Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Melkie Getnet Tadesse | -- | 3844 | 2023-04-27 11:53:53 | | | |
| 2 | Camila Xu | Meta information modification | 3844 | 2023-04-28 03:59:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tadesse, M.G.; Lübben, J.F. Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/43567 (accessed on 07 March 2026).
Tadesse MG, Lübben JF. Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/43567. Accessed March 07, 2026.
Tadesse, Melkie Getnet, Jörn Felix Lübben. "Hydrogel-Based Flexible Supercapacitors for Wearable Applications" Encyclopedia, https://encyclopedia.pub/entry/43567 (accessed March 07, 2026).
Tadesse, M.G., & Lübben, J.F. (2023, April 27). Hydrogel-Based Flexible Supercapacitors for Wearable Applications. In Encyclopedia. https://encyclopedia.pub/entry/43567
Tadesse, Melkie Getnet and Jörn Felix Lübben. "Hydrogel-Based Flexible Supercapacitors for Wearable Applications." Encyclopedia. Web. 27 April, 2023.
Copy Citation
Hydrogels are often known as three-dimensional networks of polymer chains capable of holding a large quantity of water while also being water insoluble. Research into “hydrogels” is currently a growing topic of scientific interest due to their diverse applications and eco-friendly properties.
flexible supercapacitors
wearable electronics
hydrogels
1. Introduction
Hydrogels are often known as three-dimensional networks of polymer chains capable of holding a large quantity of water while also being water insoluble. Research into “hydrogels” is currently a growing topic of scientific interest due to their diverse applications and eco-friendly properties. Hydrogels have broad applications such as drug delivery [1], tissue engineering [2], wound healing [3], hygiene product development [4] and supercapacitors’ development [5]. During the last few decades, the research and development in the area of wearable clothing has been dynamically increasing. However, the challenge in the power supply is still remains a challenge. Due to this fact, a lightweight, flexible, durable, with excellent electro-mechanical properties battery source is avoidably required [6]. All the components in wearable clothing are required to provide functionality and comfortability (weight, tactile sensation, thermal comfort, flexibility and stretchability) to the wearer during service times [7]. Hydrogels are unique due to its bio-compatibility, higher content and are often used as electrolyte materials [8].
Smart textiles have diverse applications such as heat storage [9], communications devices [10], piezoelectric energy harvesting [11], thermoelectric energy harvesting [12], electroluminescence applications [13], energy storage [14] and thermoregulated clothing [15]. For the aforementioned applications, sustainable production of the power source is strongly fundamental. There are many ways that energy storage can be achieved. These energy storages can be achieved by various mechanisms such as thermal [16], battery-based [17], an electrochemical energy storage device [18], and supercapacitor energy storage devices [19]. Among the list given above, supercapacitor-based energy storage devices have been given attention in recent times [18]. This is because not only supercapacitors provide broad applications such as in hybrid vehicles, smartphone components and energy harvesting devices but also deliver superior performance [20]. Supercapacitors can be made real by using different approaches using self-charging power textiles [21], or incorporating conducting materials into substrates such using graphene (GO) [22], poly propylene (PPy) [23], poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) [24] and functional hydrogels [25].
2. Hydrogels
Non-renewable fossil fuel-based energy sources are still commonly encountered and remain a challenge in sustainable environment. Scholars all over the world are struggling to replace them, using sustainable means to protect the environment from the pollution caused by emissions from the fossil fuels. One of the solutions could be using supercapacitors for energy storage applications from bio-based products. Supercapacitors are light in weight and flexible, and can charge faster, are more durable, and can be used in wearable applications [26]. The development of supercapacitors based on hydrogel materials demonstrated a dynamic growth in the research arena. Figure 1 illustrates how the development grows rapidly each year.
