Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Graziano Serrao | -- | 3403 | 2023-05-04 11:24:29 | | | |
| 2 | Dean Liu | -1 word(s) | 3402 | 2023-05-05 03:29:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ottenhausen, M.; Greco, E.; Bertolini, G.; Gerosa, A.; Ippolito, S.; Middlebrooks, E.H.; Serrao, G.; Bruzzone, M.G.; Costa, F.; Ferroli, P.; et al. Craniovertebral Junction Instability after Oncological Resection. Encyclopedia. Available online: https://encyclopedia.pub/entry/43756 (accessed on 03 March 2026).
Ottenhausen M, Greco E, Bertolini G, Gerosa A, Ippolito S, Middlebrooks EH, et al. Craniovertebral Junction Instability after Oncological Resection. Encyclopedia. Available at: https://encyclopedia.pub/entry/43756. Accessed March 03, 2026.
Ottenhausen, Malte, Elena Greco, Giacomo Bertolini, Andrea Gerosa, Salvatore Ippolito, Erik H. Middlebrooks, Graziano Serrao, Maria Grazia Bruzzone, Francesco Costa, Paolo Ferroli, et al. "Craniovertebral Junction Instability after Oncological Resection" Encyclopedia, https://encyclopedia.pub/entry/43756 (accessed March 03, 2026).
Ottenhausen, M., Greco, E., Bertolini, G., Gerosa, A., Ippolito, S., Middlebrooks, E.H., Serrao, G., Bruzzone, M.G., Costa, F., Ferroli, P., & La Corte, E. (2023, May 04). Craniovertebral Junction Instability after Oncological Resection. In Encyclopedia. https://encyclopedia.pub/entry/43756
Ottenhausen, Malte, et al. "Craniovertebral Junction Instability after Oncological Resection." Encyclopedia. Web. 04 May, 2023.
Copy Citation
The craniovertebral junction (CVJ) is a complex transition area between the skull and cervical spine. Pathologies such as chordoma, chondrosarcoma and aneurysmal bone cysts may be encountered in this anatomical area and may predispose individuals to joint instability.
atlanto-occipital joint
atlantoaxial fusion
spinal fusion
1. Radiological Criteria of CVJ Instability
The symptoms induced by CVJ instability may range from neck pain and restricted neck movements to sensory and motor abnormalities and gait instability [1]. The concept of CVJ instability is based on both bony abnormalities and on excessive laxity/loss of insertion of the atlanto-occipital and atlantoaxial ligaments. The combination of a hypoplastic dens in association with anomalies of the posterior arch of the atlas increases the risk for craniocervical instability. Injuries to the anterior atlanto-occipital membrane, the apical and alar ligaments, the cruciate ligaments and the tectorial membrane result in instability since these ligaments provide most of the stability to the atlanto-occipital joint [2]. Thin-section multidetector dynamic CTs of the craniocervical region with sagittal and coronal sections, lateral radiographs of flexion/extension and dynamic MRIs of flexion/extension are the studies of choice to evaluate the stability of the CVJ [3]. The radiological measurements most commonly used for the diagnosis of CVJ instability include (Figure 1):
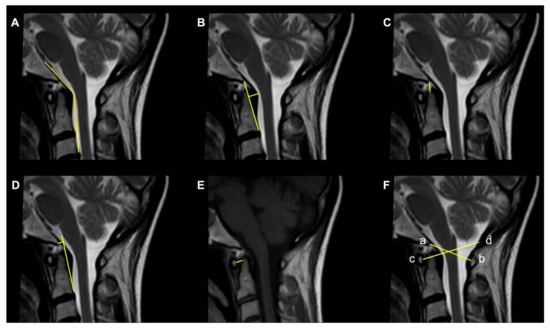
Figure 1. Commonly used radiological parameters to predict CVJ instability. (A) Clivoaxial Angle (CXA). (B) Grabb–Oakes line. (C) Basion–Dens Interval (BDI). (D) Basion–Axial Interval (BAI). (E) Atlantodental Interval (ADI). (F) Powers ratio: ab/cd.
-
The Clivoaxial Angle (CXA), which is the angle between the clivus line and the posterior axial line, examines the brainstem deformity induced by the odontoid process. A CXA of 135 degrees or less is considered “potentially pathological” [4].
-
The Grabb–Oakes line, which is the perpendicular distance from the dura to the line drawn from the basion to the posterior inferior edge of the C2 vertebra. It is a measure of the encroachment of the odontoid process into the upper spinal canal (basilar invagination) and investigates ventral brainstem compression. A measurement ≥9 mm is considered pathological [1].
-
The Basion–Dens Interval (BDI) measures the vertical distance between the basion and the dens and is considered pathological if ≥10 mm [5].
