Human gut microbes make up the most complex and essential micro-ecosystem in the body. Leukemia arises from clonal proliferation of abnormal hematopoietic stem cells, leading to disruption of normal marrow function and marrow failure, with an incidence of 0.97%, which is characterized by uncontrolled proliferation of the malignant clone and marrow failure. An anaplastic disease originating in lymph nodes or lymphoid tissue, lymphoma is a heterogeneous group of neoplastic diseases. Multiple myeloma (MM) is a terminally differentiated malignant clonal disease of plasma cells with an incidence of 0.41% of all human tumors, manifested by bone marrow clonal plasma cell infiltration and the presence of monoclonal M protein in peripheral blood and (or) urine. Gut microbes are closely related to the initiation and progression of hematological malignancies. Direct and indirect mechanisms influence the initiation and progression of hematological malignancies by intestinal microbes.
1. Leukemia
1.1. Intestinal Microbial Imbalance and the Initiation and Progression of Leukemia
1.1.1 Imbalance of Intestinal Flora Activates Inflammatory Factors to Promote the Initiation of Leukemia
Previous studies have shown that TET gene mutations can promote the development of many tumors, especially tumors in the hematopoietic system
[1][2][3]. TET 2 is a tet-methylcytosine dioxygenase 2, which can catalyze the conversion of 5-methylcytosine (5-mC) into 5-hydroxymethylcytosine (5-hmC). It plays a crucial role in DNA demethylation as an enzyme, which is essential for maintaining stem cell pluripotency
[4]. The absence of TET 2 can cause intestinal barrier damage in a TET 2 knockout mouse model, lead to intestinal microbial translocation, such as translocation of
Lactobacillus reuteri, then, the immune system is activated, resulting in increased stem cell self-renewal, which polarizes the development of the myeloid cells
[1][2][3][5][6], that eventually trigger pre-leukaemic myeloproliferation (PMP) and promote the initiation of leukemia
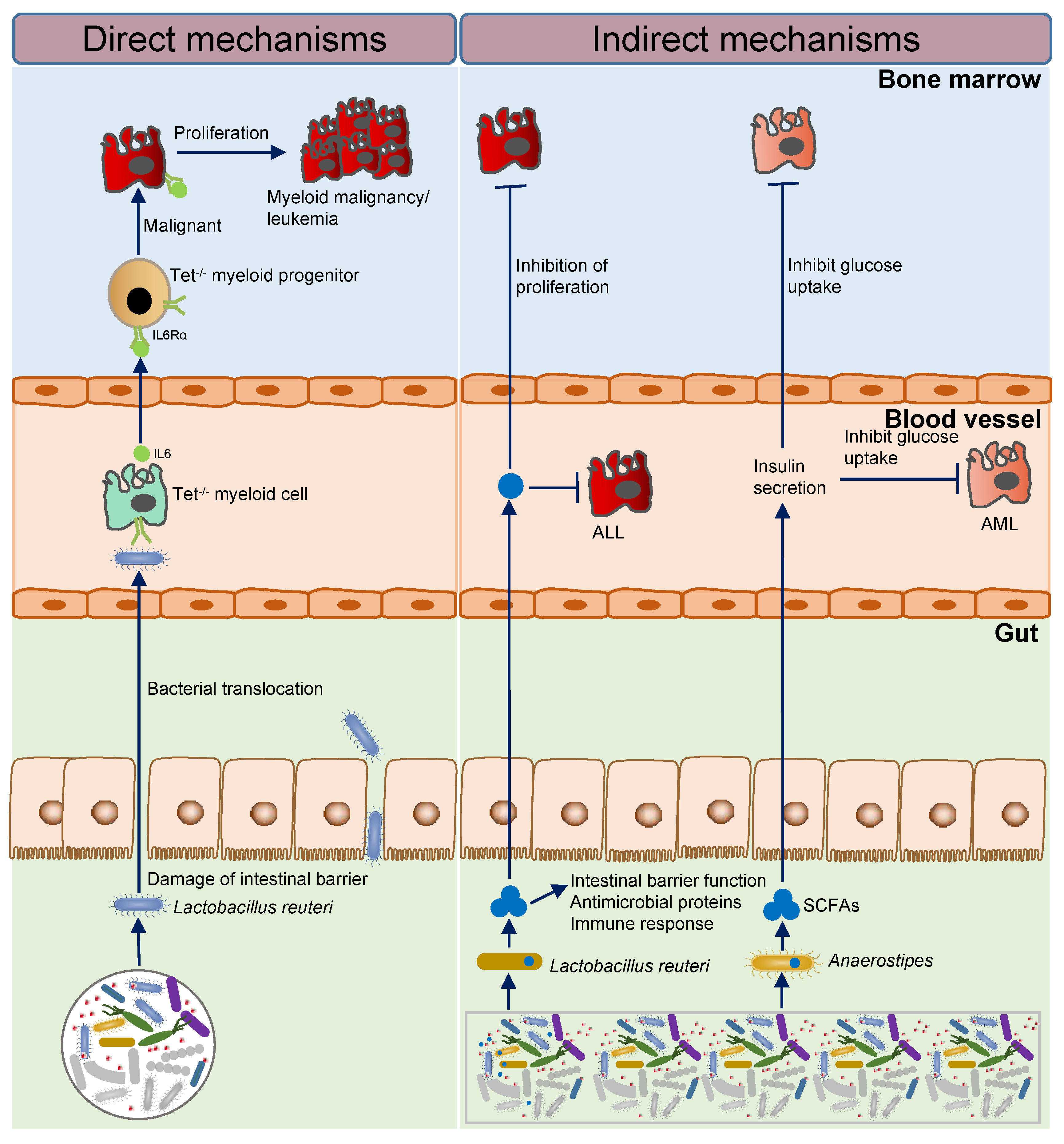
