Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dilshan Sandaruwan Premathilake | -- | 3430 | 2023-04-28 11:02:22 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3430 | 2023-05-04 04:05:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Premathilake, D.S.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R.; Vaccari, M. Lithium-ion Battery (LIB) Recycling. Encyclopedia. Available online: https://encyclopedia.pub/entry/43616 (accessed on 12 January 2026).
Premathilake DS, Botelho Junior AB, Tenório JAS, Espinosa DCR, Vaccari M. Lithium-ion Battery (LIB) Recycling. Encyclopedia. Available at: https://encyclopedia.pub/entry/43616. Accessed January 12, 2026.
Premathilake, Dilshan Sandaruwan, Amilton Barbosa Botelho Junior, Jorge Alberto Soares Tenório, Denise Crocce Romano Espinosa, Mentore Vaccari. "Lithium-ion Battery (LIB) Recycling" Encyclopedia, https://encyclopedia.pub/entry/43616 (accessed January 12, 2026).
Premathilake, D.S., Botelho Junior, A.B., Tenório, J.A.S., Espinosa, D.C.R., & Vaccari, M. (2023, April 28). Lithium-ion Battery (LIB) Recycling. In Encyclopedia. https://encyclopedia.pub/entry/43616
Premathilake, Dilshan Sandaruwan, et al. "Lithium-ion Battery (LIB) Recycling." Encyclopedia. Web. 28 April, 2023.
Copy Citation
The search for global CO2 net zero requires adapting transport vehicles to an electrification system for electric vehicles. In addition, the consumption of electric devices, and consequently batteries, has risen over the years. In order to achieve a circular economy, the spent batteries must be recycled.
LIB recycling
decentralization of recycling
LIB pretreatment
1. Battery Characterization
As one of the major determinants of the recycling steps, understanding the chemistry of the battery is vital. In any LIB battery, it is possible to identify cells, modules, and packs as the main parts of it. Further, each cell contains a cathode, anode, organic electrolyte and a separator covered by a housing (or case). The separator is wetted by electrolyte salts. The cells are connected in series or in parallel to make up the module according to the usage of the battery. A combination of two arrays may be also possible for some batteries. A module will be contained in a house made from an insulating material for battery safety. A pack of batteries can contain several modules interconnected [1]. It seems that the size, shape and content of the battery are highly dependent on the application of the battery. However, categorizing the LIBs based on their cathode material is more useful to make a good judgment.
Lithium cobalt oxide (LiCoO2) (LCO) cathode batteries can be identified as the first LIBs that use a liquid electrolyte [2][3][4]. However, applications of LCO batteries are limited to small electronic devices such as mobile phones or laptops. While LiCoO2 acts as the cathode material in LCO batteries, graphite acts as the anode material with a conductive polymer as the electrolyte [3][4]. Though graphite as the anode material has not changed much in the past years since its first introduction, cathode material has undergone various changes. LCO cathode material is easy to produce and has a stable discharging which makes it a favorable material for the cathode. However, having high Li and Co proportions make it undesirable due to extensive environmental burden, less economic viability and human health concern occurring during the extraction period of Li and Co. In addition, the performance of the battery is average compared to the other available LIB battery types. This makes it urgent to further modify the cathode materials in future LIBs [3][5].
Accordingly, it is possible to identify LIBs with lower Co content with enhanced performance. Lithium manganese oxide (LiMn2O4) (LMO) cathode is a material used as an LIB cathode with no Co involved. It is also the reason for the low cost of the same. LMO batteries have better performance than LCO batteries. For example, LMO has a higher charge rate and higher voltage than LCO batteries which makes it applicable in portable devices other than smaller electronic equipment. LMO batteries are also seen combined with other battery types to use for EVs [6]. However, as some authors elaborate, LMO has a short lifetime along with a medium energy density, which makes it unfavorable in some ways [7]. Due to these factors, LMO batteries have a low market share.
