Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Siti Nur Hidayah Jamil | -- | 1777 | 2023-05-04 11:46:34 | | | |
| 2 | Dean Liu | -1 word(s) | 1776 | 2023-05-05 03:33:23 | | | | |
| 3 | Dean Liu | Meta information modification | 1776 | 2023-05-05 03:33:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jamil, S.N.H.; Ali, A.H.; Feroz, S.R.; Lam, S.D.; Agustar, H.K.; Mohd Abd Razak, M.R.; Latip, J. Mechanism of Action of Curcumin. Encyclopedia. Available online: https://encyclopedia.pub/entry/43759 (accessed on 01 March 2026).
Jamil SNH, Ali AH, Feroz SR, Lam SD, Agustar HK, Mohd Abd Razak MR, et al. Mechanism of Action of Curcumin. Encyclopedia. Available at: https://encyclopedia.pub/entry/43759. Accessed March 01, 2026.
Jamil, Siti Nur Hidayah, Amatul Hamizah Ali, Shevin Rizal Feroz, Su Datt Lam, Hani Kartini Agustar, Mohd Ridzuan Mohd Abd Razak, Jalifah Latip. "Mechanism of Action of Curcumin" Encyclopedia, https://encyclopedia.pub/entry/43759 (accessed March 01, 2026).
Jamil, S.N.H., Ali, A.H., Feroz, S.R., Lam, S.D., Agustar, H.K., Mohd Abd Razak, M.R., & Latip, J. (2023, May 04). Mechanism of Action of Curcumin. In Encyclopedia. https://encyclopedia.pub/entry/43759
Jamil, Siti Nur Hidayah, et al. "Mechanism of Action of Curcumin." Encyclopedia. Web. 04 May, 2023.
Copy Citation
Curcumin, one of the major ingredients of turmeric (Curcuma longa), has been widely reported for its diverse bioactivities, including against malaria and inflammatory-related diseases. Curcumin’s low bioavailability limits its potential as an antimalarial and anti-inflammatory agent. Therefore, research on the design and synthesis of novel curcumin derivatives is being actively pursued to improve the pharmacokinetic profile and efficacy of curcumin.
curcumin derivatives
antimalaria
anti-inflammatory
structure–activity relationship
1. Host Proteins as Molecular Targets of Curcumin
Since malaria causes dysregulation in the inflammatory response, the elucidation of the mechanisms of action of malarial infection is important in order to understand the pathways and binding targets for inhibition. One potential mechanism involved in regulating the pathophysiology of malaria was identified to involve the Toll-like receptor (TLR) signaling pathways. TLRs, which are located on the cell surface, recognize the released Plasmodium DNA and trigger the initiation of immune responses through the activation of NF-κB [1][2]. The transcription factor NF-κB is a critical signaling protein involved in various inflammatory responses and gene expressions [3][4][5]. NF-κB is found in a dormant state in the cytoplasm and will only be transcribed when it is activated and translocated into the nucleus [2][6]. This transcription factor is the main target protein that directly regulates the pro- and anti-inflammatory cytokines within the body, including COX-2, TNF, IL-1, IL-6, IL-8, IL-10, and chemokines [1][7][8][9]. The dysregulation of these cytokines and chemokines will lead to the expression of malarial symptoms such as fever, and if left untreated, will lead to severe malaria (Figure 1). Therefore, NF-κB has become the most targeted factor in the development of antimalarial agents. Another study also established and reported that P. falciparum infection leads to the elevation of TNF production, which is the main malarial pathogenic factor, and, hence, could lead to a high risk of severe malaria and even death [10][11][12].
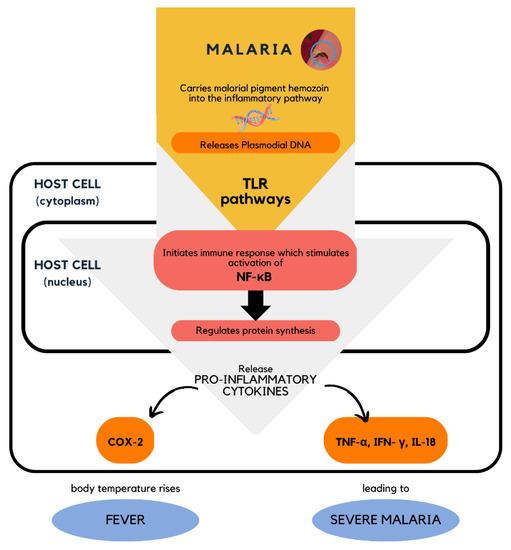
Figure 1. Cellular level mechanism of action of malarial infection in the host cell involving the activation of NF-κB [1].