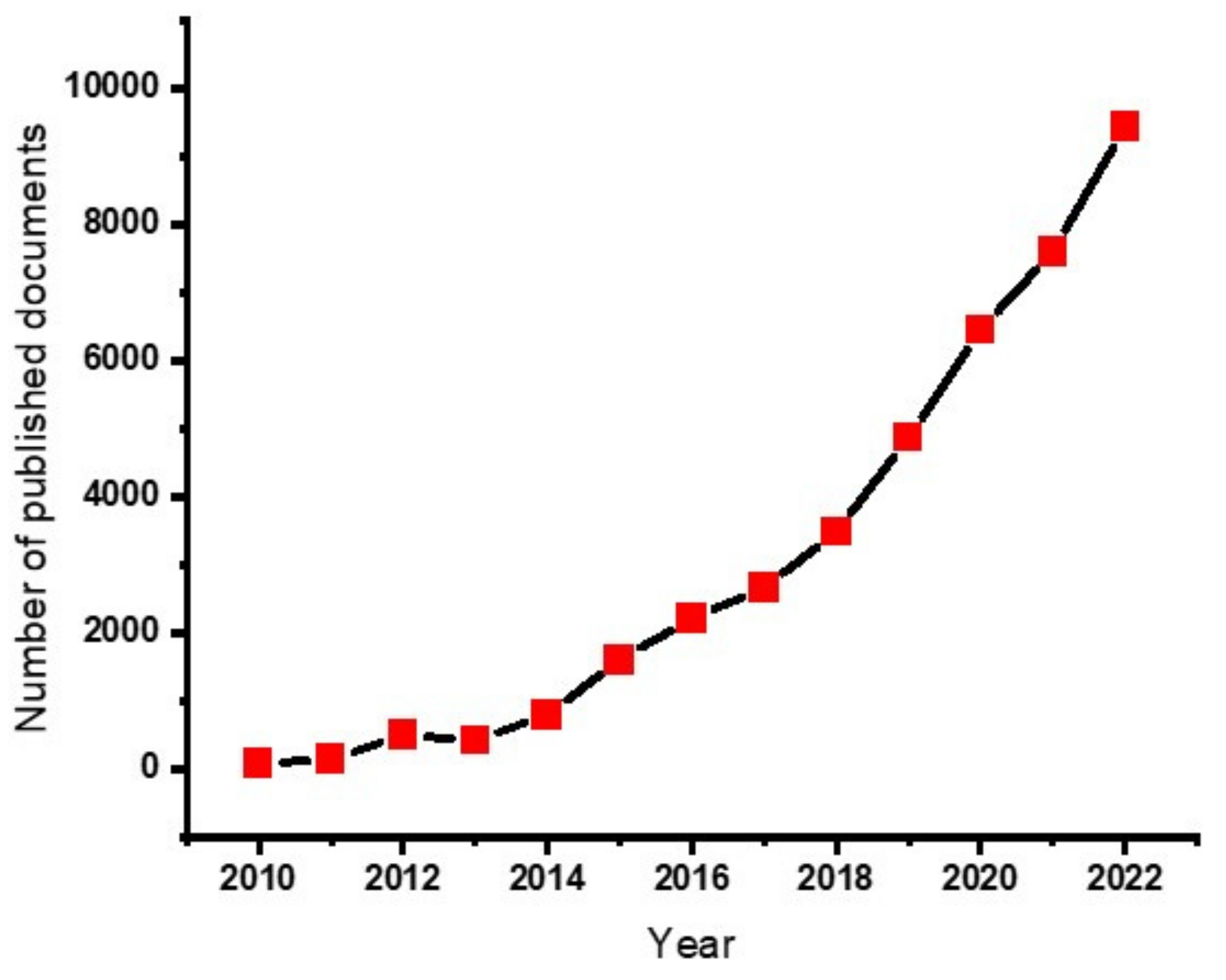
Figure 1. Number of published papers on the https://app.dimensions.ai/ data base with the search of “hydrogels for supercapacitor” (the data is accessed on 20 January 2023).
The publication record gives an indication that the use of hydrogels for the production of hydrogels is increasing and can be a future of energy storage applications.
