Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lei, P.; Zhang, W.; Ma, J.; Xia, Y.; Yu, H.; Du, J.; Fang, Y.; Wang, L.; Zhang, K.; Jin, L.; et al. Utilization of Zebrafish Nano-/Microparticles Toxicity in Water. Encyclopedia. Available online: https://encyclopedia.pub/entry/43621 (accessed on 07 February 2026).
Lei P, Zhang W, Ma J, Xia Y, Yu H, Du J, et al. Utilization of Zebrafish Nano-/Microparticles Toxicity in Water. Encyclopedia. Available at: https://encyclopedia.pub/entry/43621. Accessed February 07, 2026.
Lei, Pengyu, Wenxia Zhang, Jiahui Ma, Yuping Xia, Haiyang Yu, Jiao Du, Yimeng Fang, Lei Wang, Kun Zhang, Libo Jin, et al. "Utilization of Zebrafish Nano-/Microparticles Toxicity in Water" Encyclopedia, https://encyclopedia.pub/entry/43621 (accessed February 07, 2026).
Lei, P., Zhang, W., Ma, J., Xia, Y., Yu, H., Du, J., Fang, Y., Wang, L., Zhang, K., Jin, L., Zhong, J., & Sun, D. (2023, April 28). Utilization of Zebrafish Nano-/Microparticles Toxicity in Water. In Encyclopedia. https://encyclopedia.pub/entry/43621
Lei, Pengyu, et al. "Utilization of Zebrafish Nano-/Microparticles Toxicity in Water." Encyclopedia. Web. 28 April, 2023.
Copy Citation
A large amount of nano-/microparticles (MNPs) are released into water, not only causing severe water pollution, but also negatively affecting organisms. Therefore, it is crucial to evaluate MNP toxicity and mechanisms in water. There is a significant degree of similarity between the genes, the central nervous system, the liver, the kidney, and the intestines of zebrafish and the human body.
zebrafish
nano-/microparticles
toxicity evaluation
1. Introduction
Due to the continuous development of human activities, plastic products have become an integral part of our daily lives. However, with large quantities of plastic waste being discharged into water bodies, the global environment is expected to release 33 billion tons plastics of by 2050. In the course of environmental evolution, it will gradually decompose into tiny plastic particles called microplastics (MPs) (100–5000 nm) and nanoplastics (NPs) (<100 nm) [1][2]. Among the most common nano-/microparticles (MNPs) are polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET) [3]. Due to the small size, large surface area, strong adsorption capacity, and permeability of MNPs, their distribution and action mode in water are different from traditional organic pollutants [4][5][6][7]. High persistence and extreme distribution are unique characteristics of MNPs compared to other polluting suspended substances in the environment. As a result, MNPs are potentially harmful to freshwater and marine ecosystems [8][9]. Plastic debris has already caused an estimated EU 21 billion of severe economic damage in the world’s oceans. At the same time, due to their high surface area and hydrophobicity, MNPs can act as carriers of pathogens and organic pollutants, together with which they aggravate toxicity [10][11][12][13]. In addition, MNPs can enter the food chain circulation directly or indirectly, causing metabolic disorders and intestinal microbiota disorders in the human body. In addition, they cause neurotoxicity, reproductive toxicity, immunotoxicity, etc., which constitute serious threats to human health (Figure 1) [11][14][15].
