
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carolyne Kipkoech | -- | 2196 | 2023-05-02 15:13:29 | | | |
| 2 | Dean Liu | -1 word(s) | 2195 | 2023-05-04 03:39:42 | | |
Video Upload Options
The consumption of insects as an alternative protein source is acceptable as a sustainable alternative to mainstream protein sources. Apart from containing a high protein content, insects also have dietary fiber in the form of chitin, which helps to enrich gut microbiota. The importance of the gut microbiome in general health has recently been underlined for humans, farm animals, pets, poultry, and fish. The advances in 16S RNA techniques have enabled the examination of complex microbial communities in the gastrointestinal tract, shedding more light on the role of diet in disease and immunity. The gut microbiome generates signals influencing the normal nutritional status, immune functions, metabolism, disease, and well-being. The gut microbiome depends on dietary fiber; hence, their diversity is modulated by diet, a relevant factor in defining the composition of gut microbiota. Small shifts in diet have demonstrated an enormous shift in gut microbiota. Edible insects are an excellent source of protein, fat, and chitin that could influence the gut microbiota as a prebiotic. Chitin from insects, when consumed, contributes to a healthy gut microbiome by increasing diversity in fecal microbiota. Moreover, a high fiber intake has been associated with a reduced risk of breast cancer, diverticular disease, coronary heart disease, and metabolic syndrome.
1. Introduction
2. Edible Insects
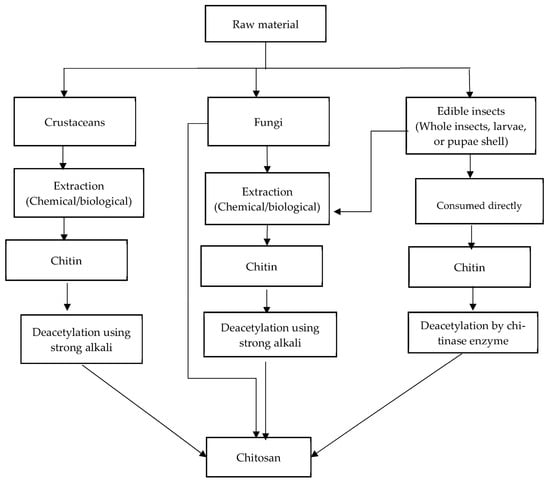
3. Chitin and Chitosan as a Dietary Fiber
References
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823.
- Van Huis, A.; Rumpold, B.; van der Fels-Klerx, H.; Tomberlin, J. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948.
- Sogari, G.; Riccioli, F.; Moruzzo, R.; Menozzi, D.; Sosa, D.A.T.; Li, J.; Liu, A.J.; Mancini, S. Engaging in entomophagy: The role of food neophobia and disgust between insect and non-insect eaters. Food Qual. Prefer. 2023, 104, 104764.
- European Commission. Authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022, 7.
- Gasco, L.; Jozefiak, A.; Henry, M. Beyond the protein concept: Health aspects of using edible insects on animals. J. Insects Food Feed 2021, 7, 715–741.
- Aguilar-Toala, J.E.; Cruz-Monterrosa, R.G.; Liceaga, A.M. Beyond Human Nutrition of Edible Insects: Health Benefits and Safety Aspects. Insects 2022, 13, 1007.
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305.
- van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576.
- Lumanlan, J.C.; Williams, M.; Jayasena, V. Edible insects: Environmentally friendly sustainable future food source. Int. J. Food Sci. Technol. 2022, 57, 6317–6325.
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2020, 15, 123013.
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036.
- Ramos-Bueno, R.P.; Gonzalez-Fernandez, M.J.; Sanchez-Muros-Lozano, M.J.; Garcia-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477.
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403.
- Slocinska, M.; Marciniak, P.; Rosinski, G. Insects antiviral and anticancer peptides: New leads for the future? Protein Pept. Lett. 2008, 15, 578–585.
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155.