Lithium iron phosphate (LiFePO4) (LFP) is another alternative that uses no Co in its cathode material. Further, the structure of the LFP cathode gives it an additional stability which enhances its total lifetime. Low environmental degradation caused during the extraction period is another plus point for these cathode materials. However, low energy density and relatively low potential make it undesirable in many high-end applications like EVs. However, the demand for LFP batteries has risen due to its low cost and extended lifetime involved [3][5].
Commonly, LCO, LMO and LFP cathode battery types are involved with low to medium energy densities in relation to the other battery types found in the market. In contrast, lithium nickel cobalt manganese oxide (LiNixCoyMnzO2) (NCM) cathode materials and lithium nickel cobalt aluminum oxide (LiNixCoyAlzO2) (NCA) have been identified for their high specific capacities and high energy densities due to the availability of Ni in the structure [8][9]. Since both NCM and NCA types use Co, they are implicated in relatively high environmental degradation and human safety issues. However, a lower Co ratio makes the impacts relatively lower than LCO battery types. It seems that high energy density is the main source that made these batteries the dominant battery types in the market. Nevertheless, the same criterion made them applicable in EVs. One notable difference among these two battery types is the expected life span. According to current studies, NCM batteries have a higher lifetime than NCA batteries, which makes NCM batteries have a higher market share than NCA batteries [7].
Doose et al. (2021) suggests that the trend of LIBs goes towards NCM cathode types due to low Co content. NCM 811 has a molar ratio of Ni:Co:Mn as 8:1:1, which makes it better in environmental performance due to less Co content, whereas NCM 622 has a molar ratio of 6:2:2 and NMC 111 has the ratio of 1:1:1 [10]. However, some authors show that higher content of Ni reduces the performance of the battery drastically in many aspects. For instance, reduced lifetime, reduced heat resistance and a few other technical problems (voltage decay, low initial coulombic efficiency, capacity loss, termination of transitional metals) can be listed [4][11][12]. So, NCM 622 can be identified as a relatively better performer in both environmental and technical aspects. A comprehensive summary of cathode types is discussed in Table 1. It shows the chemical formula, possible voltage ranges, energy densities and applications.
Table 1. Comprehensive summary of different LIB battery types based on cathode material. Data source: [13][14].
| Battery type (Based on Cathode Material) | LCO | LMO | LFP | NCA | NCM |
|---|---|---|---|---|---|
| Chemical Formula | LiCoO2 | LiMn2O4 | LiFePO4 | LiNixCoyAlzO2 | LiNixCoyMnzO2 |
| Operating Voltage (V/Cell) | 3.0–4.2 | 3.0–4.2 | 2.0–3.65 | 3.0–4.2 | 3.6–4.0 |
| Energy Density (Wh/kg) | 150–200 | 100–150 | 90–160 | 200–260 | 160–230 |
| Applications | Small devices (laptops, mobile phones) | Small devices, EVs with combination of NCM | Smaller portable devices, limited applications in EVs | EVs (Tesla vehicles) | EVs (many manufacturers), small appliances, power station applications |
| Remarks | Low safety, high cost and medium performance | Medium safety, medium performance, low lifetime | Low cost, medium performance, high thermal resistance | Medium safety and cost, high performance | Medium safety and cost, high performance |
Graphite or composite carbon being the anode material of the LIBs has not significantly changed over the past years. However, there are other materials used in the anode other than the graphite or composite carbon, which will be discussed soon under this section. Graphite or carbon-based composite in the anode material is very popular due to the properties of these materials and structures. Mainly, their safety for humans when not contaminated, structural and chemical stability under different temperatures, and economical quality are some reasons for their repeated application as the anode material. Other than that, low working potential and high theoretical capacities (~0.15 V vs. Li+/Li and 372 mAh·g−1, respectively) of these materials are also reasons to become popular for this aspect [15][16][17].