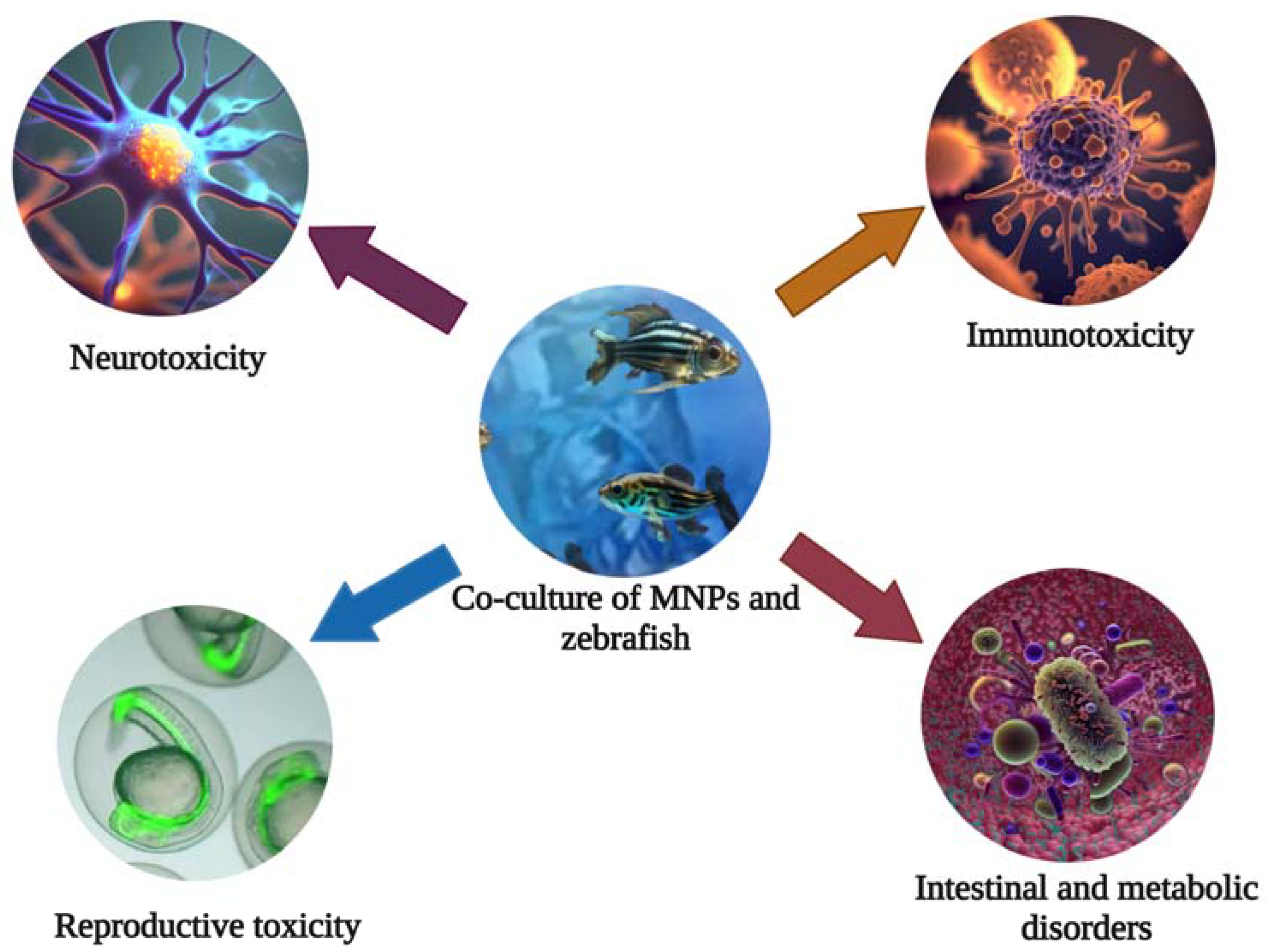
Figure 1. The zebrafish can be used for toxicity evaluation and mechanistic studies of the nervous, reproductive, and immune systems of MNPs.
Therefore, to assess the toxicity of MNPs in water, many toxicity evaluation methods have been developed, including in vitro and in vivo methods. In vitro tests include chemical analysis and cytotoxicity tests [16][17]. These methods have limitations such as not reflecting real environmental conditions and complicated operation, and are time-consuming and laborious. A variety of animal models have been used to evaluate MNP toxicity in vivo, including mice, rats, rabbits, guinea pigs, and fish [18][19][20][21]. The zebrafish is a small freshwater fish. (I) Because of its small size, vitality, and reproductive ability, it is easy to raise and manage [22]; (II) its short life cycle, from fertilized egg to adult in about three months, allows for rapid large-scale experiments [23]; (III) Zebrafish larvae are transparent, and the development process of their internal structures and organs can be directly observed through a microscope. Therefore, they emerge in the field of toxicity evaluation of pollutants such as MNPs, heavy metals, pharmaceuticals, and algae in water [24].
2. Reliability of the Zebrafish for Toxicity Evaluation
Traditionally, mammals have been used to assess toxicity. Although the mammalian model has many advantages, it also has a number of disadvantages, such as its high cost, lengthy process time, and ethical and moral issues. Conversely, zebrafish are known for their fast population growth, short reproductive cycle, ease of experimental operation, high survival rate, and low feeding and maintenance costs [25][26]. Figure 2 illustrates the main advantages of using zebrafish in MNP research MNPs. These advantages include: (I) the genome of zebrafish has been completely sequenced, and the gene similarity to humans is as high as 87%. In addition, the pathological state of many diseases and genes related to disease etiology are highly conserved in humans [27][28]; (II) The transparent nature of zebrafish embryos and larvae provides an experimental advantage compared to other model organisms for studying the accumulation sites of fluorescence-labeled MNPs particles. In addition, transgenic zebrafish strains have become effective biological models for studying metabolic and immune diseases [29][30]; (III) The blood–brain barrier of zebrafish is similar to that of humans, and has been well used for central-nervous-system drug screening [31]; (IV) The nervous system of the zebrafish, which includes the central nervous system and the peripheral nervous system, has social behaviors similar to human perception, movement, and emotion. Zebrafish have become widely used models in behavioral neuroscience, especially as disease models for Parkinson’s disease (PD), Alzheimer’s disease (AD), and depression [32][33][34]; (V) Zebrafish have similar metabolic organs and physiological structures to humans, such as the liver, kidney, and intestine. These organs can metabolize and eliminate harmful substances as shown in Figure 2 [35][36][37].
