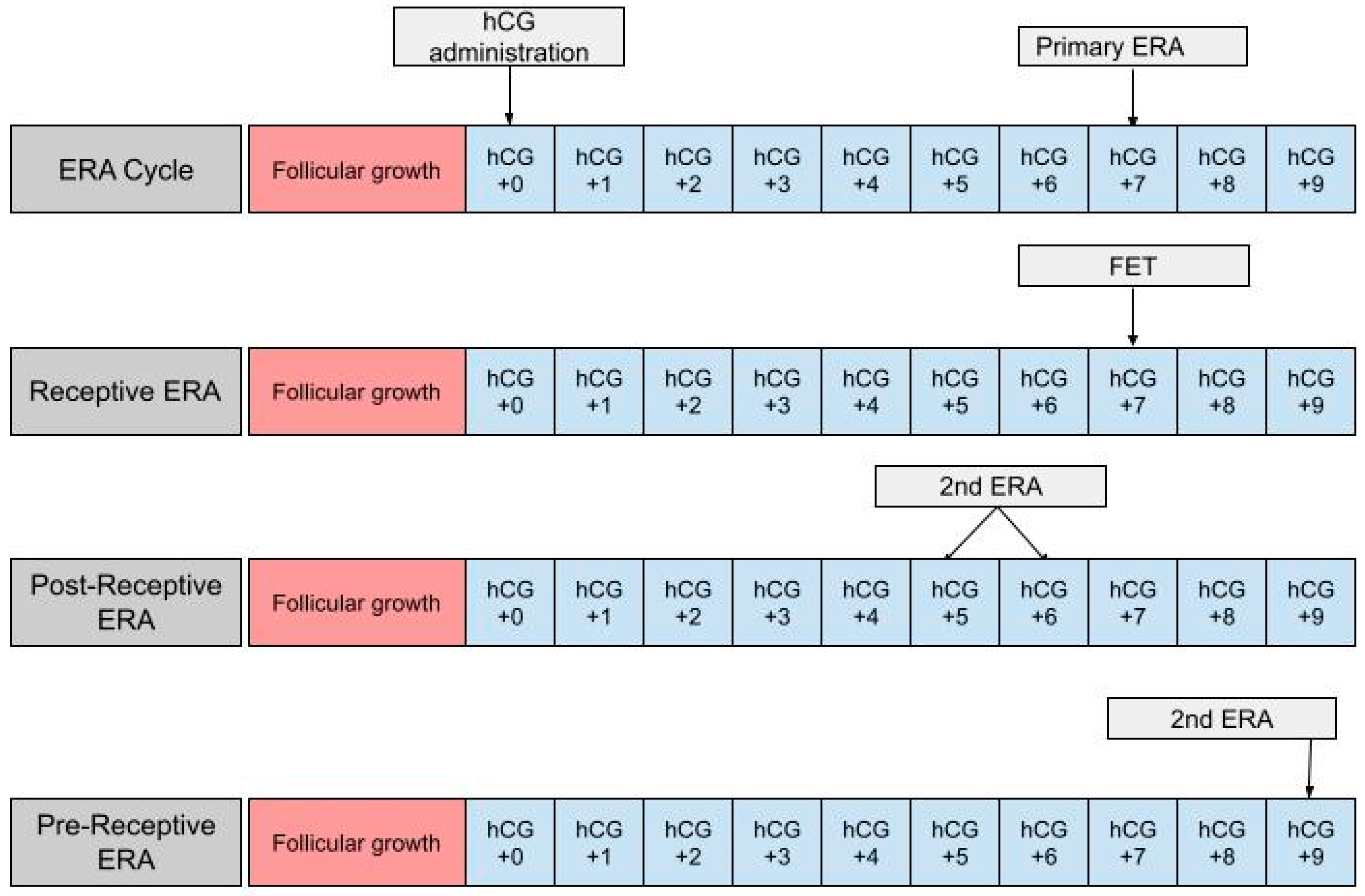
Figure 1. The first row shows the timing of the ERA biopsy in a modified natural cycle. hCG +0 is the day of the hCG trigger shot. The second row shows the timing of the frozen embryo transfer (FET) if the ERA yields a receptive result. The final two rows show the timing of the second ERA biopsy if the result of the initial ERA is post-receptive or pre-receptive, respectively.