-
The Basion–Axial Interval (BAI) is the distance from the tip of the basion to the posterior axial line and is pathological if ≥12 mm [5].
-
The translational BAI and translational BDI are the change in mm of the BAI and BDI between the flexion and extension positions of the head [1].
-
The Atlantodental Interval (ADI) is the distance between the posterior surface of the anterior atlas ring and the anterior surface of the odontoid process. An ADI >5 mm in adults and >4 mm in children is an indication for surgery [6].
-
The Condyle–C1 interval (CC1) measures the distance between the occipital condyle and C1 at four equidistant points and is pathological in children if >4 mm, with a high diagnostic accuracy [6].
-
The Powers ratio is calculated by measuring the distance between the basion and the posterior arch of the atlas and then dividing it by the distance between the opisthion and the anterior arch of the atlas [7].
The Traynelis classification classifies occipitocervical dissociation patterns into three types according to the direction of dislocation of the occiput relative to C1: type I (anterior displacement), type II (superior–inferior displacement) and type III (posterior displacement) [8]. A limitation of this classification system is that rotatory or coronal malalignments are not taken into consideration. The Harborview classification described three levels of severity of CVJ instability. In Stage 1, there is sufficient preservation of ligamentous integrity, and a conservative treatment is indicated. In Stage 2, the craniocervical alignment is within 2 mm of normal. In Stage 3, there is a craniocervical malalignment of >2 mm, which requires internal fixation [9]. Horn et al. proposed another grading scale based on both CT and MRI scans. Grade I is defined by normal CT measurements (described above) but moderately abnormal MRI findings, and in these cases, a conservative treatment is indicated. Grade II is characterized by minimal abnormalities in the CT measurements but grossly abnormal MRI findings regarding the atlanto-occipital joints, tectorial membrane, alar ligaments or cruciate ligaments. In these cases, a surgical intervention is indicated [10].
Mechanical instability of the CVJ may result from osteolytic destruction and dislocation induced by the tumor or from the surgical and radiation treatments. However, the CVJ instability in these cases rarely follows the injury patterns seen in the trauma population, and specific guidelines for the neoplastic setting in this region are not yet defined [11]. The Spinal Instability Neoplastic Score (SINS) was developed by the Spine Oncology Study Group (SOSG) in 2010 and assesses and scores six variables: location, characterization of pain (from 1 to 3), type of bone lesion (lytic, mixed, blastic), radiographic spinal alignment (normal, deformity, subluxation), degree of vertebral body destruction (insolvent, degree of collapse) and involvement of posterolateral structures (unilateral, bilateral). The total score can range from 0 to 18, and instability is defined by a score of ≥13. However, a score between 7 and 12, which indicates a potentially unstable lesion, is an indication for surgical consultation [12]. A lytic destruction or resection of 70% of a unilateral condyle, 50% of bilateral condyles or extensive removal of the posterior elements and facets are also suggested in the literature as indications for occipitocervical fixation (OCF) [13].
2. Surgical Approaches to the CVJ
Several approaches are available in the surgical armamentarium to deal with a lesion harboring into or near the CVJ. The selection of the optimal surgical strategy is paramount for the success of the operation, as this maximizes the chances of achieving the surgical goals and minimizing the surgical related morbidity. In the table reported below (Table 1),
Table 1. Advantages and disadvantages for each surgical approach.
| Surgical Approach | Advantages | Disadvantages |
|---|---|---|
| Transoral | Provide direct anterior access to the CJV from the lower portion of the clivus to C3 Furnish a safe trajectory for extradural midline lesions, avoiding traction and/or manipulation of critical anatomical structures (e.g., cranial nerves, vertebral arteries, brainstem) Permit an excellent decompression of the ventral medulla and upper cervical spinal cord, especially in irreducible ventral pathology |
High risk of morbidity including swallowing and respiratory complication, CSF leakage and meningitis in case of intradural pathology Invasive and destructive approach for the surrounding structures (e.g., soft palate and oropharyngeal mucosa or bony structures in case a wider exposure is needed); this instance can be reduced with the endoscopic transoral approach. |
| Endoscopic Endonasal |
Provide a direct anterior access to the CJV from the clivus to the odontoid process Furnish a safe trajectory for extradural midline lesions, avoiding traction and/or manipulation of critical anatomical structures (e.g., cranial nerves, vertebral arteries, brainstem) Minimally invasive approach that reduces the mortality and morbidity related to the standard transoral approach |
Less exposure in the sagittal plane, especially below the axis, compared to the transoral approach Risk of CSF leakage and infection although less frequent than with the transoral approach |