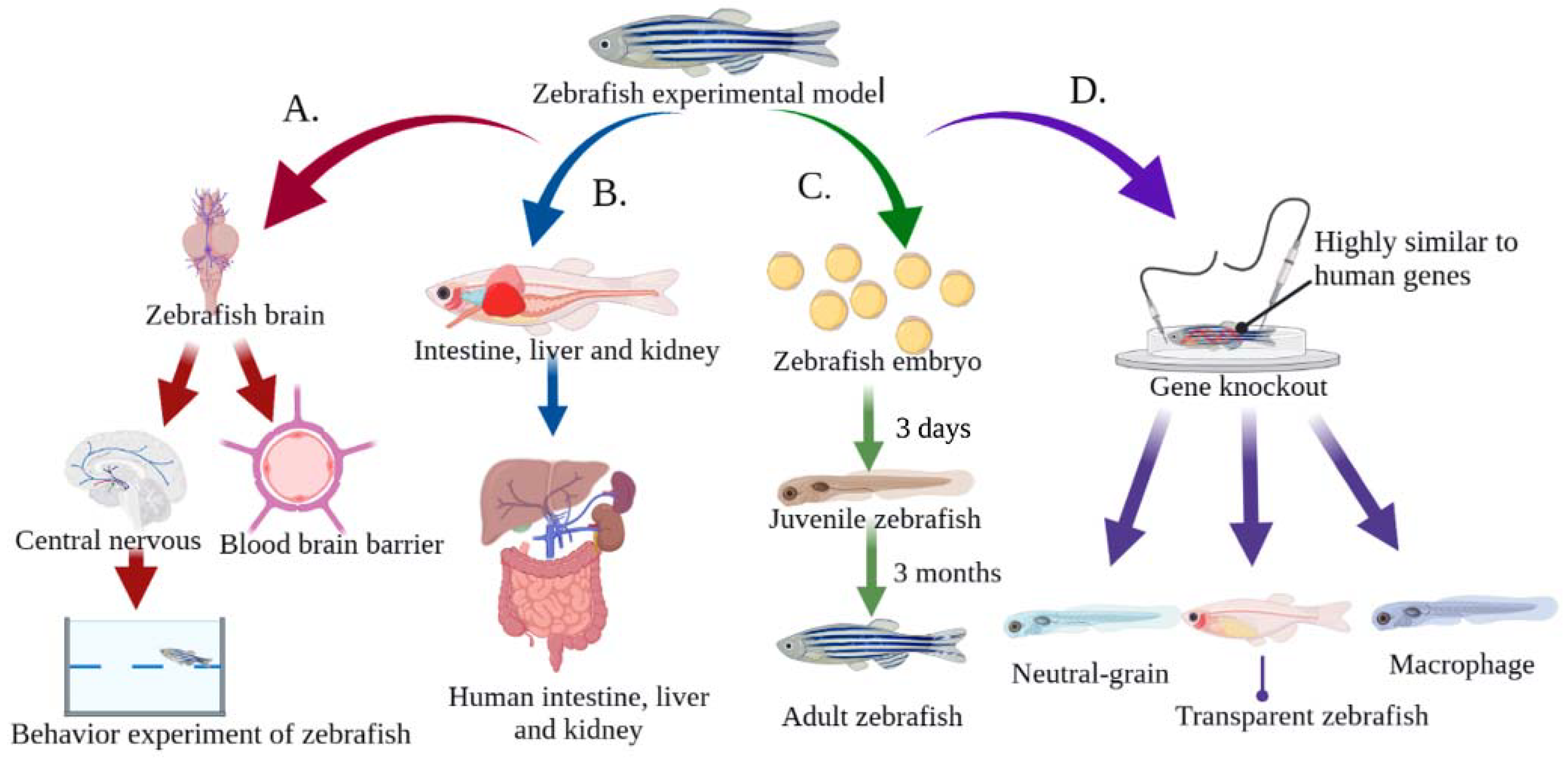
Figure 2. Advantages of zebrafish for toxicity evaluation of MNPs. (A) The blood–brain barrier, central nervous system, and social behavior are similar to those of humans, making it an ideal animal model for studying neurotoxicity; (B) The intestine, liver and kidney are similar to those of humans, which makes them suitable for studying MNPs metabolism and immune diseases; (C) The reproductive and developmental toxicity of MNPs can be easily studied due to the short reproductive cycle of zebrafish and the large number of eggs laid. In addition, the embryos are transparent, so a microscope can be used to observe the cell division and organ formation process; (D) The genome of zebrafish has been fully sequenced, and is highly consistent with the human genome, and can be easily manipulated by genetic manipulation such as gene knockout and gene overexpression.