Curcumin has been proven to suppress the activation and translocation of NF-κB into the nucleus, thus, controlling the level of inflammatory cytokines and the larger inflammatory response. Its ability to interact and inhibit various proteins helps in the elucidation and modulation of the pathophysiology of diseases at the molecular level, including malaria [13][14][15]. The interaction of curcumin with proteins is facilitated by its structural flexibility conferred by the presence of the unsaturated diketo group at the center of curcumin [8][13] (Figure 2).
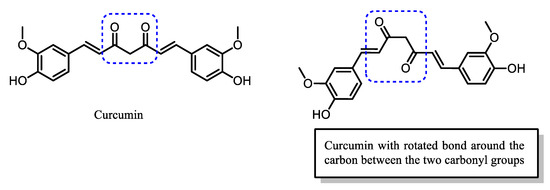
Figure 2. The structural flexibility of curcumin allows for bond rotation around the α-carbon, bridging the two carbonyl groups.
A recent study by Ali et al. on TLR pathways involving the control of the protein kinases Akt and glycogen synthase kinase-3β (GSK3β) also proved NF-κB as a downstream target that regulates anti-inflammatory cytokines [16]. Based on in vivo studies, curcumin was demonstrated to directly inhibit the host GSK3β, leading to the phosphorylation of NF-κB, hence, modulating the regulation of pro- (TNF-α, IFN-γ, and IL-18) and anti-inflammatory (IL-4 and IL-10) cytokine levels [16]. The immunomodulating effect of curcumin in reducing pro-inflammatory cytokine expression can potentially prevent severe and cerebral malaria [17][18]. As evidence to this claim, several in vitro studies have shown that curcumin downregulates pro-inflammatory cytokine production and the expression of cell adhesion molecules in TNF-activated human endothelial cells observed at the trophozoites stage of P. falciparum transmission [17]. Furthermore, the inhibition of NF-κB by curcumin also suppresses the generation of reactive oxygen species (ROS), which attenuates the inflammatory response [19][20][21].
2. Parasite Proteins as Molecular Targets of Curcumin
With regard to parasite proteins, curcumin induces ROS generation in parasite cells, which affects the function of the PfGCN5 histone acetyltransferases (HATs) p300/CREB-binding protein (CBP), thus, inhibiting histone acetylation and the transcription process in the parasite [22]. The generation of ROS is an important antimalarial mechanism as it induces protein and DNA damage within parasite cells, leading to their death [16][23][24]. The specific inhibition of parasite HAT by curcumin prevents the acetylation of K9 and K14 of histone H3. Cui et al. also reported that the antiplasmodial activity of curcumin is attributed, at least in part, to the production of ROS and the downregulation of PfGCN5 HAT activity [25].
Another mechanism of action of curcumin is by disrupting the transmission and development of Plasmodium parasites at the erythrocytic stage. As erythrocytes burst to release more merozoites, heme is also released. However, the released heme is highly toxic to the merozoites. Thus, the parasites will be stimulated to convert hematin into its detoxified polymeric form, hemozoin [26][27][28]. Curcumin treatment was reported to inhibit the formation of hemozoins in vitro, as observed through transmission electron microscopy in a study using the P. falciparum 3D7 strain [29]. This proved that the antimalarial activity of curcumin is similar to that of quinine and chloroquine [28].
Uncontrolled parasite transmission in the body can develop into fatal severe anemia or cerebral malaria, whose pathophysiology involves the inflammatory response [15][30]. The excessive stimulation of pro-inflammatory cytokines by the parasite subsequently leads to the sequestration of parasites in the brain [31]. Several reports have indicated the ability of curcumin to eliminate parasites at the trophozoite stage, synergistically and effectively better than artemisinin [32][33][34][35]. This observation was also proven using chloroquine-sensitive (CQS) and chloroquine-resistant (CQR) P. falciparum strains, with a proposed mechanism of curcumin action against the parasite proteins PfRIO2-kinase and PfGCN5 HAT [33][35][36].