| Posterior | Provide a safer surgical corridor for intradural tumors compared to anterior approaches in terms of CSF leakage and infections Extreme versatile approach to treat several types of dorsal lesions (e.g., meningiomas, schwannomas, intramedullary tumors) Capability to perform posterior fixation procedure within the same surgical time |
Risk of neurovascular injury during dissection procedures Risk of postoperative cervical pain Limited access for the resection of lesions extending into intradural and extradural compartments |
| Posterolateral | Provide a safer surgical corridor for intradural tumors compared to anterior approaches in terms of CSF leakage and infections Extreme versatile approach to treat several types of dorsal lesions (e.g., meningiomas, schwannomas, intramedullary tumors) Capability to perform posterior fixation procedure within the same surgical time Allows the resection of lesion extending into intradural and extradural compartments |
Higher risk of neurovascular injury during surgical exposure compared to posterior approach More challenging compared to the posterior approach, requires adequate surgical expertise |
3. Anterior Surgical Approaches to the CVJ
3.1. Transoral Approach
The transoral (or buccopharyngeal) approach is one of the most commonly used surgical approaches to decompress the craniovertebral junction affected by ventral, irreducible and extradural pathological lesions [14][15][16]. This approach has the ability to expose the anterior region of the foramen magnum from the basilar portion of the occipital bone to the vertebral body of C3 [16][17]. It was first described by Kanavel in 1917 who used it to remove a projectile located at the craniocervical junction. Subsequently, the microsurgical technique was popularized and refined by some expert neurosurgeons such as Alan Crockard and Arnold Menezes. From the preoperative point of view, it is necessary that patients who are candidates for this intervention carry out any drainage of the oral cavity to minimize the oral bacterial load [14][15][16]. It is also advisable to check the functionality of the mixed nerves and possibly proceed with a prophylactic tracheotomy. The maximum opening of the oral cavity should be promptly investigated especially in pediatric patients or in patients with macroglossia (down syndrome), and in such cases, an extended approach may be required (such as a mandibulotomy or glossotomy) [17]. The patient is positioned supine with the head extended and intubated via the orotracheal or tracheal route (this process is video-assisted, especially in patients with craniocervical instability), and gauzes are placed in the laryngopharynx to prevent the passage of blood into the stomach. After disinfecting the oral cavity with chlorhexidine and performing intravenous antibiotic therapy, the oral retractor (e.g., Crockard or Spetzler–Sonntag) is positioned. Hsu et al. recommend releasing the tongue from retraction at an interval of thirty minutes to avoid the risk of lingual edema from venolymphatic compression [15]. This buccopharyngeal approach can be associated with extensions to obtain a wider surgical window and in selected cases with a reduced buccal opening (“extended transoral approach”) [17][18][19][20].
Performing a mandibulotomy facilitates the surgical exposure of the region, reduces the operative distances and increases the angle of the craniocaudal and lateral exposure, which allows for optimal control from the middle third of the clivus to the C2–C3 intervertebral space. Performing a mandibuloglossotomy increases the sagittal exposure from the upper third of the clivus to the C4–C5 intervertebral space together with the reduction in the operative distance.
A palatotomy increases the rostral exposure to the upper third of the clivus without changing the operative distance or caudal or lateral exposure. A Le Fort I maxillotomy allows for lower jaw and hard palate mobilization, which allows for greater rostral control up to the sphenoid sinus and greater lateral control. The obstacle in the caudal exposure of this approach can be overcome by combining a median split of the maxillary bones, the hard palate and the soft palate [17][18]. Although, these approaches and transoral variants involve a decrease in the distance of the working canal, and increased surgical exposure is often associated with increased complications in the postoperative period [21][22]. A velopalatine incision and functional Passavant ring infringement may increase the risk of velopalatine insufficiency, whose symptoms and clinical signs include a nasal voice, dysphagia and fluid regurgitation. The incision of the tongue can lead to impaired motility of the tongue and therefore dysarthria. These procedures are also associated with an increase in the intubation period and the return to independent oral nutrition [16]. An important complication of this approach is the risk of liquorrhea; in the presence of this, it is necessary to resort to an aggressive repair treatment to avoid the onset of infectious conditions, which can lead to devastating consequences [15]. A lateral surgical exposure of the joint could lead to damage to the four cerebral arterial axes (carotid arteries and vertebral arteries), especially in the presence of anatomical variants, such as the retropharyngeal position of the carotid arteries; a correct preoperative and intraoperative radiological classification is therefore necessary with the use of a neuronavigator.