Zebrafish are also being used in toxicity assessment, according to many studies. Zebrafish have been shown to exhibit physiological responses and behavioral abnormalities as a result of exposure to environmental pollutants that are similar to those of mammals. As a result, zebrafish have been widely utilized in toxicity assessments, drug screening, and other fields, demonstrating their reliability for evaluating the biological effects of MNPs.
3. Toxicity of MNPs in Water to Zebrafish
Currently, the zebrafish used to evaluate MNP biotoxicity are manipulated by the exposure method [38][39], injecting MNPs directly into the aquaculture water or adding MNPs to the feed, and then exposing the zebrafish to the suspension for a specified period of time. Zebrafish behavior and physiological indicators can be observed during this period. As shown in Figure 3, acute toxicity of MNPs to zebrafish was evaluated by observing changes in growth, reproduction, behavior, and physiology. In addition, water-flow can also control MNP concentration and time to simulate dynamic MNP exposure environments.
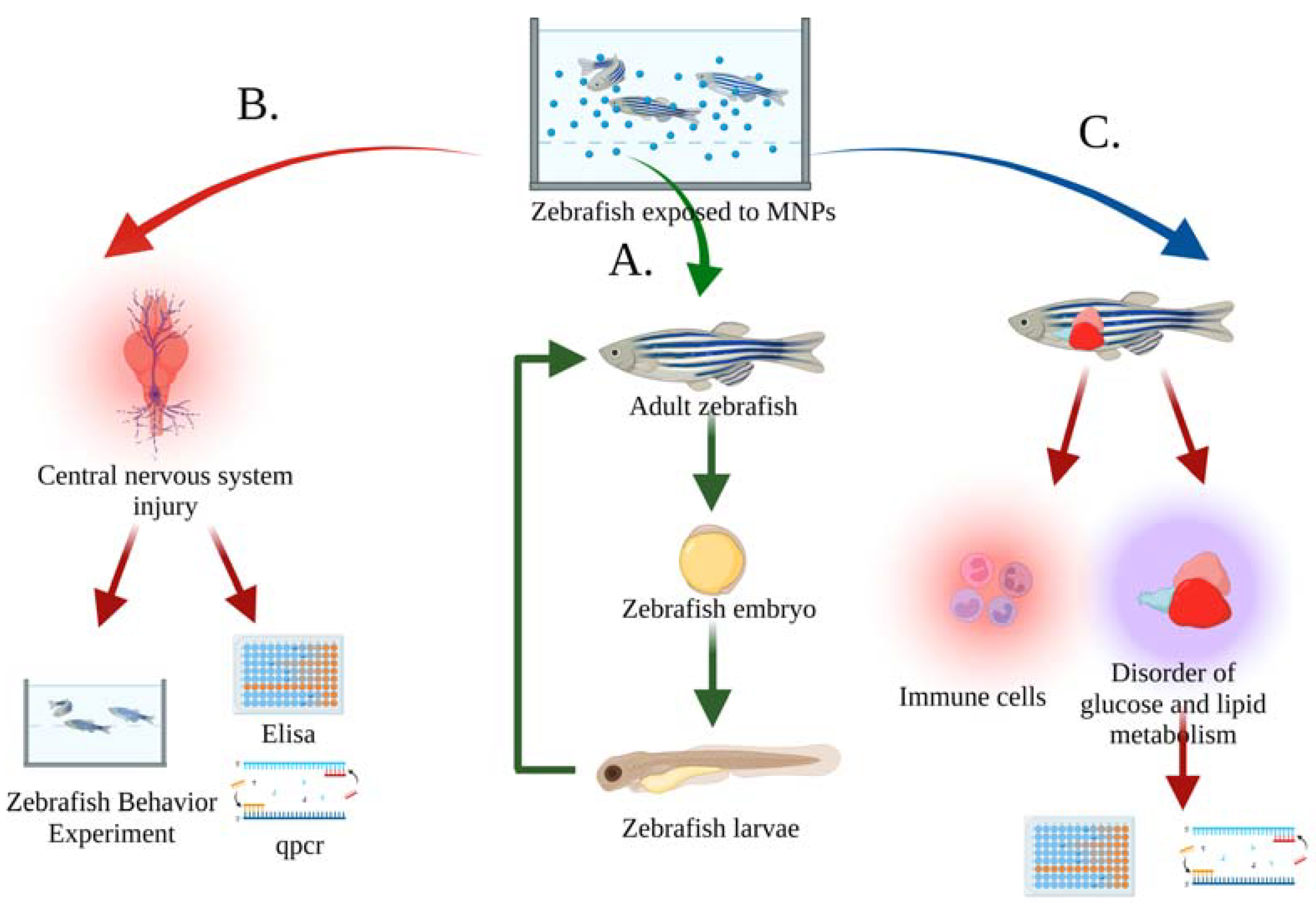
Figure 3. Evaluation index of MNP toxicity in zebrafish in a water body. (A) Evaluation of the toxicity of MNPs on growth and reproduction by the damage and apoptosis of sperm, testis, and oocytes of adult zebrafish, as well as the increased rate of malformation and mortality of embryos [40][41][42]; (B) Evaluation of the behavioral and neurological toxicity of MNPs by increasing oxidative-stress level and apoptosis in the zebrafish brain, as well as behavioral experiments to detect memory, learning, and mental disorders in zebrafish [43][44][45]; (C) Evaluation of the toxicity of MNPs on the metabolism and immune system by the upregulation of immune-related gene expression and apoptosis, as well as the reduction of hepatic glucose and lipid metabolism, glucose, α-ketoglutarate, and lipid-related indicators [46][47][48].