In contrast, the lithium titanium oxide (LTO) battery type uses LTO as the anode material instead of carbon [18][19]. The use of LTO has some advantages over graphite, such as generating a higher power with a low energy loss and durability [18][19][20]. However, high cost in manufacturing of such batteries make it unfavorable for commercial use. Other than these, new materials for anode materials are being investigated. Among them, graphene, silicon composite or silicon-based nanomaterials, and Li metal are available. These new research studies will address the problems involved with current anode materials such as heat generation in LMO and LFP cathodes or rupture generation occurring due to repeated charge cycles [21]. Titanium niobium oxide (TNO) has been studied for application as anode material [20]. For instance, TNO/NMC batteries have up to 14,000 cycles at 80% capacity retention, which is considered as another option with LTO/NMC batteries [21].
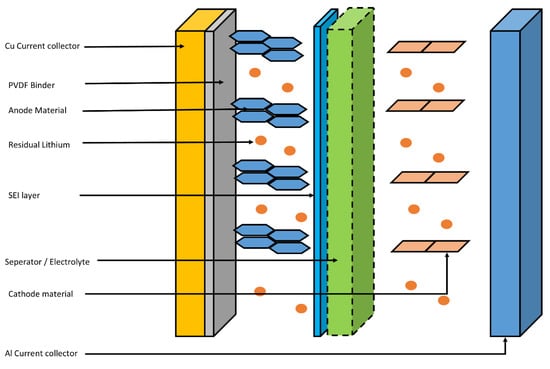
Other than the cathode and anode materials, in a spent LIB battery, some hazardous wastes can also be found. This can be easily understood by understanding the cross-sectional structure of a spent LIB. Figure 1 shows the structure of the spent LIB.
For instance, graphite is attached to the Cu current collector through polyvinylidene fluoride (PVDF) layer in LIB. Additionally, between the surface of the graphite and the electrolyte, the solid electrolyte interface (SEI) is being built through a reaction between the graphite surface and the Li from electrolyte during the recharge and discharge cycles. Usually, electrolyte is made up of Li salts such as LiPF6 or LiClO4 dissolved in organic solvents. As an organic solvent, ethylene or ethylene carbonate is used [21][22]. Authors suggest that, at the EoL of the LIB, the spent anode material can contain residuals from all these electrolytes and binders, as well as Cu foil with a higher degree of degradation to the anode material [23][24][25].
According to the chemistry of the battery, improper disposal of these materials can have a significant impact on the environment and human health. Of course, the soil and natural water body contamination are the first step of a series of chemical reactions that are waiting to follow. In particular, metallic Li can have explosive reactions with natural waters. Other metals included can affect the biodiversity of the contaminated sites. Moreover, organic materials, electrolyte, and waste graphite, upon disposal into the environment, can have a series of reactions and can lead to the release of toxic substances into the environment according to the following reaction series.
LiPF6 → PF5 + LiF
LiPF6 + H2O → 2HF + OPF3 + LiF
(C2H5O)2CO + H2O → CO2 + 2C2H5OH
(CH3O)2CO + H2O → CO2 + 2CH3OH
Importantly, these substances are highly water soluble and can cause health impact on humans and on other living beings as well. Due to these factors, proper disposal, or more importantly, material recovery from spent LIBs is necessary to implement circular economy concepts in battery manufacturing. However, designing a common recycling process for all the LIBs seems to be an impossible task given the varieties of battery chemistries. Nevertheless, current research studies suggest common frameworks that can be used as prototypes when designing an LIB recycling process. Based on this, current battery recycling companies have adopted their recycling facilities considering only one battery chemistry type. This is mainly since mixing different chemistries for recycling can imply a negative effect on the outcome. However, separate recycling is costly and reduces the overall recycling rate.
2. Material Collection
An efficient waste collection system is always the key for a successful recycling route. Accordingly, establishing an effective and efficient collection system for spent LIBs will aid in maximizing the recycling capacity and economic benefits. Further, decisions on environmental and economic aspects can be easily taken upon knowing the collection capacities.