The Knoevenagel condensate curcumin derivatives mentioned earlier were suggested to target the PfATP6 parasite protein to explain their schizont inhibition activity. This was based on the established target of the reference antimalarial drug, artemisinin, which shares the same binding pocket on PfATP6 as curcumin [32][37]. The study applied PreADMET predictions, which demonstrated the attachment and interaction of curcumin and its derivatives with the active site of PfATP6.
The identification of therapeutic targets involved in malarial infection can provide a better understanding of the inhibitory mechanism of antimalarial agents (Figure 3) [38][39]. The current knowledge on the action of curcumin derivatives is limited, as their host-targeting mechanisms have not been entirely established (Table 1) [14][40][41]. Therefore, future research that can explain an in-depth understanding of the molecular-level mechanism of action of curcumin and its bioactive derivatives will not only help in the development of potent antimalarial and anti-inflammatory agents [42] but also for other diseases [43][44][45].
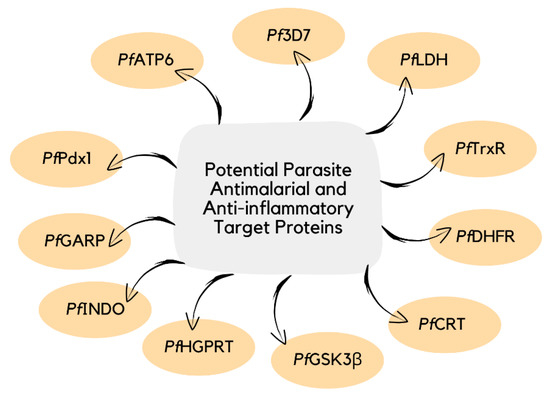
Figure 3. Examples of potential parasite target proteins involved in the pathophysiology of malaria.
Table 1. Antimalarial and anti-inflammatory activities of curcumin and its derivatives.
| Activity | In Vitro/In Vivo/In Silico Evidence | References |
|---|---|---|
| Antiplasmodium PfATP6 | Curcumin (1) reduced P. falciparum viability, causing parasitic cell proliferation to decrease.
|
[37] |
| Curcumin (1) and its derivatives (9, 14, 15, 19, 21, 23, 27, and 28) showed 100% inhibition of P. falciparum growth upon a 50 μ�g/mL dose of treatment. | [32] | |
Molecular docking results validated binding of curcumin (1) and its derivatives to PfATP with favorable free binding energy.
(higher than both artemisinin (–6.73 kcal/mol) and curcumin (–5.25 kcal/mol), hence, better interaction with the protein).
|
[32] | |
| In vitro study using CQR P. falciparum showed potent antimalarial activity of curcumin (1), with reported IC50 value of ~5 μ�M. In vivo treatment of P. berghei-infected mice with 100 mg/kg curcumin showed:
|
[46][47] | |
| Curcumin treatment on P. berghei-infected C57BI/6 mice delayed mice death by 10 days and prevented cerebral malaria. Dose: 50 mg/kg, twice daily for 6 days. |
[48] | |
| Curcumin exhibited antimalarial activity in P. berghei-infected mice. Dose: 300 mg/kg daily for 4 days (60.22% parasitemia inhibition). Dose: 80 mg/kg daily for 4 days (60.21% chemosuppressive effect). |
[49][50] | |
| Antiplasmodium Pf3D7 | Curcumin (1) showed potential inhibition of parasite transmission at the trophozoite stage. Curcumin derivative (monocarbonyl curcumin)
|
[51] |
| Antiplasmodium PfDXR | In silico and in vitro studies validated synergistic binding of curcumin (1) to PfDXR protein with fosmidomycin.
(46)—57%. |
[52] |
| Antiplasmodium PGCN5 HAT |
In vitro study suggested curcumin (1) as a potent inhibitor of p300/CBP (CREB-binding protein) as tested on four P. falciparum strains.
|
[16] |
| Antiplasmodium PfTrxR |
In vitro study using CQS (D6 clone) and CQR (W2 clone) P. falciparum strains showed that curcumin (1) inhibited PfTrxR protein with an IC50 value of 2 μM. | [53] |
| Antiplasmodium PfHGPRT PfSAHH |
In silico simulation using Molegro Virtual Docker (MVD) and admetSAR showed high binding energy of curcumin (1) to the protein.
|
[41][54] |
| Antimalaria ROS |
In vitro study showed that curcumin (1) induced intracellular ROS production related to PPARɣ/Nrf2 activation.