Hydrogel-based electrolytes demonstrated a promising result in supercapacitors’ applications. The hydrogels are highly hydrophilic substances with well-defined structures and do not dissolve in water. Hydrogels can be obtained from various sources, such as macromolecules extracted from animal collagen [27], plants [28] and seaweed [29]. The basic foundation for the hydrogel is polysaccharides and proteins with fundamental constitutes of glycosidic and amino acid repeating units, respectively. The basic properties of the hydrogel is capturing very huge amounts of water molecules in its 3D networks [30]. Hydrogels can be classified based on polymeric composition, configuration, types of crosslinking, physical appearance and electrical charge [31], as summarized in Figure 2.
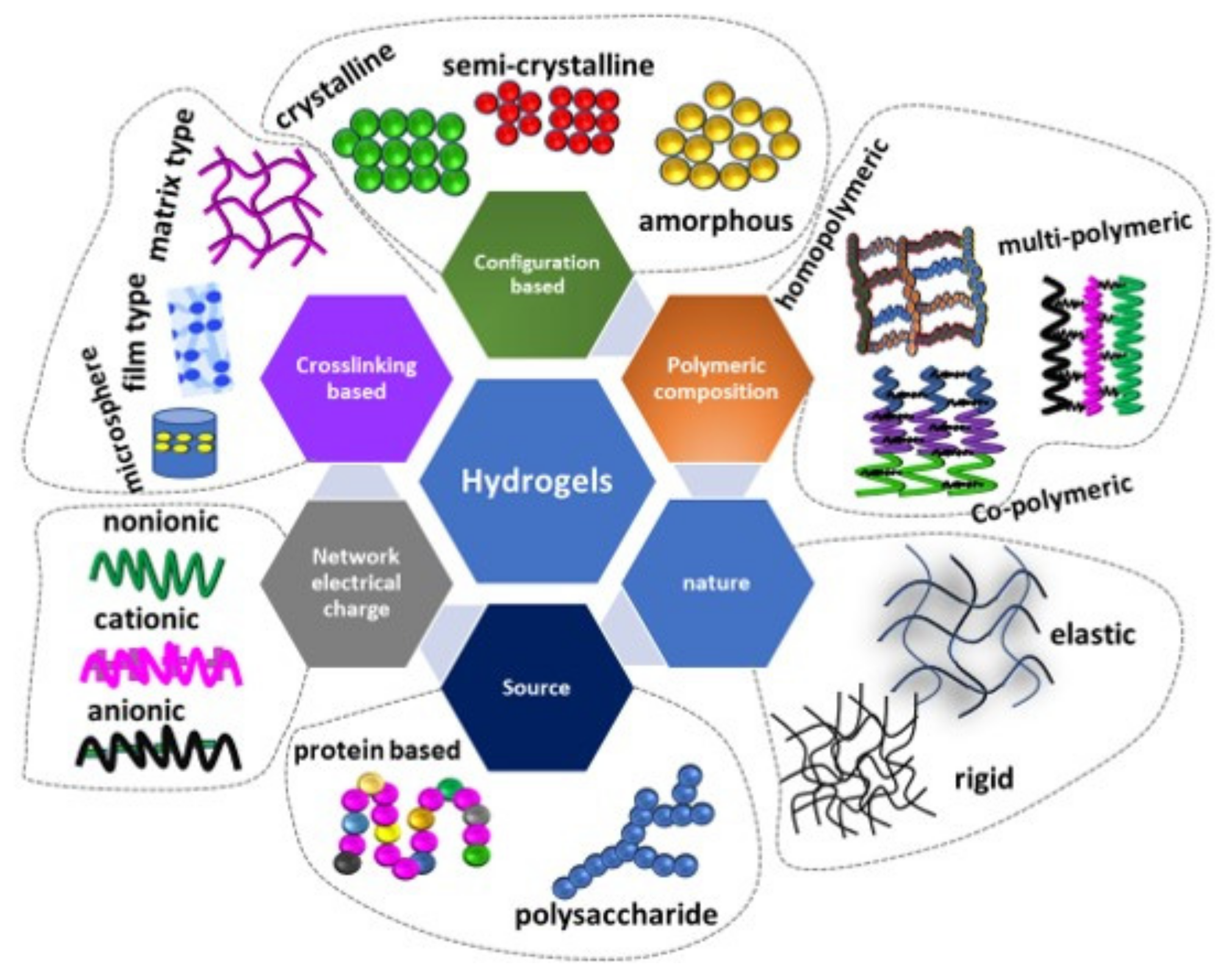
Figure 2. Review on the different classification of hydrogels.