Availability of a range of LIB products in the market is one major problem for collection. Nevertheless, LIBs have a variety of applications, due to their design, size, content, shapes, and capacities which are also changing. These factors make waste collection more difficult and complex for LIBs [26][27][28]. It is possible to distinguish three market segments for LIBs, such as small-scale electrical equipment (SSEE) (household scale), stationary energy storages (SES) and EVs. SSEE markets and SES markets are well established, and as discussed previously, an increase can be expected in EV markets, and hence an increase in spent EV LIBs. Given the differences in the three market types, the types of LIBs in a market segment are significantly different from others. As a matter of fact, different collection routes need to be established for each of these market types. SSEE, or simply, household electrical equipment batteries can be collected at locations established by the manufacturers or at retailers/supermarkets. However, large-scale LIBs (SES and EVs) need the attention of expert/trained personnel for dissembling from the equipment prior to collection [29][30].
The availability of the LIBs for collection at the end of their lifetime is vital. As per the literature, the largest proportion are available in domestic-scale equipment (SSEE). Usually, LIBs from SSEE last for 3–10 years before coming to the waste stream [30]. However, this is far more than the usual lifetime of the equipment. Most domestic scale electric equipment passes a hibernation period (inactive stage) after its service period before being added to the waste stream, extending their true lifetime. The lifetime of the EVs depends on many factors such as the annual mileage, charging frequency and condition, and the type of battery; manufacturers usually expect the battery to last for 8–10 years. In running distance, this is around 160,000 km for most of the EV types (Toyota, Nissan, BMW, etc.) [31][32]. Further, Yang et al. (2018) have predicted that EV lifetime can be varied in the range of 5–13 years in different USA cities, considering average driving conditions using predictive tools. Additionally, this can be elevated by another 10–12 years by giving them a second lifetime in SES applications (also depending on the application) [31].
According to these long-life EV batteries and the infancy stage of the EV sector, near-term priority is worth giving to mature, small-scale electric equipment batteries. However, designing recycling processes, collection mechanisms and applications for the upcoming huge waste stream of EV batteries is also important to manage these hazardous wastes from now on [33][34]. Presently, only a small fraction of electronic waste is being collected and recycled properly. Hence, a larger amount is being neglected and ends up in landfills, adding more toxic substances to natural soils and waters [35]. In 2020, Europe collected and recycled the highest fraction of e-waste (42.5%). In contrast, Asia has the second-highest waste recycling with 11.4%. America and Oceania stayed at 9.4% and 8.8%, respectively. The least recycling is recorded in Africa, which is 0.9%. So, as an average, only 17.4% of total e-waste is subjected to collection and recycling on the global scale [35]. Moreover, the generation is the highest in Asia, responsible for 24.9 Mt followed by 12 Mt from Europe and 7.7 Mt from North America [35]. For this reason, it is a clear fact that the waste generation to waste collection rate is not balanced, which leads to a lot of e-waste ending up in landfills. Due to this, it is vital to plan effective collection routes for such waste streams and use the available resources for this cause. Moreover, designing, installing and establishing structures to recycle EV batteries and SES batteries is also important at early stages to face the rapidly growing markets of the same.
As a solution for this problem of collection, proper labeling can be identified. Providing information about the battery content, applications, or secondary applications on a label or in a source that can be easily accessible will be useful. Training personnel at dismantling facilities to identify batteries using the labels or the brand can also be an alternative.
3. Material Sorting
Sorting of spent LIBs to forward them to pretreatment is as complex as collection due to large mix of materials (different NMC-cathode batteries, LCO, NCA, LFP…). However, sorting is mandatory for LIBs before undertaking any further steps due to the same factor. According to the majority of authors, the most suitable sorting method for spent LIBs is categorizing them according to the battery chemistry [36][37]. Differentiated sorting of LIBs at the household level is a bit ambiguous and it is difficult to achieve a high collection rate. It would also require an additional workload from the consumer end which is highly unlikely to be obtained in this case. Alternatively, sorting of spent batteries can be done at the recycling facilities in the reverse logistic process. Yet, this would increase the transportation costs for sorted batteries as most battery recycling facilities are not designed to receive all types of waste batteries. As an answer for this, establishment of consolidated treatment facilities with different recycling routes for a range of battery types can be put forward. The cost for such plants would be high and not economically feasible to achieve through the private sector. In contrast, scattered or partitioned recycling facilities are ideal to overcome the complexity of the material mix. For instance, separated pretreatment plants can handle the preliminary steps of the recycling routes for all types of batteries. Sorting of the batteries, separating of the casing, discharging the batteries and separation of battery modules can be some of the steps involved in a pretreatment facility. Afterwards, sorted and partially treated battery types can be forwarded to other facilities for further treatment and recycling. This can reduce the high cost involved in a single treatment plant and can enhance the total efficiency of the recycling process [33][36].