3.1. Growth and Reproduction of Zebrafish
Exposure of zebrafish to MNPs in aqueous solution has adverse effects on the growth and reproduction of zebrafish: (I) It may cause damage to the chorionic membrane of zebrafish embryos or changes in water quality (such as hypoxia induction) [41], which may lead to premature hatching of embryos, and their inability to survive for a long time, resulting in an increase in the mortality of larvae [49]; (II) Zebrafish larvae were unable to survive for long in an aqueous solution containing 2 mg/mL MNPs, and their mortality rate increased to 32.4%; (III) Exposed larvae displayed edema of the yolk sac and pericardium, curvature of the spine, curvature of the tail, and a larger area of visual vesicles; (IV) When zebrafish embryos were exposed to 100 ppm of PET-NPs in aqueous solution, the survival rate decreased to 65%, while at 200 ppm the survival rate was almost zero [50]; (V) A previous study exposed adult zebrafish to PS-NPs for one month, and a large accumulation of PS-NPs, as well as an increase in reactive oxygen species (ROS) was observed in the gonads and liver of the zebrafish [51]. It was found that high concentrations of MNPs resulted in apoptosis of male zebrafish testis, as well as a significant reduction in basement-membrane thickness, resulting in oxidative stress to female zebrafish oocytes, leading to apoptosis [40], and affecting egg morphology and yolk area of zebrafish offspring, resulting in malformations [52]; (VI) Upon exposure to PE-MPs in water for 15 days, adult zebrafish accumulated a large amount of PE-MP in their bodies, causing DNA damage to red blood cells and nuclear abnormalities, which may lead to genetic damage. In addition, the activities of superoxide dismutase (SOD) and catalase (CAT) decreased, resulting in an imbalance in the redox state [53].
3.2. The Behavior and Nervous System of Zebrafish
According to studies, MNPs can cross the blood–brain barrier [54], and they accumulate in large amounts in the heads of zebrafish, causing an increase in oxidative stress levels and apoptosis in the brain [43][44], in addition to a decrease in the activity of acetylcholinesterase (AChE) in the brain of the zebrafish (the activity regulates brain function and is considered a biomarker of neurotoxicity in zebrafish), as well as inhibition of the synthesis of neurotransmitters (such as dopamine, melatonin, aminobutyric acid, and 5-hydroxytryptamine) [55][56], causing serious harm to the nervous system and inducing neurobehavioral disorders [57]. One study found that zebrafish swimming behavior and range of motion were significantly reduced when exposed to PC-MPs and PE-MPs in water. There were also emetic and electrophysiology abnormalities, learning and memory problems [58][59], and severe epileptic behaviors [45]. Meanwhile, avoidance and anxiety behaviors were observed in zebrafish larvae [60]. The above studies suggest that MNP exposure may negatively affect the nervous system and behavior of zebrafish. This provides a rapid method for assessing MNP toxic effects on aquatic organisms’ nervous systems and behavior.