The critical properties of hydrogel material are that it is highly sensitive to the environment, as it is hydrophilic polymers. It can swell and de-swell in water and can keep a large volume of water in swollen state [31]. This makes hydrogel materials sensitive to environmental stimuli such as electrical, temperature, sound and ionic substances. Due to this fact, hydrogel materials are perfect candidates for several applications.
3. Hydrogels for Supercapacitor Production
The increase in the demand of energy storage materials provide an opportunity of using supercapacitors to be used for wearable clothing applications. In this regard, hydrogel-based supercapacitors are new emerging technologies for wearable electronics due to its flexibility, stretchability and lightweightdness properties [25]. Various conducting polymer-based hydrogels demonstrate great potential for supercapacitor applications due to excellent electrical conductivity, flexibility, electrolytic properties, excellent solid–liquid interface and self-healing ability [32]. This section provides various insights on the various hydrogel electrolytes for supercapacitor application where further used for the production of wearable electronics. High efficiency, with high energy density energy storage materials for wearable electronics, is seldom required. The applications of hydrogels in the supercapacitor can be either as an electrode or electrolyze or can be both at the same time.
3.1. Electrochemical Properties of Hydrogels
Hydrogels continue to be used in potential applications for supercapacitor production due to their bio-compatibility [33]. In addition to their bio-compatibility, their electrochemical performance plays an important role in producing energy storage materials [34]. In order to measure the electrochemical properties of hydrogels, first the hydrogel shall be converted into electrodes. The electrodes can then be characterized by measuring the electrical conductivity of the electrode material first hand to observe whether the material possesses electrical conductivity. The electrical conductivity can be measured using four probe principles [35]. Then, the most applicable way of measuring the electrochemical performance of the hydrogels is measuring the specific capacitance, and the charge-discharge capability at various current densities [33]. The most important equipment to measure the electrochemical characteristics of the hydrogel is the electro impedance spectroscopy (EIS). It is familiar that EIS can help to demonstrate potentiostats and galvanostats, which measure the cyclic voltammetry (CV) characteristics and charge-discharge capability of the hydrogel materials. Meng, X., et al. stated that the galvanostatic charge–discharge testing results in various current densities and specific capacitance using potentiostats’ principles. Furthermore, the authors have used Nyquist plots to explore the electrochemical kinetics of the electrodes. In general, the electrochemical measurements have been measured almost in similar manner, which is using EIS instruments.
3.2. Carbon-Based Hydrogels
The incorporation of carbon-based nanomaterials in the production of hydrogels has been given recent attention for various applications. Carbon-based hydrogels can be further classified into graphene-based hydrogels, polymer-based hydrogels and bio-mass-derived hydrogels [36]. These hydrogels are carbon-based [37] because they comprise carbon in their constituent compound, and they can be used for the production of supercapacitors, as they are conductive in nature. Carbon-based hydrogels from natural resources provide an option to obtain the sustainable manufacturing of supercapacitors [38].
3.2.1. Graphene-Based Hydrogels
Graphene-based hydrogels are perfect candidates for the synthesis of supercapacitors due to their excellent rate capabilities, ultrahigh energy density and durability. Ju et al. [39] have reported the preparation of very high performance supercapacitors from ethylenediamine (EDA) functionalized graphene hydrogels (FGHs) as electrode materials. The specific energy, cycle life and capacity are very important parameters required for the production of high-performance supercapacitors. The fundamental properties of materials to be used in supercapacitor applications is its electrochemical performance.