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Tech. 1999, 10, 37–51.
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Basri, S.M.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828.
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64.
- Ma, J.; Faqir, Y.; Tan, C.; Khaliq, G. Terrestrial insects as a promising source of chitosan and recent developments in its application for various industries. Food Chem. 2022, 373, 131407.
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795.
- Kipkoech, C.; Kinyuru, J.; Imathiu, S.; Roos, N. Use of house cricket to address food security in Kenya: Nutrient and chitin composition of farmed crickets as influenced by age. Afr. J. Agric. Res. 2017, 12, 3189–3197.
- Garino, C.; Mielke, H.; Knuppel, S.; Selhorst, T.; Broll, H.; Braeuning, A. Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor). Food Chem. Toxicol. 2020, 142, 111460.
- Derrien, C.; Boccuni, A. Current status of the insect producing industry in Europe. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 471–479.
- European Commission. Approval of chitosan hydrochloride as basic substance in accordance with Regulation (EC) No 1107/2009; European Commission, Ed.; European Commission, European Union: Washington, DC, USA, 2014; pp. 5–7.
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; Franco, L.D.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290.
- Kemboi, V.J.; Kipkoech, C.; Njire, M.; Were, S.; Lagat, M.K.; Ndwiga, F.; Wesonga, J.M.; Tanga, C.M. Biocontrol Potential of Chitin and Chitosan Extracted from Black Soldier Fly Pupal Exuviae against Bacterial Wilt of Tomato. Microorganisms 2022, 10, 165.
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A.; Danmaigoro, A.; Nicholas, N.P. Gut health and serum growth hormone levels of broiler chickens fed dietary chitin and chitosan from cricket and shrimp. Poult. Sci. 2019, 98, 745–752.
- Kamilya, D.; Khan, M.I.R. Chitin and chitosan as promising immunostimulant for aquaculture. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 761–771.
- Zhang, J.; Wan, J.; Wu, G.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Low-molecular-weight chitosan relieves enterotoxigenic Escherichia coli-induced growth retardation in weaned pigs. Int. Immunopharmacol. 2020, 78, 105798.
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269.
- Gong, T.; Zhou, Y.; Zhang, L.; Wang, H.; Zhang, M.; Liu, X. Capsaicin combined with dietary fiber prevents high-fat diet associated aberrant lipid metabolism by improving the structure of intestinal flora. Food Sci. Nutr. 2023, 11, 114–125.
- Abbott, A. Scientists bust myth that our bodies have more bacteria than human cells. Nat. News 2016.
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782.
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627.
- Maukonen, J.; Saarela, M. Human gut microbiota: Does diet matter? Proc. Nutr. Soc 2015, 74, 23–36.
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of mediterranean diet on human gut microbiota. Nutrients 2021, 13, 7.
- Rio-Aige, K.; Azagra-Boronat, I.; Massot-Cladera, M.; Selma-Royo, M.; Parra-Llorca, A.; Gonzalez, S.; Garcia-Mantrana, I.; Castell, M.; Rodriguez-Lagunas, M.J.; Collado, M.C.; et al. Association of Maternal Microbiota and Diet in Cord Blood Cytokine and Immunoglobulin Profiles. Int. J. Mol. Sci. 2021, 22, 1778.
- Teng, N.M.Y.; Price, C.A.; McKee, A.M.; Hall, L.J.; Robinson, S.D. Exploring the impact of gut microbiota and diet on breast cancer risk and progression. Int. J. Cancer 2021, 149, 494–504.
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285.
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The gut microbiota in internal medicine: Implications for health and disease. Neth. J. Med. 2015, 73, 61–68.
- Lee, Y.K. Effects of diet on gut microbiota profile and the implications for health and disease. Biosci. Microbiota Food Health 2013, 32, 1–12.
- Walker, W. Dysbiosis. In The Microbiota in Gastrointestinal Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 227–232.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232.
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749.
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22.
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85.
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148.
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66.