|
[19][55] |
| Antimalaria | In vitro study using NF54 intraerythrocytic-form P. falciparum strain reported highly potent antiparasitic activity of curcumin (1).
|
[56] |
In vitro study using 3D7 clone strain of P. falciparum reported synergistic antimalarial effect of curcumin (1) with dihydroartemisinin and reduction in hemozoin formation upon several consecutive treatments of curcumin.
|
[24] | |
| In vitro study showed the effectiveness of curcumin–artemisinin combination therapy with additive interaction in killing P. falciparum. In vivo study using P. berghei-infected mice showed 100% survival upon treatment. Dose: 750 µg. |
[56] | |
| In vivo study on P. berghei ANKA-infected mice revealed treatment of curcumin (1) reduced parasitemia level and increased survival rate. Dose: 50 mg/kg daily. |
[57] | |
In vitro study shows reported IC50:
Dose: 5 and 10 mg/kg. |
[58] | |
| Anti-inflammatory COX-2 |
In vitro study using DPPH radical-scavenging assay showed anti-inflammatory activity of curcumin and its derivatives. Reported IC50 value and % inhibition:
|
[59] |
| Molecular docking using FlexX program validated COX-2 as a target protein and showed binding of curcumin (1) and curcumin derivatives (2, 3). Favorable interactions:
|
[59] | |
| Anti-inflammatory NF-κB |
In vivo study on P. berghei ANKA-infected mice upon treatment of curcumin (1) showed inhibition of NF-κB activation, which reduced expression of adhesion molecules and suppressed pro-inflammatory cytokines level. Dose: 100 mg/kg daily for 4 days. |
[60] |
| Anti-inflammatory GSK3β |
In vivo study on P. berghei NK65-infected rats upon treatment of curcumin (1) showed inhibition of host GSK3β, leading to the phosphorylation of NF-κB and regulation of pro- (decrease in serum TNF-α and IFN-γ levels) and anti-inflammatory (IL-10 and IL-4) cytokines. Dose: 3, 10, and 30 mg/kg. |
[11] |
| Anti-inflammatory | In vivo study on P. berghei NK65- and ANKA-infected mice upon treatment of curcumin (1). Reported activity:
|
[61] |
References
- Schumann, R.R. Malarial fever: Hemozoin is involved but Toll-free. Proc. Natl. Acad. Sci. USA 2007, 104, 1743–1744.
- Chan, M.M.Y. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995, 49, 1551–1556.
- Zambre, A.P.; Kulkarni, V.M.; Padhye, S.; Sandur, S.K.; Aggarwal, B.B. Novel curcumin analogs targeting TNF-induced NF-κB proliferation in human leukemic KBM-5 cellsB activation. Bioorg. Med. Chem. 2006, 14, 7196–7204.
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. The molecular targets and therapeutic uses of curcumin in health and disease. AEMB 2007, 595, 1–75.
- Siebenlist, U.; Franzoso, G.; Brown, K. Structure, regulation and function of NF-kappaB. Annu. Rev. Cell Dev. Biol. 1994, 10, 405–455.
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. AEMB 2007, 595, 127–148.
- Xu, Y.X.; Pindolia, K.R.; Janakiraman, N.; Noth, C.J.; Chapman, R.A.; Gautam, S.C. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997, 25, 413–422.
- Surh, Y.J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097.
- Paulino, N.; Paulino, A.S.; Diniz, S.N.; de Mendonça, S.; Gonçalves, I.D.; Flores, F.F.; Santos, R.P.; Rodrigues, C.; Pardi, P.C.; Suarez, J.A.Q. Evaluation of the anti-inflammatory action of curcumin analog (DM1): Effect on iNOS and COX-2 gene expression and autophagy pathways. Bioorg. Med. Chem. 2016, 24, 1927–1935.
- Perlmann, P.; Troye-Blomberg, M. Malaria blood-stage infection and its control by the immune system. Folia Biol. 2000, 46, 210–218.
- Jang, M.K.; Sohn, D.H.; Ryu, J.H. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-α release from Curcuma zedoaria. Planta Med. 2001, 67, 550–552.
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Nakamura, A.; Yoshikawa, M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 5891–5898.
- Dasgupta, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Maneeruttanarungroj, C.; Swift, S.E.; Lyons, T.M.; Tirado-Rives, J.; Jorgensen, W.L.; Yuthavong, Y.; Anderson, K.S. Exploiting structural analysis, in silico screening, and serendipity to identify novel inhibitors of drug-resistant falciparum malaria. ACS Chem. Biol. 2009, 4, 29–40.