3.3. Metabolism and Immune System of Zebrafish
MNPs are capable of crossing biological barriers and entering the circulatory system, where they reduce energy reserves through mechanical destruction, ultimately affecting the immune system [61]. (I) It was found that zebrafish exposed to MNPs had significant increases in mRNA and protein levels of genes associated with the innate immune system (interleukin-1α (IL-1α), IL-1β, nuclear factor-κb (NF-κb), and interferon) and apoptosis (casp3A and BCL2) [62]. Moreover, the number of white blood cells decreased, the accumulation of neutrophils in intestinal epithelium increased, and the immune function was abnormal [63]. At the same time, the metabolic system of zebrafish was adversely affected, resulting in abnormal liver and intestinal metabolism; (II) Zebrafish embryos exposed to PE-MPs showed an interference in the metabolism of triglycerides, total cholesterol, non-esterified fatty acids, total bile acids, glucose, and pyruvate; (III) When adult male zebrafish were exposed for 21 days to PS-MPs, a significant reduction in body weight was observed as well as a reduction in transcription levels associated with glucose and lipid metabolism in the liver. Furthermore, glucose, pyruvate, and alpha-ketoglutaric acid levels in the liver decreased. The liver also decreased in genes related to fatty-acid metabolism and amino-acid metabolism; (IV) In adult zebrafish exposed for 21 days to aqueous solutions of PE-MPs and PP-MPs, MPs induced oxidative stress, resulting in lipid peroxidation as well as stimulation of autophagy and apoptosis signal transduction pathways [64][65]. In combination, exposure to MNPs disrupts the immune system and glucose and lipid metabolism in zebrafish [46].
References
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455.
- Banerjee, A.; Shelver, W.L. Micro- and nanoplastic induced cellular toxicity in mammals: A review. Sci. Total Environ. 2021, 755, 142518.
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29.
- Yang, H.; He, Y.; Yan, Y.; Junaid, M.; Wang, J. Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere. Nanomaterials 2021, 11, 2747.
- Sarkar, B.; Dissanayake, P.D.; Bolan, N.S.; Dar, J.Y.; Kumar, M.; Haque, M.N.; Mukhopadhyay, R.; Ramanayaka, S.; Biswas, J.K.; Tsang, D.C.W.; et al. Challenges and opportunities in sustainable management of microplastics and nanoplastics in the environment. Environ. Res. 2022, 207, 112179.
- Lei, P.; Chen, H.; Ma, J.; Fang, Y.; Qu, L.; Yang, Q.; Peng, B.; Zhang, X.; Jin, L.; Sun, D. Research progress on extraction technology and biomedical function of natural sugar substitutes. Front. Nutr. 2022, 9, 952147.
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic Mechanism and Biological Detoxification of Fumonisins. Toxins 2022, 14, 182.
- Zhang, B.; Chao, J.; Chen, L.; Liu, L.; Yang, X.; Wang, Q. Research progress of nanoplastics in freshwater. Sci. Total Environ. 2021, 757, 143791.
- Horton, A.A. Plastic pollution: When do we know enough? J. Hazard. Mater. 2022, 422, 126885.
- Lionetto, F.; Corcione, C.E.; Rizzo, A.; Maffezzoli, A. Production and Characterization of Polyethylene Terephthalate Nanoparticles. Polymers 2021, 13, 3745.
- Menon, V.; Sharma, S.; Gupta, S.; Ghosal, A.; Nadda, A.K.; Jose, R.; Sharma, P.; Kumar, S.; Singh, P.; Raizada, P. Prevalence and implications of microplastics in potable water system: An update. Chemosphere 2023, 317, 137848.
- Liu, Z.; Xia, X.; Lv, X.; Song, E.; Song, Y. Iron-bearing nanoparticles trigger human umbilical vein endothelial cells ferroptotic responses by promoting intracellular iron level. Environ. Pollut. 2021, 287, 117345.
- Liu, Z.; Lv, X.; Yang, B.; Qin, Q.; Song, E.; Song, Y. Tetrachlorobenzoquinone exposure triggers ferroptosis contributing to its neurotoxicity. Chemosphere 2021, 264, 128413.
- Zhang, Q.; He, Y.; Cheng, R.; Li, Q.; Qian, Z.; Lin, X. Recent advances in toxicological research and potential health impact of microplastics and nanoplastics in vivo. Environ. Sci. Pollut. Res. Int. 2022, 29, 40415–40448.
- Bhat, M.A.; Gedik, K.; Gaga, E.O. Atmospheric micro (nano) plastics: Future growing concerns for human health. Air Qual. Atmosphere Health 2023, 16, 233–262.
- Huang, J.; Dong, G.; Liang, M.; Wu, X.; Xian, M.; An, Y.; Zhan, J.; Xu, L.; Xu, J.; Sun, W.; et al. Toxicity of micro(nano)plastics with different size and surface charge on human nasal epithelial cells and rats via intranasal exposure. Chemosphere 2022, 307, 136093.
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene nanoplastics alter the cytotoxicity of human pharmaceuticals on marine fish cell lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65.