3.2. Transoral Endoscopic Approach
The relevance of the comorbidities associated with the transoral approach and its extensions has led the scientific community to identify new minimally invasive ways to manage the craniovertebral junction pathology [23][24][25]. The advancement of endoscopic techniques has allowed surgeons to perform minimally invasive procedures in the face of more aggressive and disabling traditional interventions at the craniovertebral junction. Frempong-Boadu et al. demonstrated for the first time the feasibility of the endoscope-assisted transoral approach for the treatment of joint anomalies [23]. Since then, there have been several anatomical studies and case reports. The use of the endoscopic technique has the advantage of a better visualization of the operative field while minimizing surgical exposure. As previously illustrated, some extended variations can be employed to achieve greater rostral control of the clivus. The transoral use of the endoscope has allowed researchers to spare the incision of the hard palate together with the other bone structures, which guarantees a rostral approach up to the spheno-occipital junction [24][25].
3.3. Endoscopic Endonasal Approach
The endoscopic endonasal approach (EEA) to the craniovertebral junction (CVJ) is a minimally invasive surgical technique that allows for access to the CVJ through the nasal cavity (Figure 2).
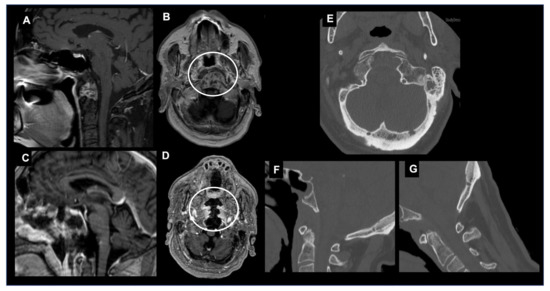
Figure 2. Endoscopic endonasal approach (EEA) to a CVJ chordoma. Sagittal (A) and axial (B) T1-weighted images after contrast injection showing a craniovertebral junction chordoma invading the C1 anterior arch, transverse ligament and tip of the odontoid. The patient underwent a gross total removal through an EEA. Postoperative sagittal (C) and axial (D) T1-weighted images after contrast injection confirmed the entity of resection and the integrity of C1-C2 joint. (E) Axial CT scan showing the occipital condyle integrity >90%. Dynamic cervical spine CT scans in maximal extension (F) and flexion (G) showing no abnormal movements and excluding any postoperative CVJ instability.
This approach has been used to treat a variety of conditions affecting the CVJ, including tumors, congenital anomalies and trauma. One of the advantages of the EEA is that it avoids the need for a large incision in the neck or skull, which can lead to less pain, a faster recovery and improved cosmetic outcomes [26][27][28]. Several surgical advantages have been attributed to the EEA in comparison to classical craniotomy or transfacial microsurgical techniques, and they have showed a reduced rate of morbidity and mortality [29][30]. In 2005, Kassam was the first neurosurgeon to use this technique to perform an odontoidectomy [31]. Subsequently, the pioneering work of the Pittsburgh group has been followed by numerous case reports and clinical series that all showed a reduction in the mortality rate and morbidity compared to the classic transoral approach [32][33][34][35]. The main advantages of the EEA to the craniovertebral junction derive from the surgical angle of attack and the surgical incision located at the level of the nasopharynx rather than the oropharynx. The nose and paranasal sinuses provide excellent rostral access and a rostrocaudal angle of attack for the treatment of most pathologies affecting this anatomical region; this is particularly true in patients with basilar invagination or when the odontoid has ascended upwards [36].
The foramen magnum and the superior portion of the cervical spine are located immediately behind the nasopharynx, which can be reached easily and directly through the nasal corridor. The nasopharynx has a significantly reduced proportion of virulent bacterial flora and neural plexuses that coordinate swallowing relative to the oropharynx [37]. The palate is never affected by the approach since the rostrocaudal angle of attack is parallel to that of the soft palate, and thus it does not require its transgression. The angle of approach and the caudal limit of access of the EEA to the CVJ is determined by the rhinopalatine line [38][39]. The lateral exposure of the EEA is limited by the parapharyngeal internal carotids and the jugular foramen. The transnasopharynx–transodontoid approach is an extension of the transclival approach. This approach can, however, be performed independently with preservation of the clivus since, in most cases, the pathology is confined to the level of the cervical spine; moreover, the exposure of the sphenoid sinus is not always required [40][41][42]. Furthermore, the possibility of stabilizing the CVJ through the use of transarticular screws positioned endoscopically via the endonasal have been described in anatomical studies [43][44]. In some situations, lateral exposure at the level of the foramen magnum is required [45]. This is particularly true in conditions affecting the occipital condyle and extending into the jugular foramen. In these situations, it is advisable to provide an appropriate lateral surgical window by removing the occipital condyle and partially resectioning the Eustachian tube. The reaming process of the occipital condyle can be performed until the hypoglossal canal is exposed. Careful neurophysiological monitoring of the cranial nerves is therefore essential to prevent the risk of deficits and to maintain the function of the hypoglossal nerve intact. If the medial occipital condylectomy is unilateral, by keeping the contralateral alar ligament intact, there is no risk of craniovertebral instability.