While sorting is an essential part of the pretreatment process, identifying the battery chemistry can only be done through a laboratory analysis. So, appropriate labeling for the battery from the manufacturer can be useful in sorting. The labeling can contain the essential details of the battery such as battery chemistry, date of manufacture, name and location of the manufacturer and application area of the battery, for instance. Through these details, the sorting process can be performed effectively, safely and reliably. Moreover, it can save resources involved in laboratory analysis [33].
Current battery labeling practices in the EU and in the USA only instruct how to handle and dispose batteries. For example, it provides instructions to not dispose of the spent battery with household waste. Moreover, availability of metals such as Hg, Cd and Pb will also be noted in the labeling [38].
A new set of labeling requirements was published by the EU commission in 2020 with repealing directive 2006/66/EC and amending Regulation (EU) No 2019/1020, also known as the EU battery passport and electronic information exchange system. Though the directive came into effect in January 2022, a set of deadlines have been implemented for adaptation of different objectives. Accordingly, by 1st of January 2026, all the batteries that enter the EU market will register in an online electronic exchange system where the public can access and refer to all the information about the battery. Moreover, there will be engraved or printed a QR code which will indicate information about battery lifetime, charge capacity and presence of hazardous metals. The QR code will be linked with the battery passport where online traceability and management of the battery can be implemented. Through the identifier, the online digital file of the battery will be frequently updated by the economic operators of the battery throughout its lifetime. In addition, it will include information about the status of the battery, repairs or repurposing done. Moreover, the QR code labeling will also be linked with carbon footprint declaration of the battery entering into force on 1 July 2024 and carbon footprint performance, starting from no later than 1 January 2026 for industrial or EV batteries with a capacity above 2 kWh. In addition, effective from 1 January 2027, the percentage of availability of recovered Li, Co, Pb and Ni in the battery with the set guidelines must also be provided along with other active minerals in the battery [39][40].
Compared to the EU, the USA has no dedicated rules applied to labeling of LIBs for the purpose of sorting. Instead, rules and regulations for normal battery labeling will take effect on LIBs as well. Accordingly, batteries must contain information about the battery type (Ni-Cd or Pb-acid) which will be helpful in disposing and recycling. Further, recycling symbols must be shown on the battery. However, specifically LIBs in the USA should contain an additional label for transportation safety purposes. This label will be useful in obtaining certificates for safety tests and provide information on packaging and transport volume limitations [41][42].
China, on the other hand, has imposed labeling guidelines for LIBs with the Interim Provisions on the Traceability Management of Power Battery Recovery and Utilization of New Energy Vehicles in 2018. According to this measure, a platform should be maintained to trace the entire lifecycle stages of the LIBs that enter the market. The platform will provide information about production, use, disposal, recycling or repurposing of the LIB in question [43][44]. This interim measure will act like the battery passport decree in the EU.
In summary, an effective collection of spent LIBs is mandatory for better recycling outcomes. Optimum use of resources and new technologies for collection of EOL LIBs is ideal to match the rising demand of EV market. At the same time, decentralized pretreatment plants for waste LIBs could be beneficial in enhancing sorting, discharging and dismantling. Therefore, sorted, pretreated LIBs or battery parts can be forwarded to dedicated recycling and recovery facilities. Policies play a vital role as a motivation factor for recycling of LIBs. Proper establishment of policies can considerably increase the efficiency of the recycling and recovering capacities.
References
- Yun, L.; Linh, D.; Shui, L.; Peng, X.; Garg, A.; Le, M.L.P.; Asghari, S.; Sandoval, J. Metallurgical and Mechanical Methods for Recycling of Lithium-Ion Battery Pack for Electric Vehicles. Resour. Conserv. Recycl. 2018, 136, 198–208.