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic-Mediated Pathophysiological Changes in Rodents, Rabbits, and Chickens: A Review. J. Food Prot. 2021, 84, 1480–1495.
- Lim, D.; Jeong, J.; Song, K.S.; Sung, J.H.; Oh, S.M.; Choi, J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere 2021, 262, 128330.
- Ma, C.; Chen, Q.; Li, J.; Li, B.; Liang, W.; Su, L.; Shi, H. Distribution and translocation of micro- and nanoplastics in fish. Crit. Rev. Toxicol. 2021, 51, 740–753.
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public. Health 2020, 17, 1509.
- Chen, J.; Kong, A.; Shelton, D.; Dong, H.; Li, J.; Zhao, F.; Bai, C.; Huang, K.; Mo, W.; Chen, S.; et al. Early life stage transient aristolochic acid exposure induces behavioral hyperactivity but not nephrotoxicity in larval zebrafish. Aquat. Toxicol. 2021, 238, 105916.
- Lin, B.; Ma, J.; Fang, Y.; Lei, P.; Wang, L.; Qu, L.; Wu, W.; Jin, L.; Sun, D. Advances in Zebrafish for Diabetes Mellitus with Wound Model. Bioengineering 2023, 10, 330.
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707.
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 163, 5–12.
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417.
- Clément, Y.; Torbey, P.; Gilardi-Hebenstreit, P.; Crollius, H.R. Enhancer-gene maps in the human and zebrafish genomes using evolutionary linkage conservation. Nucleic Acids Res. 2020, 48, 2357–2371.
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503.
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzopyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930.
- Trevisan, R.; Voy, C.; Chen, S.; Di Giulio, R.T. Nanoplastics Decrease the Toxicity of a Complex PAH Mixture but Impair Mitochondrial Energy Production in Developing Zebrafish. Environ. Sci. Technol. 2019, 53, 8405–8415.
- Li, Y.; Chen, T.; Miao, X.; Yi, X.; Wang, X.; Zhao, H.; Lee, S.M.-Y.; Zheng, Y. Zebrafish: A promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood-brain barrier. Pharmacol. Res. 2017, 125, 246–257.
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75.
- Otsuna, H.; Hutcheson, D.A.; Duncan, R.N.; McPherson, A.D.; Scoresby, A.N.; Gaynes, B.F.; Tong, Z.; Fujimoto, E.; Kwan, K.M.; Chien, C.-B.; et al. High-resolution analysis of central nervous system expression patterns in zebrafish Gal4 enhancer-trap lines. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2015, 244, 785–796.
- Cole, G.J.; Zhang, C.; Ojiaku, P.; Bell, V.; Devkota, S.; Mukhopadhyay, S. Effects of ethanol exposure on nervous system development in zebrafish. Int. Rev. Cell Mol. Biol. 2012, 299, 255–315.
- Goessling, W.; Sadler, K.C. Zebrafish: An important tool for liver disease research. Gastroenterology 2015, 149, 1361–1377.
- Gao, Y.; Jin, Q.; Gao, C.; Chen, Y.; Sun, Z.; Guo, G.; Peng, J. Unraveling Differential Transcriptomes and Cell Types in Zebrafish Larvae Intestine and Liver. Cells 2022, 11, 3290.
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92.
- Sulukan, E.; Şenol, O.; Baran, A.; Kankaynar, M.; Yıldırım, S.; Kızıltan, T.; Bolat, İ.; Ceyhun, S.B. Nano-sized polystyrene plastic particles affect many cancer-related biological processes even in the next generations; zebrafish modeling. Sci. Total. Environ. 2022, 838, 156391.
- Zhang, R.; Silic, M.R.; Schaber, A.; Wasel, O.; Freeman, J.L.; Sepúlveda, M.S. Exposure route affects the distribution and toxicity of polystyrene nanoplastics in zebrafish. Sci. Total. Environ. 2020, 724, 138065.
- Chatterjee, A.; Maity, S.; Banerjee, S.; Dutta, S.; Adhikari, M.; Guchhait, R.; Biswas, C.; De, S.; Pramanick, K. Toxicological impacts of nanopolystyrene on zebrafish oocyte with insight into the mechanism of action: An expression-based analysis. Sci. Total. Environ. 2022, 830, 154796.
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total. Environ. 2018, 643, 324–334.
- Malafaia, G.; de Souza, A.M.; Pereira, A.C.; Gonçalves, S.; da Costa Araújo, A.P.; Ribeiro, R.X.; Rocha, T.L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total. Environ. 2020, 700, 134867.
- Teng, M.; Zhao, X.; Wu, F.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Yan, S.; Tian, S. Charge-specific adverse effects of polystyrene nanoplastics on zebrafish (Danio rerio) development and behavior. Environ. Int. 2022, 163, 107154.