3.4. Instability after EEA
However, one of the potential complications of the transclival–transodontoid EEA is the postoperative instability of the CVJ (Figure 3).
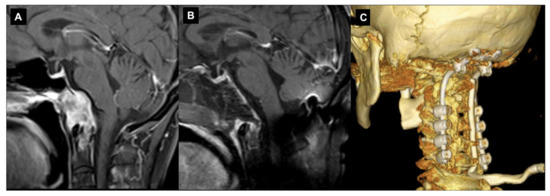
Figure 3. Endoscopic endonasal approach to CVJ chordoma and occipitocervical fixation. Sagittal preoperative (A) and postoperative (B) T1-weighted MR images after contrast injection showing the chordoma infiltration of C0-C1-C2 complex joint and a gross total resection. In the same surgical setting, an occipitocervical fixation was performed. A 3D reconstruction of the postoperative CT (C).
This can occur when the stability of the CVJ is compromised by the surgical procedure or preoperatively by tumor growth, which leads to symptoms such as neck pain, weakness and spinal cord compression. The risk of postoperative instability can be reduced by careful preoperative planning, proper surgical techniques and appropriate postoperative management [46]. There are several different techniques that have been used to prevent instability after the EEA. These include the use of internal fixation devices such as screws and rods in the pre- or immediate postoperative course, as well as the use of external fixation devices such as halo traction. There have been several studies published in recent years that have investigated the incidence and management of postoperative instability after applying the EEA for CVJ tumors, with a focus on condyle resection. An anatomical study showed that a lower-third clivectomy and unilateral anterior condylectomy through an EEA can cause progressive hypermobility at the CVJ. On the basis of biomechanical criteria, OCF is indicated for patients who undergo a > 75% anterior condylectomy [47]. Kooshkabadi et al. evaluated the incidence of postoperative instability after an EEA for CVJ tumors. The study included 212 patients who underwent an EEA for lower clivus lesions, and they found that around 3.3% of the patients required a fixation. They showed that an EEA resection greater than 75% of the occipital condyle significantly increased the risk of CVJ instability, which required subsequent fixation. The degree of the condyle resection was a significant factor that predisposed it to the occipitocervical instability [48]. There are also some reports, mainly related to the EEA to CVJ abnormalities and basilar invagination, on the preservation of the anterior C1 arch that avoids the need for posterior fixation with the aim of preserving the rotational movement at the C0–C2 segment and reducing the risk of a subaxial instability development [36][49][50]. Overall, these studies suggest that the entity of condyle resections, C1 anterior arch and transverse ligament preservation while using the EEA on CVJ tumors may represent two significant factors that are related to the risk of postoperative instability [46][51]. However, the evidence is not entirely consistent, and further studies with larger patient populations and longer follow-ups are needed to better understand the risk. The most consistent risk factor identified across these studies is the size and location of the tumors, specifically tumors located in the upper cervical spine and tumors with a wide base. Other factors that have been identified as risk factors for postoperative instability include the degree of resection, the surgical approach and the reconstruction methods used.
4. Posterior Surgical Approaches to the CVJ
The posterior and posterolateral approaches represent two further operative techniques for the treatment of CVJ primary and metastatic neoplastic diseases available in the neurosurgical armamentarium (Figure 4).
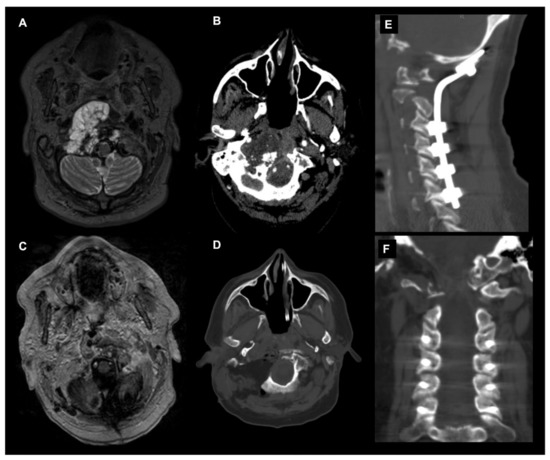
Figure 4. Combined endoscopic endonasal approach and far-lateral transcondylar and petro-occipital trans-sigmoid approach to recurrent CVJ chordoma and subsequent occipitocervical fixation. Axial T2-weighted MR image (A) and angio CT scan (B) showing a recurrent predominantly right craniovertebral junction chordoma. The chordoma infiltrates the rhinopharynx, C0-C1-C2 joint complex and the entire right occipital condyle. A combined endoscopic endonasal approach associated with far-lateral transcondylar and petro-occipital trans-sigmoid approach has been performed. (C) Axial postoperative T1-weighted after contrast injection image and (D) noncontrast CT scan disclosed a gross total resection with the destruction of the right clival–atlo–axial joint. An occipital-cervical fixation was therefore postoperatively planned and performed (E,F).