4. ERA in the Context of Hormone Replacement Therapy Frozen Embryo Transfer
Hormone replacement therapy (HRT) is the other main protocol used in frozen embryo transfer (FET). In a HRT-FET cycle, exogenous estradiol (E2) and progesterone (P) are administered at set points to enhance one’s natural cycle, optimizing the endometrium for transfer [33]. The goal of HRT is to prepare the endometrium for FET, but there is some variation in the protocols used. The HRT cycle begins with the administration of E2 on day one, two or three. E2 participates in the proliferation of the endometrium and the suppression of spontaneous follicle growth. E2 can be administered as a fixed 6 mg daily dose or in increasing increments that start at 2 mg/day and increase up to 6 mg/day [40]. There is also variability in the routes of E2 administration, including oral, vaginal, and transdermal routes. The variations in dose and route of administration have been shown to produce comparable outcomes [41]. Starting on day 12, a transvaginal ultrasound (TVUS) is used to assess whether the endometrium has reached the desired thickness of >7 mm and to confirm the absence of a leading follicle [42][43][44]. In the event of the presence of a leading follicle, the cycle is canceled, and FET cannot be performed until the following cycle. E2 administration can last up to 36 days, following which progesterone supplementation is commenced [45][46].
In contrast to E2, there is some debate in the literature regarding the dosing and routes of administration of progesterone [47]. It can be administered through oral, intramuscular, or subcutaneous routes, or as a suppository. However, a vaginal suppository remains the most widely used option for the administration of progesterone. Progesterone initiates the secretory transformation of the endometrium, preparing it for implantation [48][49]. It should be continued until a luteo–placental shift occurs and is generally continued until the 10th to 12th week of gestation [50]. Typically, the endometrium first has the possibility of being receptive on the third day after the initiation of progesterone [51]. It is the duration of progesterone that can be modified and ultimately optimized when an ERA is incorporated into a HRT-FET cycle.
In using an ERA to guide the timing for an HRT-FET cycle, a patient will ultimately need two cycles, the first being an ERA cycle, and the second being an HRT-FET cycle [23][52][53]. In the ERA cycle, the patient uses the same medications they would in an HRT-FET cycle, mimicking the uterine conditions in an eventual transfer cycle (Figure 2). However, instead of embryo transfer occurring, an endometrial biopsy can be performed on day P +5 and sent for an ERA. The results of the ERA are used to personalize the date of the embryo transfer (and/or the initiation of progesterone) to the individual receptivity of each patient’s endometrium. Following the endometrial biopsy, the ERA report can return one of three results: a receptive, pre-receptive, or post-receptive result. If the ERA report concludes that the patient is receptive, then the endometrium is ready for embryo transfer and FET is performed on the day that the ERA is indicated as receptive, in the following cycle. If the result is nonreceptive, then gene profile analysis results reveal whether the endometrium is pre-receptive or post-receptive. In the next cycle, a second ERA is performed on day P +6 or P +7 if the report states that the patient was pre-receptive. Alternatively, the ERA is performed on P +3 or P +4 if the patient is considered post-receptive. If the patient is considered receptive following the second ERA, then FET is performed on the same cycle day that the ERA is reported to be receptive, in the following cycle [23]. While the above-mentioned process is the most accurate method with which to obtain the patient’s WOI, in clinical practice it is up to the discretion of the provider and patient whether or not to perform a second ERA at all, following the initial nonreceptive result. In some cases, embryo transfer us performed without confirming the receptiveness of the endometrium with a second ERA cycle.