[1][2][4][7]. Mechanically, small-intestinal barrier dysfunction (reduced ZO-1 and upregulation of defence response genes), which occurs spontaneously or upon intestinal damage, results in bacterial translocation and to high levels of IL-6. Systemic microbial signals can bypass bacterial translocation in Tet2-deficient mice. Microbial-induced IL-6 is sensed by Tet2
−/− myeloid progenitor (MP) cells that overexpress IL-6Rα and are highly sensitive to IL-6 (Stat3 (pY705)). The MPs then expand upon IL-6 signals and differentiate preferentially into mature myeloid cells. This cycle results in the development of PMP. Treatment with antibiotics or neutralizing anti-IL-6 antibody can revert PMP, indicating that microbial inflammatory signals are required for PMP in the context of Tet2 deficiency
[8]. This research proved that intestinal flora-dependent inflammation is necessary for the development of PMP; however, it remains to be determined whether inflammation caused by intestinal flora can result in PMP developing into leukemia.
1.1.2. Short-Chain Fatty Acid Producing Bacteria Inhibit the Progression of Leukemia
Cancer-associated
cachexia (CAC) is a multifactorial syndrome of persistent skeletal muscle mass loss, characterized by reduced intake and abnormal metabolism, leading to the breakdown of protein and a breaking of synthesis balance
[9]. The role of gut microbiota in the treatment of cancer and related cachexia was reported by Laure B. Bindels et al., it was discovered that the abundance of
Lactobacillus spp. in the intestinal flora of ALL mice decreased, while the abundance of
Enterobactereae and
Paraacteroides goldsteinii/ASF 519 increased. ALL mice were injected with synbiotic or SCFA-producing bacteria
Lactobacillus reuteri, and synbiotic or SCFA-producing bacteria were found to restore
Lactobacillus abundance accompanying reduced
Enterobacteriaceae levels, resulting in enhanced survival. The mechanism may be that synbiotic biotics or short-chain fatty acid-producing bacteria can improve intestinal barrier function (e.g. ZO-1, Muc2), increase the expression of intestinal antibacterial genes (e.g. Lasozyme, TCF4), and promote immune response (e.g. TNFa, CD3g) in ALL mice. This research suggested that nonintestinal tumors affect the gut microbial ecosystem, and intestinal homeostasis can also be preserved by specific gut microorganisms that produce short chain fatty acids
[10].