- Sarkar, F.H.; Li, Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2004, 555, 53–64.
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000.
- Ali, A.H.; Sudi, S.; Basir, R.; Embi, N.; Sidek, H.M. The antimalarial effect of curcumin is mediated by the inhibition of glycogen synthase kinase-3 β. J. Med. Food. 2017, 20, 152–161.
- Mimche, P.N.; Taramelli, D.; Vivas, L. The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar. J. 2011, 10, 510.
- Kaiser, K.; Texier, A.; Ferrandiz, J.; Buguet, A.; Meiller, A.; Latour, C.; Peyron, F.; Cespuglio, R.; Picot, S. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J. Infect. Dis. 2006, 193, 987–995.
- Cui, L.; Miao, J.; Cui, L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: Inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 2007, 51, 488–494.
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional curcumin mediate multitherapeutic effects. J. Food Sci. 2017, 82, 2006–2015.
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98.
- Hassan, F.U.; Rehman, M.S.U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514.
- Bhaumik, S.; Anjum, R.; Rangaraj, N.; Pardhasaradhi, B.V.V.; Khar, A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999, 456, 311–314.
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–570.
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494.
- Kingston, D.G.I.; Cassera, M.B. Antimalarial natural products. Prog. Chem. Org. Nat. Prod. 2022, 117, 1–106.
- Hsu, C.H.; Cheng, A.L. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480.
- Alumasa, J.N.; Gorka, A.P.; Casabiance, L.B.; Comstock, E.; de Dios, A.C.; Roepe, P.D. The hydroxyl functionality and a rigid proximal N are required for forming a novel non-covalent quinine-heme complex. J. Inorg. Biochem. 2011, 105, 467.
- Tjahjani, S.; Syafruddin; Tjokropranoto, R. Interaction of alphamangostin and curcumin with dihydroartemisinin as antimalaria in vitro. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012017.
- Hunt, N.H.; Golenser, J.; Chan-Ling, T.; Parekh, S.; Rae, C. Immunopathogenesis of cerebral malaria. Int. J. Parasitol. 2006, 36, 569–582.
- Schofield, L.; Grau, G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735.
- Dohutia, C.; Chetia, D.; Gogoi, K.; Bhattacharyya, D.R.; Sarma, K. Molecular docking, synthesis and in vitro antimalarial evaluation of certain novel curcumin analogues. Braz. J. Pharm. Sci. 2018, 53, 1–14.
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902.
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. The potency of heterocyclic curcumin analogues: An evidence-based review. Pharmacol. Res. 2021, 166, 105489.
- Balaji, S.N.; Ahsan, M.J.; Jadav, S.S.; Trivedi, V. Molecular modelling, synthesis, and antimalarial potentials of curcumin analogues containing heterocyclic ring. Arab. J. Chem. 2015, 12, 2492–2500.
- Parveen, A.; Chakraborty, A.; Konreddy, A.K.; Chakravarty, H.; Sharon, A.; Trivedi, V.; Bal, C. Skeletal hybridization and PfRIO-2 kinase modeling for synthesis of α-pyrone analogs as anti-malarial agent. Eur. J. Med. Chem. 2013, 70, 607–612.
- Ji, H.F.; Shen, L. Interactions of curcumin with the PfATP6 model and the implications for its antimalarial mechanism. Bioorganic Med. Chem. Lett. 2009, 19, 2453–2455.
- Eckstein-Ludwig, U.; Webb, R.J.; Van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961.
- Gui, J.S.; Jalil, J.; Jubri, Z.; Kamisah, Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019, 71, 79–89.
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. The potential use of a curcumin-piperine combination as an antimalarial agent: A systematic review. J. Trop. Med. 2021, 2021, 9135617.
- Singh, D.B.; Dwivedi, S. Structural insight into binding mode of inhibitor with SAHH of Plasmodium and human: Interaction of curcumin with anti-malarial drug targets. J. Chem. Biol. 2016, 9, 107–120.
- Zahidah, A.F.; Faizah, O.; Nur Aqilah, K.; Taty Anna, K. Curcumin as an anti-arthritic agent in collagen-induced arthritic Sprague-Dawley rats. Sains Malays. 2012, 41, 591–595.