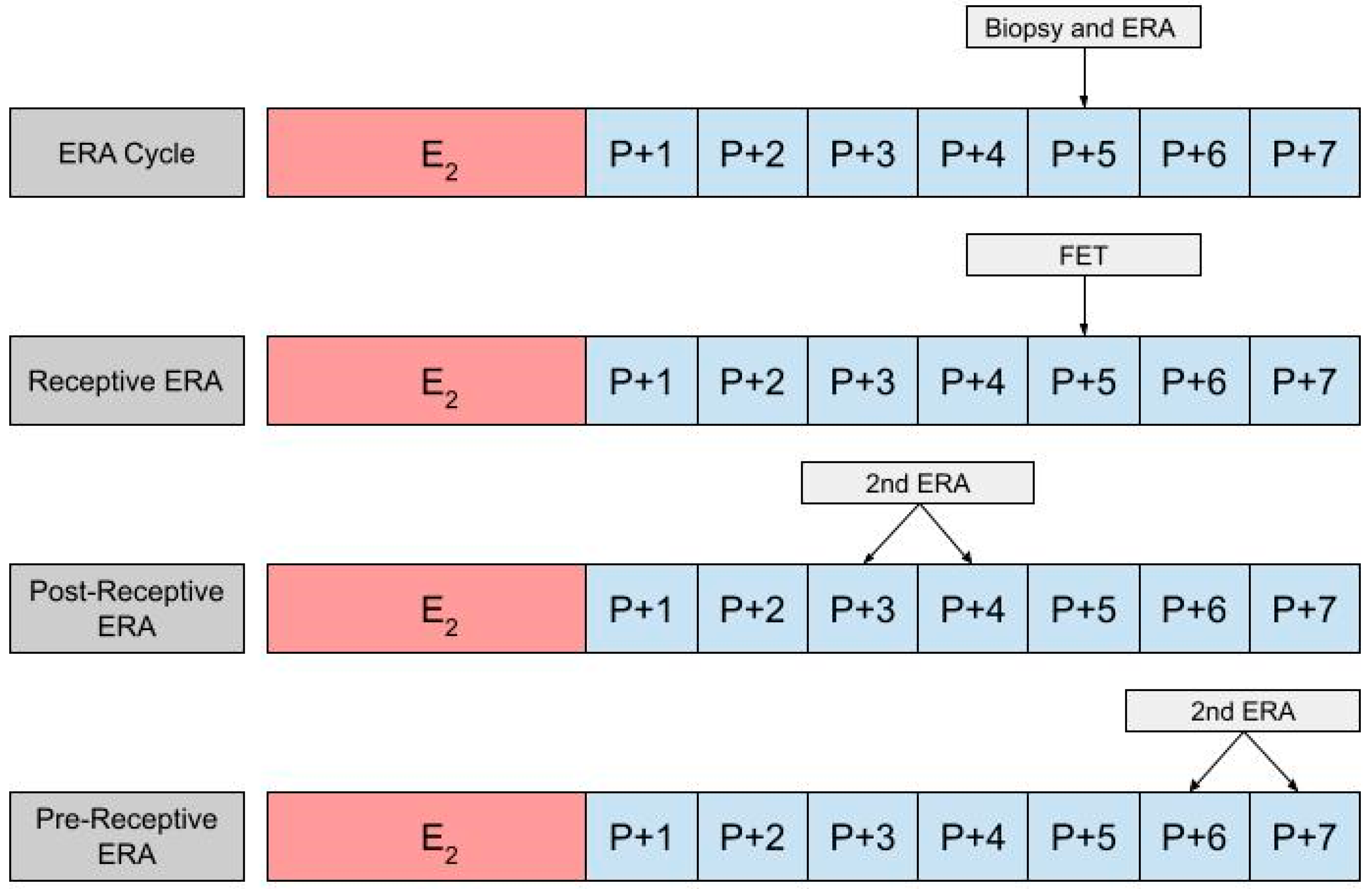
Figure 2. The first row shows the timing of the biopsy and the ERA in a cycle. The subsequent row shows the timing of the frozen embryo transfer (FET) if the ERA indicates a receptive endometrium. The final two rows show the timing of the second ERA if the result of the initial ERA is post-receptive or pre-receptive, respectively.
Igenomix (Miami, FL) is the medical testing laboratory that creates and distributes the endometrial receptivity array. The provider sends the endometrial biopsy collected from the patient to Igenomix. Igenomix analyzes the endometrial biopsy using the technique described in an earlier section and reports the receptivity status of the endometrium. As described in the prior section, providers can access the guideline for the ERA through the Igenomix website, Igenomix.com. The guideline outlines the rationale, indications, and methodology behind the ERA. In this guideline, providers can find an example of an ERA result report along with a guide on how to interpret the results, which has also been summarized in the previous paragraph. Additionally, the company has created a calculator for clinicians to effectively use the results generated in the ERA report; it aids in determining the timing of progesterone administration and embryo transfer to optimize the patient’s receptivity. The “personalized Embryo Transfer (pET) Calculator,” available exclusively on the Igenomix website, allows clinicians to select the cycle type (HRT vs. nFET) and the type of the embryo (blastocyst vs. day 3 embryo) [54].