The main variables determining the selection of the surgical approach are related to the tumor pathology, i.e., primary vs metastatic disease; anatomy, i.e., tumor location and involvement of the adjacent structures; and biomechanical considerations.
References
- Henderson, F.C.; Francomano, C.A.; Koby, M.; Tuchman, K.; Adcock, J.; Patel, S. Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg. Rev. 2019, 42, 915–936.
- Riascos, R.; Bonfante, E.; Cotes, C.; Guirgui, M.; Hakimelahi, R.; West, C. Imaging of Atlanto-Occipital and Atlantoaxial Traumatic Injuries: What the Radiologist Needs to Know. Radiographics 2015, 35, 2121–2134.
- Kasliwal, M.K.; Fontes, R.B.; Traynelis, V.C. Occipitocervical dissociation-incidence, evaluation, and treatment. Curr. Rev. Musculoskelet. Med. 2016, 9, 247–254.
- Henderson, F.C.; Henderson, F.C.; Wilson, W.A.; Mark, A.S.; Koby, M. Utility of the clivo-axial angle in assessing brainstem deformity: Pilot study and literature review. Neurosurg. Rev. 2018, 41, 149–163.
- Rojas, C.A.; Bertozzi, J.C.; Martinez, C.R.; Whitlow, J. Reassessment of the craniocervical junction: Normal values on CT. AJNR. Am. J. Neuroradiol. 2007, 28, 1819–1823.
- Pang, D.; Nemzek, W.R.; Zovickian, J. Atlanto-occipital dislocation: Part 1--normal occipital condyle-C1 interval in 89 children. Neurosurgery 2007, 61, 514–521.
- Lee, I.; Vasquez, L.; Tyroch, A.; Trier, T. Association of Atlanto-Occipital Dislocation, Retroclival Hematoma, and Hydrocephalus: Management and Survival in a Pediatric Patient. J. Neurol. Surg. Rep. 2017, 78, e46–e51.
- Traynelis, V.C.; Marano, G.D.; Dunker, R.O.; Kaufman, H.H. Traumatic atlanto-occipital dislocation. Case report. J. Neurosurg. 1986, 65, 863–870.
- Bellabarba, C.; Mirza, S.K.; West, G.A.; Mann, F.A.; Dailey, A.T.; Newell, D.W.; Chapman, J.R. Diagnosis and treatment of craniocervical dislocation in a series of 17 consecutive survivors during an 8-year period. J. Neurosurg. Spine 2006, 4, 429–440.
- Horn, E.M.; Feiz-Erfan, I.; Lekovic, G.P.; Dickman, C.A.; Sonntag, V.K.H.; Theodore, N. Survivors of occipitoatlantal dislocation injuries: Imaging and clinical correlates. J. Neurosurg. Spine 2007, 6, 113–120.
- Zuckerman, S.L.; Kreines, F.; Powers, A.; Iorgulescu, J.B.; Elder, J.B.; Bilsky, M.H.; Laufer, I. Stabilization of Tumor-Associated Craniovertebral Junction Instability: Indications, Operative Variables, and Outcomes. Neurosurgery 2017, 81, 251–258.
- Fisher, C.G.; Dipaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229.
- Fiani, B.; Jarrah, R.; Shields, J.; Durrani, S.; Panico, N.; Mualem, W.; Nathani, K.R.; Pasko, K. A Comprehensive Overview of Pediatric Neoplasms at the Craniocervical Junction: Meningiomas, Schwannomas, and Chordomas. Cureus 2022, 14, e31083.
- Crockard, H.A. The transoral approach to the base of the brain and upper cervical cord. Ann. R. Coll. Surg. Engl. 1985, 67, 321–325.
- Hsu, W.; Wolinsky, J.-P.; Gokaslan, Z.L.; Sciubba, D.M. Transoral Approaches to the Cervical Spine. Neurosurgery 2010, 66, A119–A125.
- Menezes, A.H. Surgical approaches: Postoperative care and complications “transoral–transpalatopharyngeal approach to the craniocervical junction”. Child’s Nerv. Syst. 2008, 24, 1187–1193.
- Youssef, A.S.; Sloan, A.E. Extended Transoral Approaches. Neurosurgery 2010, 66, A126–A134.
- Youssef, A.S.; Guiot, B.; Black, K.; Sloan, A.E. Modifications of the transoral approach to the craniovertebral junction: Anatomic study and clinical correlations. Neurosurgery 2008, 62, 145–154.
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222.
- Perrini, P.; Benedetto, N.; Guidi, E.; Di Lorenzo, N. Transoral approach and its superior extensions to the craniovertebral junction malformations: Surgical strategies and results. Neurosurgery 2009, 64, 331–342, discussion 342.