- Aliakbarzadeh, F.; Rafiee, M.; Khodagholi, F.; Khorramizadeh, M.R.; Manouchehri, H.; Eslami, A.; Sayehmiri, F.; Mohseni-Bandpei, A. Adverse effects of polystyrene nanoplastic and its binary mixtures with nonylphenol on zebrafish nervous system: From oxidative stress to impaired neurotransmitter system. Environ. Pollut. 2023, 317, 120587.
- Mak, C.W.; Ching-Fong Yeung, K.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442.
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of polyethylene microplastics on the microbiome and metabolism in larval zebrafish. Environ. Pollut. 2021, 282, 117039.
- Cheng, H.; Duan, Z.; Wu, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Zhang, H.; Wei, Y.; Sun, H. Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae. Environ. Int. 2022, 161, 107128.
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279.
- Sökmen, T.Ö.; Sulukan, E.; Türkoğlu, M.; Baran, A.; Özkaraca, M.; Ceyhun, S.B. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology 2020, 77, 51–59.
- Bashirova, N.; Poppitz, D.; Klüver, N.; Scholz, S.; Matysik, J.; Alia, A. A mechanistic understanding of the effects of polyethylene terephthalate nanoplastics in the zebrafish (Danio rerio) embryo. Sci. Rep. 2023, 13, 1891.
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410.
- Tarasco, M.; Gavaia, P.J.; Bensimon-Brito, A.; Cordelières, F.P.; Santos, T.; Martins, G.; de Castro, D.T.; Silva, N.; Cabrita, E.; Bebianno, M.J.; et al. Effects of pristine or contaminated polyethylene microplastics on zebrafish development. Chemosphere 2022, 303, 135198.
- Araújo, A.P.d.C.; da Luz, T.M.; Rocha, T.L.; Ahmed, M.A.I.; e Silva, D.D.D.M.; Rahman, M.M.; Malafaia, G. Toxicity evaluation of the combination of emerging pollutants with polyethylene microplastics in zebrafish: Perspective study of genotoxicity, mutagenicity, and redox unbalance. J. Hazard. Mater. 2022, 432, 128691.
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194.
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total. Environ. 2017, 609, 1312–1321.
- Lin, J.; Xiao, Y.; Liu, Y.; Lei, Y.; Cai, Y.; Liang, Q.; Nie, S.; Jia, Y.; Chen, S.; Huang, C.; et al. Leachate from plastic food packaging induced reproductive and neurobehavioral toxicity in zebrafish. Ecotoxicol. Environ. Saf. 2022, 231, 113189.
- Chen, Q.; Lackmann, C.; Wang, W.; Seiler, T.-B.; Hollert, H.; Shi, H. Microplastics Lead to Hyperactive Swimming Behaviour in Adult Zebrafish. Aquat. Toxicol. 2020, 224, 105521.
- Yu, H.; Chen, Q.; Qiu, W.; Ma, C.; Gao, Z.; Chu, W.; Shi, H. Concurrent water- and foodborne exposure to microplastics leads to differential microplastic ingestion and neurotoxic effects in zebrafish. Water Res. 2022, 219, 118582.
- Santos, D.; Luzio, A.; Matos, C.; Bellas, J.; Monteiro, S.M.; Félix, L. Microplastics alone or co-exposed with copper induce neurotoxicity and behavioral alterations on zebrafish larvae after a subchronic exposure. Aquat. Toxicol. 2021, 235, 105814.
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033.
- Rabezanahary, A.N.A.; Piette, M.; Missawi, O.; Garigliany, M.-M.; Kestemont, P.; Cornet, V. Microplastics alter development, behavior, and innate immunity responses following bacterial infection during zebrafish embryo-larval development. Chemosphere 2023, 311, 136969.
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 15775.
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878.
- Bobori, D.C.; Dimitriadi, A.; Feidantsis, K.; Samiotaki, A.; Fafouti, D.; Sampsonidis, I.; Kalogiannis, S.; Kastrinaki, G.; Lambropoulou, D.A.; Kyzas, G.Z.; et al. Differentiation in the expression of toxic effects of polyethylene-microplastics on two freshwater fish species: Size matters. Sci. Total. Environ. 2022, 830, 154603.
- Wang, H.; Wang, Y.; Wang, Q.; Lv, M.; Zhao, X.; Ji, Y.; Han, X.; Wang, X.; Chen, L. The combined toxic effects of polyvinyl chloride microplastics and di(2-ethylhexyl) phthalate on the juvenile zebrafish (Danio rerio). J. Hazard. Mater. 2022, 440, 129711.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
784
Revisions:
2 times
(View History)
Update Date:
28 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