References
- Scott, R.T., Jr.; Ferry, K.; Su, J.; Tao, X.; Scott, K.; Treff, N.R. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: A prospective, blinded, nonselection study. Fertil. Steril. 2012, 97, 870–875.
- Heger, A.; Sator, M.; Pietrowski, D. Endometrial Receptivity and its Predictive Value for IVF/ICSI-Outcome. Geburtshilfe Und Frauenheilkd. 2012, 72, 710–715.
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. The use of preimplantation genetic testing for aneuploidy (PGT-A): A committee opinion. Fertil. Steril. 2018, 109, 429–436.
- Simopoulou, M.; Sfakianoudis, K.; Maziotis, E.; Tsioulou, P.; Grigoriadis, S.; Rapani, A.; Giannelou, P.; Asimakopoulou, M.; Kokkali, G.; Pantou, A.; et al. PGT-A: Who and when? A systematic review and network meta-analysis of RCTs. J. Assist. Reprod. Genet. 2021, 38, 1939–1957.
- Twisk, M.; Mastenbroek, S.; van Wely, M.; Heineman, M.J.; Van Der Veen, F.; Repping, S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst. Rev. 2006, CD005291.
- Kliman, H.J.; Frankfurter, D. Clinical approach to recurrent implantation failure: Evidence-based evaluation of the endometrium. Fertil. Steril. 2019, 111, 618–628.
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746.
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617.
- Moustafa, S.; Young, S. Diagnostic and therapeutic options in recurrent implantation failure. F1000Research 2020, 9, 208.
- Dorostghoal, M.; Ghaffari, H.-O.; Moramezi, F.; Keikhah, N. Overexpression of Endometrial Estrogen Receptor-Alpha in The Window of Implantation in Women with Unexplained Infertility. Int. J. Fertil. Steril. 2018, 12, 37–42.
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 1975, 122, 262–263.
- Kliman, H.J.; McSweet, J.C.; Grunert, G.M.; Cardone, V.R.; Cadesky, K.; Keefe, D.L. The endometrial function test (EFT) directs care and predicts ART outcome. Fertil. Steril. 2002, 78, S17.
- Kliman, H.J.; Honig, S.; Walls, D.; Luna, M.; McSweet, J.C.; Copperman, A.B. Optimization of endometrial preparation results in a normal endometrial function test® (EFT®) and good reproductive outcome in donor ovum recipients. J. Assist. Reprod. Genet. 2006, 23, 299–303.
- Soriano, D.; Adler, I.; Bouaziz, J.; Zolti, M.; Eisenberg, V.H.; Goldenberg, M.; Seidman, D.S.; Elizur, S.E. Fertility outcome of laparoscopic treatment in patients with severe endometriosis and repeated in vitro fertilization failures. Fertil. Steril. 2016, 106, 1264–1269.
- Littman, E.; Giudice, L.; Lathi, R.; Berker, B.; Milki, A.; Nezhat, C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil. Steril. 2005, 84, 1574–1578.
- Macer, M.L.; Taylor, H.S. Endometriosis and Infertility: A Review of the Pathogenesis and Treatment of Endometriosis-associated Infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549.
- Díaz-Gimeno, P.; Horcajadas, J.A.; Martinez-Conejero, J.A.; Esteban, F.J.; Alama, P.; Pellicer, A.; Simon, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60.e15.
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The Sequence of the Human Genome. Science 2001, 291, 1304–1351.
- Gómez, E.; Ruíz-Alonso, M.; Miravet, J.; Simón, C. Human Endometrial Transcriptomics: Implications for Embryonic Implantation. Cold Spring Harb. Perspect. Med. 2015, 5, a022996.
- Altmäe, S.; Esteban, F.J.; Stavreus-Evers, A.; Simón, C.; Giudice, L.; Lessey, B.A.; Horcajadas, J.A.; Macklon, N.S.; D’Hooghe, T.; Campoy, C.; et al. Guidelines for the design, analysis and interpretation of ‘omics’ data: Focus on human endometrium. Hum. Reprod. Update 2014, 20, 12–28.