For the ultrahigh efficiency of supercapacitor applications, the base materials have to possess high porosity, very high surface area, excellent mechanical properties and fast electron transfer. Due its three-dimensional architectures, graphene-based hydrogels fulfil these fundamental properties [40]. The preparation method has a great influence on its final output. Chen et al. [41] reported the preparation of graphene-based hydrogels with nickel hydroxide and obtained a capacitance as high as 3138.5 F. This value is very sufficient to store energy and is equivalent to the theoretical capacitance. This implies that hybrid hydrogels using graphene are excellent materials for supercapacitor applications.
Utilizing 3D architectural graphene structures for the production of supercapacitors using various mechanisms with different composite materials provides excellent performance in terms of specific capacitance value. Graphene-based hydrogels received tremendous attention in recent times for such applications. Flexibility, high energy density, and superior capacitance with acceptable charge-discharge capability demonstrate a promising performance.
3.2.2. Carbon Nanotubes
Carbon-based nanomaterials possess excellent and exceptional mechanical strength, ultrahigh electrical conductivity, high specific surface area and sole hierarchical structures [42]. Due to the above-mentioned facts, carbon-based nanomaterials are excellent candidates for supercapacitor applications. Carbon-nanotubes (CNTs) are carbon nanomaterials with no exception for such applications. Recent publications indicated that supercapacitors based on CNT is growing. Figure 3 shows the growing trends of the use of CNTs hydrogels for the production of supercapacitors. Carbon nanotubes’ (CNTs) electrodes have been fabricated using polyvinylidene fluoride (PVDF) and polyvinyl alcohol (PVA) as a gel electrolyte, which help to obtain a capacitance value of 173 F/g [43].
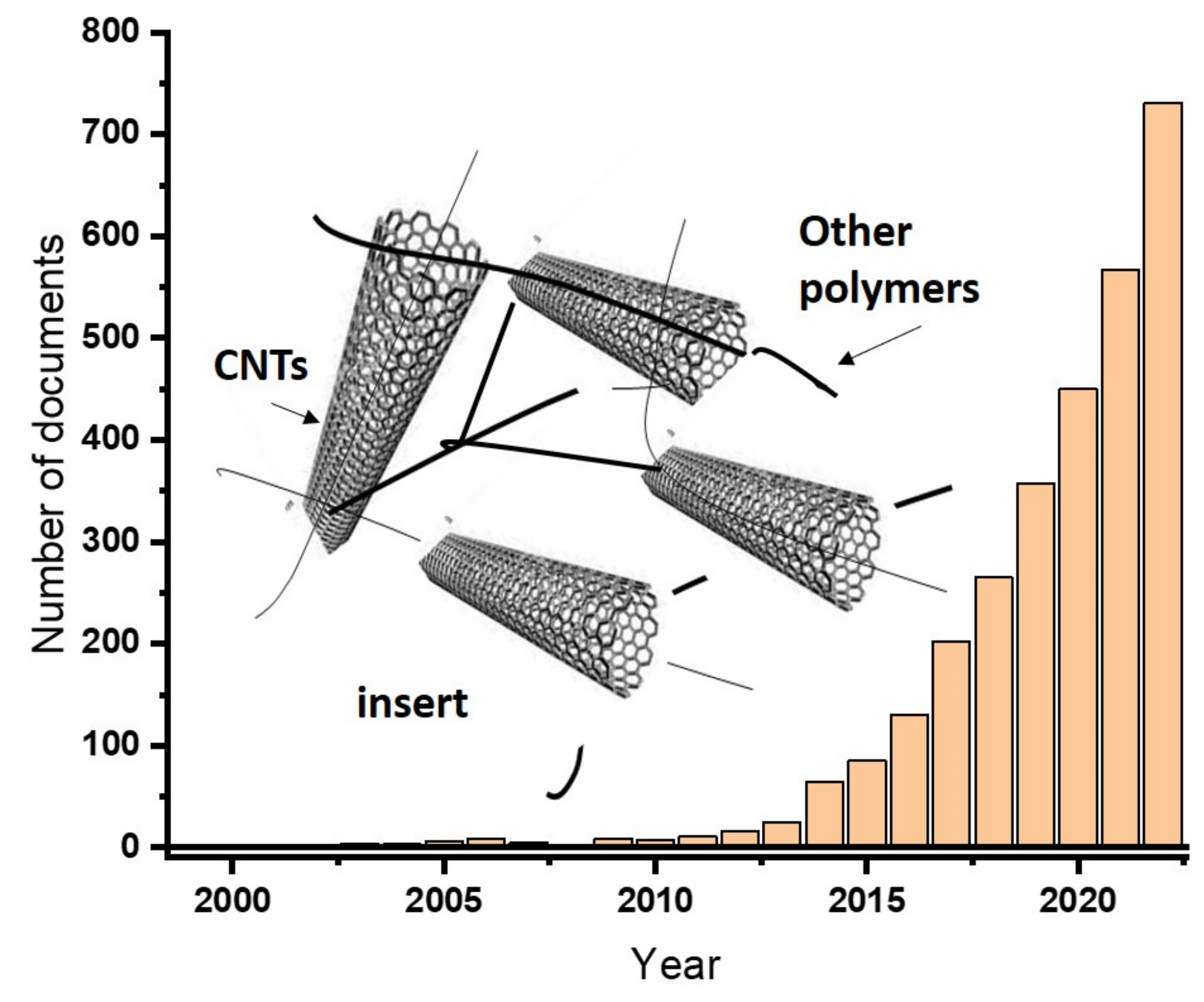
Figure 3. The increasing trends on the use of carbon nanotubes in the production of supercapacitors searched on the topic of “carbon nanotube-based hydrogels for supercapacitor applications” (as obtained from ScienceDirect databases (accessed on 12 December 2022). The insert representing the hydrogels is formed by CNTs.
The preparation of supercapacitors using CNTs also includes the formation of different composites using different polymers.