It is well known that cell bioenergetics in malignant cells involve more glucose consumption than in normal somatic cells. To successfully compete for a limited amount of whole-body glucose, malignant cells need to reduce glucose availability in normal tissues such as adipose tissue and muscle, both of which consume a significant amount of glucose. This systematic rebalancing of biological processes is known as adaptive homeostatic, and similar biological processes have been reported previously, where stimulatory signals, such as aging or environmental stress, lead to an expansion or contraction of the homeostatic range.
[11][12]. However, the effects of intestinal microbes and tumor cells on glucose utilization are rarely reported. Haobin Ye et al. used 16S rRNA sequencing to find that in the feces of AML mice, SCFA-producing bacterias
Lachnospiraceae and
Bacteroidales S24-7 families were significantly reduced, and the abundance of
Anaerostipes, which generate butyric acid, was also decreased. Additionally, there was a significant decrease in SCFAs such as butyric acid and propionic acid in the stool samples of leukemic mice. Furthermore, leukemic mice were treated with butyrate or propionate to test its functional relevance, and whether intestinal epithelial integrity was partially restored or not was recorded. Strikingly, butyrate suppressed tumor burden in bone marrow and gonadal adipose tissue, and propionate suppressed tumor burden in gonadal adipose tissue. Additionally, butyrate and propionate treatment decreased serum IGFBP1 levels, increased insulin levels, and decreased plasma glucose concentrations. The results of this research indicated that leukemia cells hijack host glucose by inducing IGFBP1 production from adipose tissue to mediate insulin sensitivity and by inducing gut dysbiosis, serotonin loss, and incretin inactivation to suppress insulin secretion. Disrupting this adaptive homeostasis attenuates leukemia progression, and that SCFAs inhibit leukemia-induced adaptive homeostasis by affecting the integrity of the intestinal epithelium and regulating IGFBP1 as well as insulin levels
[13].
Briefly, the imbalance of intestinal microbiota may promote the development of leukemia by activating inflammatory factors and activating abnormal glucose metabolism. Loss of TET 2 causes gut microbial translocation that causes PMP development and may eventually lead to leukemogenesis. The absence of SCFAs producing bacteria promotes the progression of leukemia, and transplantation of such bacteria or treatment with SCFAs can significantly inhibit leukemia progression and improve survival (Figure 1). Interestingly, there is an observation that Lactobacillus reuteri has completely opposite functions in leukemogenesis and development, as well as completely opposite results.
Figure 1. The mechanism of intestinal microbes affecting the initiation and progression of leukemia.
1.2. The Treatment Prospect for Leukemia
The gut ecosystem is the largest and most complex micro-ecosystem in the human body. Homeostasis of the intestinal microenvironment plays a major role in maintaining normal physiological functions, and the imbalance of intestinal microecology has been considered to be an important factor affecting the occurrence and development of many diseases. Therefore, researchers may relieve disease symptoms or disease progression by the combination of drugs and interventions of the intestinal flora. By comparing the structure of intestinal flora in leukemia patients and healthy donors, studying the alterations in the structure of intestinal flora in leukemia patients will contribute to the early prevention and diagnosis of leukemia. At the same time, researchers can repair the unbalanced intestinal microbes by adding probiotics and adjusting the diet structure (prebiotics, SCFAs, etc.), to improve the treatment effect of leukemia and provide neoadjuvant therapy strategies in clinical care.