- Ruiz-Alonso, M.; Blesa, D.; Simón, C. The genomics of the human endometrium. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2012, 1822, 1931–1942.
- Díaz-Gimeno, P.; Ruiz-Alonso, M.; Blesa, D.; Bosch, N.; Martínez-Conejero, J.A.; Alamá, P.; Garrido, N.; Pellicer, A.; Simón, C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil. Steril. 2013, 99, 508–517.
- Garrido-Gómez, T.; Ruiz-Alonso, M.; Blesa, D.; Diaz-Gimeno, P.; Vilella, F.; Simón, C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 2013, 100, 818–824.
- Wong, K.M.; Mastenbroek, S.; Repping, S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil. Steril. 2014, 102, 19–26.
- Zhang, J.; Du, M.; Li, Z.; Wang, L.; Hu, J.; Zhao, B.; Feng, Y.; Chen, X.; Sun, L. Fresh versus frozen embryo transfer for full-term singleton birth: A retrospective cohort study. J. Ovarian Res. 2018, 11, 59.
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C.; Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertil. Steril. 2011, 96, 344–348.
- Bhattacharya, S. Maternal and perinatal outcomes after fresh versus frozen embryo transfer—What is the risk-benefit ratio? Fertil. Steril. 2016, 106, 241–243.
- Zhao, J.; Xu, B.; Zhang, Q.; Li, Y.P. Which one has a better obstetric and perinatal outcome in singleton pregnancy, IVF/ICSI or FET?: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2016, 14, 51.
- Evans, J.; Hannan, N.J.; Edgell, T.A.; Vollenhoven, B.J.; Lutjen, P.J.; Osianlis, T.; Salamonsen, L.A.; Rombauts, L.J.F. Fresh versus frozen embryo transfer: Backing clinical decisions with scientific and clinical evidence. Hum. Reprod. Update 2014, 20, 808–821.
- Blanco-Breindel, M.F.; Singh, M.; Kahn, J. Endometrial Receptivity. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK587449/ (accessed on 27 March 2023).
- Groenewoud, E.; Cohlen, B.; Al-Oraiby, A.; Brinkhuis, E.; Broekmans, F.; de Bruin, J.; Dool, G.V.D.; Fleisher, K.; Friederich, J.; Goddijn, M.; et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum. Reprod. 2016, 31, 1483–1492.
- Agha-Hosseini, M.; Hashemi, L.; Aleyasin, A.; Ghasemi, M.; Sarvi, F.; Nashtaei, M.S.; Khodarahmian, M. Natural cycle versus artificial cycle in frozen-thawed embryo transfer: A randomized prospective trial. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 12–17.
- Mumusoglu, S.; Polat, M.; Ozbek, I.Y.; Bozdag, G.; Papanikolaou, E.G.; Esteves, S.C.; Humaidan, P.; Yarali, H. Preparation of the Endometrium for Frozen Embryo Transfer: A Systematic Review. Front. Endocrinol. 2021, 12, 688237.
- Eleftheriadou, A.; Francis, A.; Wilcox, M.; Jayaprakasan, K. Frozen Blastocyst Embryo Transfer: Comparison of Protocols and Factors Influencing Outcome. J. Clin. Med. 2022, 11, 737.
- Greenbaum, S.; Athavale, A.; Klement, A.H.; Bentov, Y. Luteal phase support in fresh and frozen embryo transfers. Front. Reprod. Health 2022, 4, 919948.
- Jia, Y.; Sha, Y.; Qiu, Z.; Guo, Y.; Tan, A.; Huang, Y.; Zhong, Y.; Dong, Y.; Ye, H. Comparison of the Effectiveness of Endometrial Receptivity Analysis (ERA) to Guide Personalized Embryo Transfer with Conventional Frozen Embryo Transfer in 281 Chinese Women with Recurrent Implantation Failure. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935634.
- Hashimoto, T.; Koizumi, M.; Doshida, M.; Toya, M.; Sagara, E.; Oka, N.; Nakajo, Y.; Aono, N.; Igarashi, H.; Kyono, K. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two-centers study. Reprod. Med. Biol. 2017, 16, 290–296.