3.3. Conductive Polymer-Based Hydrogels
Hydrogels based on conductive polymers are materials that contain conductive polymers within a cross-linked network structure of the electrically insulative polymer gel [44] and conductive network hydrogels [45]. The electrically conducting polymer hydrogels can be produced using various polymerization techniques depending on the types of monomers, and the conducting polymer hydrogels are further classified depending on the polymer type. This section discusses the use of different conducting polymers for the preparation of supercapacitors.
3.3.1. PAMPS-Based Hydrogels
Poly (2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) and ammonium molybdate [(NH4)2MoO4] (Mo) composite demonstrated an effective supercapactive performance when it is in a redox-mediated environment [46]. Conducting polymers are always combined with other compounds to form complete and compensated properties such as the polyelectrolyte additions; for example, the zwitterionic silica sulfobetaine [47]. This helps to enhance the capacitive performance. The synergic effect with the electrolyte helps to double the capacitance from the pure PAMPS. The use of PAMPS as electrode and electrolyte materials for the preparation of high performance supercapacitor was patented in JUSTIA patents [48]. The work was performed with comprising molybdate (VI) salts dispersed in a hydrogel matrix and claims 360–550 F/g at a current density in a range of 1–10 A/g, which is relatively very high when compared with other similar hydrogel-based electrodes. By combining PAMPS with other polymeric materials such as polypyrrole [49], it was possible to produce a hydrogel electrode with a high capacitance value of 698.8 F/g at 5 mV s−1. The work was performed with carbon fibers, which leads to the use of a PAMPS-based hydrogel for the production of high-performance supercapacitors for the use in smart clothing applications. There are several possibilities to form composites with PAMPS so that the mechanical and electrochemical properties are improved for supercapacitor applications.
Extensive research has been conducted to uncover the flexible energy storage for wearable electronics. Furthermore, the low cost preparation [50], which is stable at different temperatures [51], is important for wearable applications. PAMPS with a various composition will be the perfect candidate for such applications.