2. Lymphoma
2.1. Intestinal Microbial Imbalance and the Lymphomagenesis
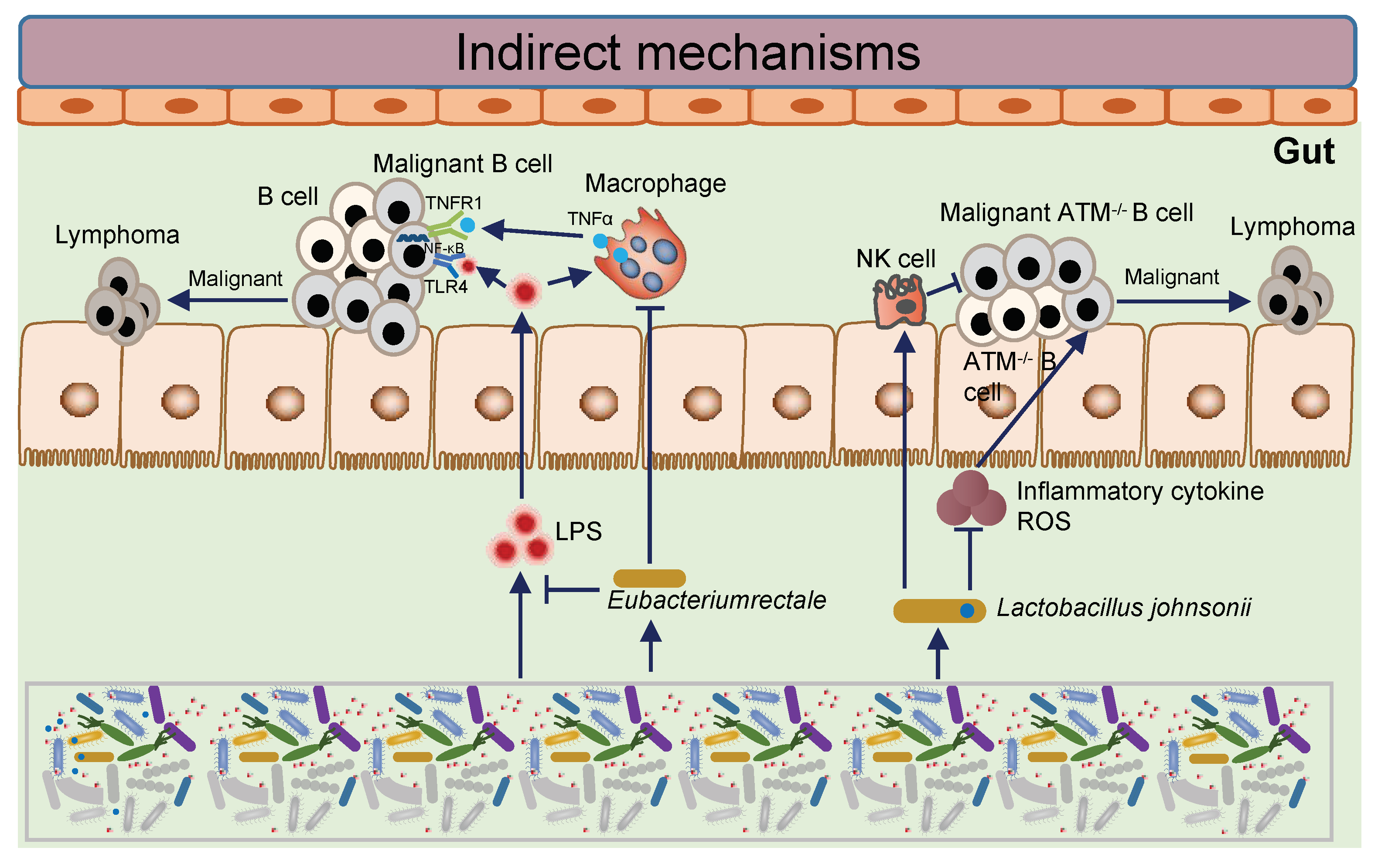
Microbe-induced tumorigenesis has been confirmed in a variety of gastrointestinal solid tumors, but was rarely studied in hematological malignancies, especially lymphoma. In order to determine the role of intestinal microbiota in the initiation and progression of lymphoma, metagenomic sequencing was performed by Haiyang Lu et al. for stool samples from patients with primary gastrointestinal lymphoma and healthy donors. The unique microbial characteristics of intestinal lymphoma were identified, with a significantly reduced amount of symbiotic microorganisms, notably
Eubacterium rectum, which produces butyrate. Furthermore, the author transferred the defective microbiota of
Eubacterium rectum from patients with intestinal lymphoma into mice and caused inflammation and the production of tumor necrosis factor (TNF). On the contrary, treatment with
Eubacterium rectum reduced TNF levels and lymphoma incidence rate in sensitized Eμ-Myc mice. In addition, lipopolysaccharide (LPS) in the resident microbiota of lymphoma patients and mice interacted with TNF signal and enhanced the NF-kB pathway through MyD88-dependent TLR4 signal, which also enhanced the survival and proliferation of intestinal B cells. However, in the GI tract, a
Eubacterium rectale-deficient gut microbiota stimulated B cells through enhanced TNF release and further sensitized B cells to LPS; these extracellular substances could bind to membrane TNFR1 and TLR4 and activate NF-kB signaling in a MyD88-dependent manner, as an extrinsic mechanism. These findings reveal the mechanism of inflammation-related lymphoma and the potential clinical principle of intestinal microbiota therapy targeting
[14].