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107.
- Warner, J. 7-Lithium-Ion Battery Packs for EVs. In Lithium-Ion Batteries; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 127–150. ISBN 9780444595133.
- El Mofid, W.; Ivanov, S.; Konkin, A.; Bund, A. A High-Performance Layered Transition Metal Oxide Cathode Material Obtained by Simultaneous Aluminum and Iron Cationic Substitution. J. Power Sources 2014, 268, 414–422.
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Sun, B.; Wang, G. Towards High-Energy-Density Lithium-Ion Batteries: Strategies for Developing High-Capacity Lithium-Rich Cathode Materials. Energy Storage Mater. 2021, 34, 716–734.
- Bharathraj, S.; Adiga, S.P.; Mayya, K.S.; Song, T.; Kim, J.; Sung, Y. Degradation-Guided Optimization of Charging Protocol for Cycle Life Enhancement of Li-Ion Batteries with Lithium Manganese Oxide-Based Cathodes. J. Power Sources 2020, 474, 228659.
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A Comprehensive Review on the Recycling of Spent Lithium-Ion Batteries: Urgent Status and Technology Advances. J. Clean. Prod. 2022, 340, 130535.
- Chen, Y.; Wang, G.X.; Konstantinov, K.; Liu, H.K.; Dou, S.X. Synthesis and Characterization of LiCoxMnyNi1−x−yO2 as a Cathode Material for Secondary Lithium Batteries. J. Power Sources 2003, 119–121, 184–188.
- Li, Y.-C.; Xiang, W.; Wu, Z.-G.; Xu, C.-L.; Xu, Y.-D.; Xiao, Y.; Yang, Z.-G.; Wu, C.-J.; Lv, G.-P.; Guo, X.-D. Construction of Homogeneously Al3+ Doped Ni Rich Ni-Co-Mn Cathode with High Stable Cycling Performance and Storage Stability via Scalable Continuous Precipitation. Electrochim. Acta 2018, 291, 84–94.
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in Ecofriendly Battery Recycling and Closed Material Cycles: A Perspective on Future Lithium Battery Generations. Metals 2021, 11, 291.
- Wang, Z.; Liu, E.; He, C.; Shi, C.; Li, J.; Zhao, N. Effect of Amorphous FePO4 Coating on Structure and Electrochemical Performance of Li1.2Ni0.13Co0.13Mn0.54O2 as Cathode Material for Li-Ion Batteries. J. Power Sources 2013, 236, 25–32.
- Xiang, W.; Zhu, C.-Q.; Zhang, J.; Shi, H.; Liang, Y.-T.; Yu, M.-H.; Zhu, X.-M.; He, F.-R.; Lv, G.-P.; Guo, X.-D. Synergistic Coupling Effect of Sodium and Fluorine Co-Substitution on Enhancing Rate Capability and Cycling Performance of Ni-Rich Cathode for Lithium Ion Battery. J. Alloys Compd. 2019, 786, 56–64.
- Mekonnen, Y.; Sundararajan, A.; Sarwat, A.I. A Review of Cathode and Anode Materials for Lithium-Ion Batteries. In SoutheastCon 2016; IEEE: Norfolk, VA, USA, 2016.
- Daniel, C.; Mohanty, D.; Li, J.; Wood, D.L. Cathode Materials Review. AIP Conf. Proc. 2014, 1597, 26–43.
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium Sulfur Batteries, a Mechanistic Review. Energy Environ. Sci. 2015, 8, 3477–3494.
- Lahiri, I.; Choi, W. Carbon Nanostructures in Lithium Ion Batteries: Past, Present, and Future. Crit. Rev. Solid State Mater. Sci. 2013, 38, 128–166.
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763.
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264.
- Nemeth, T.; Schröer, P.; Kuipers, M.; Sauer, D.U. Lithium Titanate Oxide Battery Cells for High-Power Automotive Applications—Electro-Thermal Properties, Aging Behavior and Cost Considerations. J. Energy Storage 2020, 31, 101656.