- Karam, Y.R.; Menezes, A.H.; Traynelis, V.C. Posterolateral approaches to the craniovertebral junction. Neurosurgery 2010, 66, A135–A140.
- Jones, D.C.; Hayter, J.P.; Vaughan, E.D.; Findlay, G.F. Oropharyngeal morbidity following transoral approaches to the upper cervical spine. Int. J. Oral Maxillofac. Surg. 1998, 27, 295–298.
- Frempong-Boadu, A.K.; Faunce, W.A.; Fessler, R.G. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery 2002, 51, S60–S66.
- La Corte, E.; Aldana, P.R. Endoscopic approach to the upper cervical spine and clivus: An anatomical study of the upper limits of the transoral corridor. Acta Neurochir. 2017, 159, 633–639.
- Visocchi, M.; Della Pepa, G.M.; Doglietto, F.; Esposito, G.; La Rocca, G.; Massimi, L. Video-assisted microsurgical transoral approach to the craniovertebral junction: Personal experience in childhood. Child’s Nerv. Syst. 2011, 27, 825–831.
- Kassam, A.; Snyderman, C.H.; Mintz, A.; Gardner, P.; Carrau, R.L. Expanded endonasal approach: The rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg. Focus. 2005, 19, E3.
- Kassam, A.; Snyderman, C.H.; Mintz, A.; Gardner, P.; Carrau, R.L. Expanded endonasal approach: The rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg. Focus. 2005, 19, E4.
- La Corte, E.; Aldana, P.R.; Schiariti, M.; Maccari, A.; Ferroli, P. Endoscopic approaches to the craniovertebral junction. Acta Neurochir. 2014, 156, 293–295.
- Cavallo, L.M.; Messina, A.; Cappabianca, P.; Esposito, F.; de Divitiis, E.; Gardner, P.; Tschabitscher, M. Endoscopic endonasal surgery of the midline skull base: Anatomical study and clinical considerations. Neurosurg. Focus 2005, 19, E2.
- Kasemsiri, P.; Carrau, R.L.; Ditzel Filho, L.F.S.; Prevedello, D.M.; Otto, B.A.; Old, M.; De Lara, D.; Kassam, A.B. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014, 82, S12–S21.
- Kassam, A.B.; Snyderman, C.; Gardner, P.; Carrau, R.; Spiro, R.; Sonntag, V.K.H.; Cappabianca, P.; Cavallo, L.M.; Jho, H.D. The expanded endonasal approach: A fully endoscopic transnasal approach and resection of the odontoid process: Technical case report. Neurosurgery 2005, 57, E213.
- Gempt, J.; Lehmberg, J.; Meyer, B.; Stoffel, M. Endoscopic transnasal resection of the odontoid in a patient with severe brainstem compression. Acta Neurochir. 2010, 152, 559–560.
- Gempt, J.; Lehmberg, J.; Grams, A.E.; Berends, L.; Meyer, B.; Stoffel, M. Endoscopic transnasal resection of the odontoid: Case series and clinical course. Eur. Spine J. 2011, 20, 661–666.
- Lee, A.; Sommer, D.; Reddy, K.; Murty, N.; Gunnarsson, T. Endoscopic transnasal approach to the craniocervical junction. Skull Base 2010, 20, 199–202.
- Nayak, J.V.; Gardner, P.A.; Vescan, A.D.; Carrau, R.L.; Kassam, A.B.; Snyderman, C.H. Experience with the expanded endonasal approach for resection of the odontoid process in rheumatoid disease. Am. J. Rhinol. 2007, 21, 601–606.
- Gladi, M.; Iacoangeli, M.; Specchia, N.; Re, M.; Dobran, M.; Alvaro, L.; Moriconi, E.; Scerrati, M. Endoscopic transnasal odontoid resection to decompress the bulbo-medullary junction: A reliable anterior minimally invasive technique without posterior fusion. Eur. Spine J. 2012, 21 (Suppl. S1), 55–60.
- van Abel, K.M.; Mallory, G.W.; Kasperbauer, J.L.; Moore, E.J.; Price, D.L.; O’Brien, E.K.; Olsen, K.D.; Krauss, W.E.; Clarke, M.J.; Jentoft, M.E.; et al. Transnasal odontoid resection: Is there an anatomic explanation for differing swallowing outcomes? Neurosurg. Focus 2014, 37, E16.
- Aldana, P.R.; Naseri, I.; La Corte, E. The Naso-Axial Line. Oper. Neurosurg. 2012, 71, ons308–ons314.