The ataxia-telangiectasia (A-T) syndrome is autosomal recessive disorder with an increased likelihood of lymphoid malignancies
[15]. Neoplasia occurs in approximately 30–40% of all A-T patients
[16]: more than 40% of tumors are non-Hodgkin’s B cell lymphomas, about 20% acute lymphocytic leukemias, and 5% Hodgkin’s lymphomas
[17]. Biallelic mutations in the ATM gene cause A-T, and there have been over 600 different ATM mutations described. Using an A-T mouse model, Mitsuko L. Yamamoto et al. discovered that the intestinal microbiota is a major contributor to disease penetrance, lifespan, molecular oxidative stress, and systemic leukocyte toxicity by comparing lymphoma incidence in several isogenic mouse colonies harboring different bacterial communities. The relative abundance of
Lactobacillus johnsonii decresed in A-T mice by high-throughput sequencing of 16S rRNA. By restoring
Lactobacillus johnsonii to the cancer-prone mouse colony, short-term oral transfer was causally tested for the ability to confer reduced genotoxicity. The intervention decreased systemic genotoxicity, which is associated with a reduction in basal leucocytes and cytokine-mediated inflammation, and mechanistically linked to the host cell biology of systemic genotoxicity. According to these findings, the intestinal microbiota can serve as a potential target for translational interventions in individuals at risk for B cell lymphoma, or for other diseases that are driven by genotoxicity or the molecular response to oxidative stress
[18].
In conclusion, the abundance of gut microbiota can be used as a predictor of BSI in lymphoma patients, and the deletion of butyric acid-producing bacterium Eubacterium rectum may cause lymphoma, as well as the deficiency of Lactobacillus johnsonii may cause lymphoma in A-T mice (Figure 2). However, further research on gut microbes and lymphoma needs to be performed.
Figure 2. The mechanism of intestinal microbes affecting the initiation and progression of lymphoma.
2.2. The Treatment Prospect for Lymphoma
There are few studies on intestinal microbes in lymphoma, whether their microbial composition at initial diagnosis or after treatment. Lymphoma patients usually lack Eubacterium rectum in the intestine, leading to inflammation and lymphoma development. Lymphoma initiation can be reduced by supplementing Eubacterium rectum, whose absence can be used to indicate lymphomagenesis risks. In addition, in an A-T mouse model, the deficiency of Lactobacillus johnsonii may lead to the initiation of lymphoma. As a result of lymphoma disease characteristics, the immunity of most lymphoma patients is suppressed, resulting in infection and death in severe cases; therefore, clinical lymphoma patients will use prophylactic antibiotics, and the blind use of antibiotics may make the bad intestinal microbes worse, thereby affecting the survival of patients. The research results of Emmanuel Montassier et al. have good reference and guiding significance for solving this problem and for newly diagnosed patients to assess the risk of infection and guide them regarding the possible use prophylactic antibiotics. However, this method has defects. According to a prospective clinical study, a higher relative abundance of Bacteroides eggerthii, Ruminococcus lactaris, Eubacterium spp. CAG 180, Akkermansia muciniphila, and Erysipelatoclostridium ramosum increased the probability for CR prediction, whereas higher relative abundances of Bacteroides stercoris and others increased the probability for non-response prediction. The composition of intestinal microorganisms in lymphoma patients is not clear, and whether it is universal or whether the use of antibiotics will make the imbalance of intestinal microorganisms in patients more serious are urgent problems to be solved.