- Kevin, T.T.M.; Nur Idanis, A.S.; Anastasha, B.; Mohd Faris, M.R.; Faizah, O.; Taty Anna, K. Curcumin minimises histopathological and immunological progression in the ankle joints of collagen-induced arthritis rats. Med. Health 2020, 15, 26–36.
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.I.A.M.; Mokhtar, M.H. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients 2021, 13, 3126.
- Irfandi, R.; Ilham, M.; Erwing, R.; Arafah, M.; Rompegading, A.B.; Putri, S.E.; Sartika, S.D.; Fauziah, S.; Agustina, A.S.; Akbar, H.; et al. Review on curcumin compounds in turmeric plants for the treatment of COVID-19. Int. J. Des. Nat. Ecodyn. 2022, 17, 957–965.
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-artemisinin combination therapy for malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860.
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2004, 326, 472–474.
- Waknine-Grinberg, J.H.; McQuillan, J.A.; Hunt, N.; Ginsbur, J. Modulation of cerebral malaria by fasudil and other immune-modifying compounds. Exp. Parasitol. 2010, 125, 141–146.
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Salleh, A.; Setyowati, E.Y.; Berbudi, A. Piperine enhances the antimalarial activity of curcumin in Plasmodium berghei ANKA-infected mice: A novel approach for malaria prophylaxis. Evid.-Based Complement. Altern. Med. 2022, 2022, 7897163.
- Alkandahri, M.Y.; Berbudi, A.; Subarnas, A. Evaluation of experimental cerebral malaria of curcumin and kaempferol in Plasmodium berghei ANKA-infected mice. Pharmacogn. J. 2022, 14, 905–911.
- Balasaheb, R.; Wanare, G.; Kawathekar, N.; Ranjan, R.; Kumar, N.; Sahal, D.; Singh, V. Dibenzylideneacetone analogues as novel Plasmodium falciparum inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 3034–3036.
- Yusuf, A.S.; Sada, I.; Hassan, Y.; Olomola, T.O.; Adeyemi, C.M.; Ajibade, S.O. Synthesis, antimalarial activity, and docking studies of monocarbonyl analogues of curcumin. Ovidius Univ. Ann. Chem. 2018, 29, 92–96.
- Munigunti, R.; Gathiaka, S.; Acevedo, O.; Sahu, R.; Tekwani, B.; Calderón, A.I. Determination of antiplasmodial activity and binding affinity of curcumin and demethoxycurcumin towards PfTrxR. Nat. Prod. Res. 2014, 28, 356–364.
- Singh, D.B.; Gupta, M.K.; Singh, D.V.; Singh, S.K.; Misra, K. Docking and in silico ADMET studies of noraristeromycin, curcumin and its derivatives with Plasmodium falciparum SAH hydrolase: A molecular drug target against malaria. Interdiscip. Sci. Comput. Life Sci. 2013, 5, 1–12.
- Mimche, P.N.; Thompson, E.; Taramelli, D.; Vivas, L. Curcumin enhances non-opsonic phagocytosis of Plasmodium falciparum through up-regulation of cd36 surface expression on monocytes/macrophages. J. Antimicrob. Chemother. 2012, 67, 1895–1904.
- Montesino, N.L.; Kaiser, M.; Brun, R.; Schmidt, T.J. Search for antiprotozoal activity in herbal medicinal preparations; new natural leads against neglected tropical diseases. Molecules 2015, 20, 14118–14138.
- Ullah, R.; Rehman, A.; Zafeer, M.F.; Rehman, L.; Khan, Y.A.; Khan, M.A.H.; Khan, S.N.; Khan, A.U.; Abidi, S.M.A. Anthelmintic potential of thymoquinone and curcumin on Fasciola gigantica. PLoS ONE 2017, 12, e0171267.
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front. Pharmacol. 2017, 8, 622.
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorganic Med. Chem. Lett. 2005, 15, 1793–1797.
- Memvanga, P.B.; Coco, R.; Préat, V. An oral malaria therapy: Curcumin-loaded lipid-based drug delivery systems combined with β-arteether. J. Control. Release 2013, 172, 904–913.
- Olanlokun, J.O.; Abiodun, W.O.; Ebenezer, O.; Koorbanally, N.A.; Olorunsogo, O.O. Curcumin modulates multiple cell death, matrix metalloproteinase activation and cardiac protein release in susceptible and resistant Plasmodium berghei-infected mice. Biomed. Pharmacother. 2022, 146, 112454.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
05 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