- Patel, J.A.; Patel, A.J.; Banker, J.M.; Shah, S.I.; Banker, M.R. Personalized embryo transfer helps in improving In vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J. Hum. Reprod. Sci. 2019, 12, 59–66.
- ERA-EMMA-ALICE-Manual-EndomeTRIO-Manual-USA-2021.pdf . Available online: https://www.igenomix.ca/wp-content/uploads/sites/14/2021/10/ERA-EMMA-ALICE-Manual-EndomeTRIO-Manual-USA-2021.pdf (accessed on 27 March 2023).
- Madero, S.; Rodriguez, A.; Vassena, R.; Vernaeve, V. Endometrial preparation: Effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum. Reprod. 2016, 31, 1755–1764.
- Glujovsky, D.; Pesce, R.; Fiszbajn, G.; Sueldo, C.; Hart, R.J.; Ciapponi, A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst. Rev. 2020, 2020, CD006359.
- Devroey, P.; Pados, G. Preparation of endometrium for egg donation. Hum. Reprod. Update 1998, 4, 856–861.
- Navot, D.; Laufer, N.; Kopolovic, J.; Rabinowitz, R.; Birkenfeld, A.; Lewin, A.; Granat, M.; Margalioth, E.J.; Schenker, J.G. Artificially Induced Endometrial Cycles and Establishment of Pregnancies in the Absence of Ovaries. N. Engl. J. Med. 1986, 314, 806–811.
- Borini, A.; Prato, L.D.; Bianchi, L.; Violini, F.; Cattoli, M.; Flamigni, C. CLINICAL ASSISTED REPRODUCTION: Effect of Duration of Estradiol Replacement on the Outcome of Oocyte Donation. J. Assist. Reprod. Genet. 2001, 18, 187–192.
- Bourdon, M.; Santulli, P.; Maignien, C.; Gayet, V.; Pocate-Cheriet, K.; Marcellin, L.; Chapron, C. The deferred embryo transfer strategy improves cumulative pregnancy rates in endometriosis-related infertility: A retrospective matched cohort study. PLoS ONE 2018, 13, e0194800.
- Sekhon, L.; Feuerstein, J.; Pan, S.; Overbey, J.; Lee, J.A.; Briton-Jones, C.; Flisser, E.; Stein, D.E.; Mukherjee, T.; Grunfeld, L.; et al. Endometrial preparation before the transfer of single, vitrified-warmed, euploid blastocysts: Does the duration of estradiol treatment influence clinical outcome? Fertil. Steril. 2019, 111, 1177–1185.e3.
- Zarei, A.; Sohail, P.; Parsanezhad, M.E.; Alborzi, S.; Samsami, A.; Azizi, M. Comparison of four protocols for luteal phase support in frozen-thawed Embryo transfer cycles: A randomized clinical trial. Arch. Gynecol. Obstet. 2016, 295, 239–246.
- Tabibzadeh, S. Molecular control of the implantation window. Hum. Reprod. Update 1998, 4, 465–471.
- Franasiak, J.M.; Ruiz-Alonso, M.; Scott, R.T.; Simón, C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil. Steril. 2016, 105, 861–866.
- Weissman, A. RESULTS—Frozen-Thawed Embryo Transfer—IVF-Worldwide . 2017. Available online: https://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryo-transfer.html (accessed on 25 March 2023).
- Escribá, M.-J.; Bellver, J.; Bosch, E.; Sánchez, M.; Pellicer, A.; Remohí, J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: A randomized study. Fertil. Steril. 2006, 86, 92–97.
- Bergin, K.; Eliner, Y.; Duvall, D.W.; Roger, S.; Elguero, S.; Penzias, A.S.; Sakkas, D.; Vaughan, D.A. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil. Steril. 2021, 116, 396–403.
- Riestenberg, C.; Kroener, L.; Quinn, M.; Ching, K.; Ambartsumyan, G. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil. Steril. 2021, 115, 1001–1006.
- Personalized Embryo Transfer (PET) Calculator. Igenomix. (n.d.). Available online: https://clinics.myigenomix.com/pet/calculator (accessed on 24 March 2023).
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
05 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