3. Multiple Myeloma
3.1. The Imbalance of Intestinal Flora Activates Th17 Cells to Promote the Process of Multiple Myeloma
Arianna Calcinotto et al. reported that in the mouse model Vk * MYC,
Prevotella hepatica promoted the differentiation of Th17 cells in the intestine and migrated to the bone marrow to secrete IL-17, thus promoting multiple myeloma (MM) progression, suggesting that commensal bacteria release a paracrine signaling network between innate and adaptive immunity to accelerate MM progression. It describes that B cells randomly acquire the characteristics of MM and migrate to the bone marrow after the activation of MYC, dependent on the germinal center. Meanwhile, a favorable cytokine environment induces Th17 differentiation and eosinophils (Eos) activation in the bone marrow niche, thus establishing a positive feedback loop of self-amplification and maintaining the progress of MM, and the specific intestinal microbes are conducive to the differentiation of Th17 cells, which migrate to bone marrow and further promote the eos-Th17-MM cell network
[19].
3.2. Alterations of Gut Microbiome Accelerate Multiple Myeloma Progression by Increasing the Relative Abundances of Nitrogen-Recycling Bacteria
Xingxing Jian et al. reported that the abnormality of amino acid in the bone marrow microenvironment of the MM and a large number of light chain proteins cause renal tubular damage, resulting in the accumulation of blood urea, and the proliferation of nitrogen-recycling bacteria. On the contrary, nitrogen-recycling bacteria cause urea degradation, glutamine synthesis and absorption by the MM cells, thus accelerating the tumor progression. This research revealed a new mechanism of intestinal flora alteration in accelerating MM progression, suggesting that the intervention of the intestinal flora in MM patients may help improve traditional tumor therapy [20].
In conclusion, gut microbes can promote MM progression through mechanisms such as metabolic reprogramming and immune activation, and gut microbial abundance may provide new insights into MM progression (Figure 3).
Figure 3. The mechanism of intestinal microbes affecting the progression of multiple myeloma.
3.3. The Treatment Prospect for Multiple Myeloma
Currently, there are few reports on the relationship between MM and intestinal microbes, which may be related to its low incidence and the difficulty in collecting patient specimens. The accumulation of urea in MM patients led to the increase of nitrogen-recycling bacteria, and the amino acids synthesized by nitrogen-recycling bacteria were used by MM cells to promote the MM progression, and MM development was been inhibited when targeted the nitrogen-recycling bacteria Klebsiella pneumoniae glutamine synthetase gene. The MM progression effects of gut microbes and metabolites were examined for the first time, and the gut microbial composition and metabolic situation of MM patients were investigated, which has constructive significance and provides a neoadjuvant for the diagnosis and treatment of MM. Arianna Calcinotto et al. reported that in Vk * MYC mice, Prevotella hepatica promoted the progress of MM by promoting the differentiation of Th17 cells in the intestine and migrating to the bone marrow, which has guiding significance for clinical treatment of MM. Matthew J. Pianko et al. suggested that Eubacterium hallii acts as an indicator for MRD negativity. The above research results suggest that MM may be treated with drugs targeted at a certain microbe or a combination of dietary management and chemotherapy drugs, and the curative effect and prognosis of MM can be predicted according to the abundance of a certain microbe, so as to improve the accuracy of diagnosis and reduce the medical expenses of patients. Therefore, it may be a worthwhile research project for treating MM with intestinal flora as the target.