- La Corte, E.; Aldana, P.R.; Ferroli, P.; Greenfield, J.P.; Härtl, R.; Anand, V.K.; Schwartz, T.H. The rhinopalatine line as a reliable predictor of the inferior extent of endonasal odontoidectomies. Neurosurg. Focus 2015, 38, E16.
- Messina, A.; Bruno, M.C.; Decq, P.; Coste, A.; Cavallo, L.M.; De Divittis, E.; Cappabianca, P.; Tschabitscher, M. Pure endoscopic endonasal odontoidectomy: Anatomical study. Neurosurg. Rev. 2007, 30, 189–194.
- Tan, S.H.; Ganesan, D.; Prepageran, N.; Waran, V. A minimally invasive endoscopic transnasal approach to the craniovertebral junction in the paediatric population. Eur. Arch. Otorhinolaryngol. 2014, 271, 3101–3105.
- Tanriverdi, O.; Tugcu, B.; Gunaldi, O.; Baydin, S.S.; Demirgil, B.T.; Sam, B.; Kucukyuruk, B.; Tanriover, N. The selective odontoidectomy: Endoscopic endonasal approach to the craniocervical junction. J. Craniofac. Surg. 2014, 25, 1482–1487.
- Mendes, G.A.C.; Dickman, C.A.; Rodriguez-Martinez, N.G.; Kalb, S.; Crawford, N.R.; Sonntag, V.K.H.; Preul, M.C.; Little, A.S. Endoscopic endonasal atlantoaxial transarticular screw fixation technique: An anatomical feasibility and biomechanical study. J. Neurosurg. Spine 2015, 22, 470–477.
- Forbes, J.A.; Kumar, C.; McGough, D.; Palmisciano, P.; Hussein, A.E.; Zhebrykov, D.; Gibson, J.; Andaluz, N.; Sedaghat, A.R.; Prestigiacomo, C.J.; et al. Anterior occipital condyle screw placement through the endonasal corridor: Proof of concept study with cadaveric analysis. Eur. Spine J. 2023, 32, 682–688.
- Morera, V.A.; Fernandez-Miranda, J.C.; Prevedello, D.M.; Madhok, R.; Barges-Coll, J.; Gardner, P.; Carrau, R.; Snyderman, C.H.; Rhoton, A.L.; Kassam, A.B. “Far-Medial” Expanded Endonasal Approach to the Inferior Third of the Clivus. Oper. Neurosurg. 2010, 66, ons211–ons220.
- Baldassarre, B.; DI Perna, G.; Portonero, I.; Penner, F.; Cofano, F.; Marco, R.; Marengo, N.; Garbossa, D.; Pecorari, G.; Zenga, F. Craniovertebral junction chordomas: Case series and strategies to overcome the surgical challenge. J. Craniovertebral Junction Spine 2021, 12, 420–431.
- Perez-Orribo, L.; Little, A.S.; Lefevre, R.D.; Reyes, P.R.; Newcomb, A.G.U.S.; Prevedello, D.M.; Roldan, H.; Nakaji, P.; Dickman, C.A.; Crawford, N.R. Biomechanical evaluation of the craniovertebral junction after anterior unilateral condylectomy: Implications for endoscopic endonasal approaches to the cranial base. Neurosurgery 2013, 72, 1021–1029.
- Kooshkabadi, A.; Choi, P.A.; Koutourousiou, M.; Snyderman, C.H.; Wang, E.W.; Fernandez-Miranda, J.C.; Gardner, P.A. Atlanto-occipital Instability Following Endoscopic Endonasal Approach for Lower Clival Lesions: Experience with 212 Cases. Neurosurgery 2015, 77, 888–897.
- Iacoangeli, M.; Gladi, M.; Alvaro, L.; Di Rienzo, A.; Specchia, N.; Scerrati, M. Endoscopic endonasal odontoidectomy with anterior C1 arch preservation in elderly patients affected by rheumatoid arthritis. Spine J. 2013, 13, 542–548.
- Re, M.; Iacoangeli, M.; Di Somma, L.; Alvaro, L.; Nasi, D.; Magliulo, G.; Gioacchini, F.M.; Fradeani, D.; Scerrati, M. Endoscopic endonasal approach to the craniocervical junction: The importance of anterior C1 arch preservation or its reconstruction. Acta Otorhinolaryngol. Ital. 2016, 36, 107–118.
- Palmisciano, P.; Al Fawares, Y.; Woodhouse, C.; Yang, G.; Xu, A.; D’Herbemont, S.; Hoang, S.; McGuire, J.L.; Phillips, K.M.; Cheng, J.; et al. The Impact of C1 Anterior Arch Preservation on Spine Stability after Odontoidectomy: Systematic Review and Meta-Analysis. World Neurosurg. 2022, 167, 165–175.e2.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
940
Revisions:
2 times
(View History)
Update Date:
05 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