- Meng, X. Recent Progress of Graphene as Cathode Materials in Lithium Ion Batteries. IOP Conf. Ser.: Earth Environ. Sci. 2019, 300, 042039.
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric Car Battery: An Overview on Global Demand, Recycling and Future Approaches towards Sustainability. J. Environ. Manag. 2021, 295, 113091.
- Contestabile, M.; Panero, S.; Scrosati, B. A Laboratory-Scale Lithium-Ion Battery Recycling Process. J. Power Sources 2001, 92, 65–69.
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A Review of Processes and Technologies for the Recycling of Lithium-Ion Secondary Batteries. J. Power Sources 2008, 177, 512–527.
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-Value Utilization of Graphite Electrodes in Spent Lithium-Ion Batteries: From 3D Waste Graphite to 2D Graphene Oxide. J. Hazard. Mater. 2021, 401, 123715.
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484.
- Badawy, S.M. Synthesis of High-Quality Graphene Oxide from Spent Mobile Phone Batteries. Environ. Prog. Sustain. Energy 2016, 35, 1485–1491.
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019.
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Tracing Global Lithium Flow: A Trade-Linked Material Flow Analysis. Resour. Conserv. Recycl. 2017, 124, 50–61.
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308.
- Rallo, H.; Benveniste, G.; Gestoso, I.; Amante, B. Economic Analysis of the Disassembling Activities to the Reuse of Electric Vehicles Li-Ion Batteries. Resour. Conserv. Recycl. 2020, 159, 104785.
- Glöser-Chahoud, S.; Pfaff, M.; Walz, R.; Schultmann, F. Simulating the Service Lifetimes and Storage Phases of Consumer Electronics in Europe with a Cascade Stock and Flow Model. J. Clean. Prod. 2019, 213, 1313–1321.
- Yang, F.; Xie, Y.; Deng, Y.; Yuan, C. Predictive Modeling of Battery Degradation and Greenhouse Gas Emissions from U.S. State-Level Electric Vehicle Operation. Nat. Commun. 2018, 9, 2429.
- Casals, L.C.; Amante García, B.; Canal, C. Second Life Batteries Lifespan: Rest of UsefuLife and Environmental Analysis. J. Environ. Manag. 2019, 232, 354–363.
- Gaines, L.; Richa, K.; Spangenberger, J. Key Issues for Li-Ion Battery Recycling. MRS Energy Sustain. 2018, 5, E14.
- IEA. Global EV Outlook 2020. Available online: https://www.iea.org/reports/global-ev-outlook-2020 (accessed on 3 June 2022).
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University: Bonn, Germany; United Nations Institute for Training and Research: Geneva, Switzerland; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Rotterdam, The Netherlands, 2020; ISBN 9789280891140.
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of Scale for Future Lithium-Ion Battery Recycling Infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62.
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646.
- European Commission. Regulation of the European Parliament and the Council Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No2019/1020. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020PC0798 (accessed on 6 January 2023).
- European Parliament. Directive 2008/98/ec of the European Parliament and of the Council EUR-Lex. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0098 (accessed on 3 June 2022).
- The Federal Universal Waste Regulations, in Title 40 of the Code of Federal Regulations (CFR) in Part 273 1995. Available online: https://www.ecfr.gov/current/title-40/part-261/subpart-D (accessed on 6 January 2023).
- Bureau of National Affairs (Arlington, V.). Environment Reporter: Cases; Bureau of National Affairs: Virginia, NV, USA, 1996.
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. Management Status of Waste Lithium-Ion Batteries in China and a Complete Closed-Circuit Recycling Process. Sci. Total Environ. 2021, 776, 145913.
- Li, W.; Yang, M.; Long, R.; Mamaril, K.; Chi, Y. Treatment of Electric Vehicle Battery Waste in China: A Review of Existing Policies. J. Environ. Eng. Landsc. Manag. 2021, 29, 111–122.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
840
Revisions:
2 times
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No