3.3.2. Polyaniline (PAni) Hydrogel
Polyaniline (PAni), an intrinsically conductive polymer, is an intensively studied polymer that is usually produced using oxidative polymerization means [52]. Analine monomers are the sources of PAni. Usually the conductivity of PAni varies based on the dopant variety [53]. By polymerization, PAni-based hydrogels combined with a nonconducting hydrogel matrix has been reported elsewhere [54] for the production of supercapacitor materials with a sufficient electrochemical performance. Conductive polymer-based hydrogels have a unique advantage of being hydrogels and conductive at the same time. Being flexible, biocompatible, hydrophilic and biodegradable, its electroconductivity makes polymer hydrogels perfect candidates for supercapacitor application, which combines with the lightweight and highly integrability of fabrics identified as a promising class of materials for wearable applications. Like other materials, PAni-based hydrogels are most often characterized with its electrochemical properties.
Typically, PAni are combined with other polymers to obtain better electrochemical properties. For instance, PAni has been combined with polyvinyl alcohol (PVA) [55] and has obtained a 237 mF/cm2 at current density of 0.5 mA/cm2 with excellent charge-discharge cycles. However, there is a difficulty in making a good combination of PVA and PAni; the authors employed a special crosslinking agent to obtain good results. It is also possible to produce supercapacitors with excellent mechanical properties, electrical properties and improved performance in electrochemical properties with PVA-PAni-graphene-based hydrogel composites [56].
Another work performed by Dou, P., et al., [57] combined PAni with amino trimethylene phosphonic acid and achieved an ultrahigh specific capacitance of the specific capacitance over 420 F/g. Amino trimethylene phosphonic acid acts as a dopant and gelator, which helps PAni to increase the porosity and hence the surface area, which is the fundamental quantity that plays to increase the cyclic voltammetry or capacitance of the substance. Actually, the electroconductivity due to the doping surface area because of the gelation addition has been improved by the addition of amino trimethylene phosphonic acid. The use of the hydrogel based on PAni can act as an electrolyte by combining with carboxymethylcellulose and polyacrylamide solution [58], which indicates that PAni-based hydrogels are not only used as electrodes but also electrolytes in supercapacitor production. The final output for the supercapacitor is when it is served as an energy storage for some applications. The production of the wearable sensors were reported using PAni-based hydrogels as supercapacitors [59]. The authors in this work claimed that the production of the solid electroless in the preparation of supercapacitors with a sandwich structure obtained a real capacitance of 635 mF/cm2 with hydrochloric acid as a dopant to increase the electrical conductivity. Therefore, supercapacitors produced from PAni-based hydrogels are feasible for the production of wearable electronics.
3.3.3. Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate-Based Hydrogel
Poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS) is a well-researched intrinsically-conductive polymer due to its high electromechanical properties [12]. PEDOT:PSS has received attention not only due its electrochemical properties but also its water dispersibility, ease of production, environmental stability and easy availability [60]. Similarly, PEDOT:PSS is water dispersible, as the responsible polystyrene sulfonate that makes PEDOT water dispersible; it decreases the electrical conductivity. Doping with ionic solution will help to move the hydrophilic PSS towards the surface after the water dispersion occurred [12]. This property of PEDOT:PSS makes the polymer a perfect candidate for the formation of electroconductive hydrogels by combining other polymeric compounds. For instance, the PEDOT:PSS-based hydrogel with the AlCl3-induced cross-linking was prepared using fast gelation principle and obtained a capacitance of 158 F/g at a scan rate of 50 mV/s with 84.9% capacitance retention after 2000 cycles. Ethylene glycol has been used an ionic dopant element to shift the PSS part of the conductive polymer.
The conductive hydrogel-based PEDOT:PSS has received considerable attention for the production of flexible electrodes due several reasons such as flexibility, eco-friendliness, low production cost and excellent mechanical strength [61][62]. These properties make PEDOT:PSS-based hydrogels to be selected for the applications of wearable electronics. For instance, self-healable and stretchable hydrogels [63], fiber hybrid-based with excellent volumetric capacitance [61], and conductive hydrogels [64] have been investigated for the use of energy harvesting for wearable electronics. Generally, supercapacitors for the energy harvesting-based electrically conducting PEDOT:PSS polymer have been given recent attention for various applications such as wearable electronics due to their light weight and electrochemical performance properties. Sometimes, polyvinyl alcohol (PVA) has been used as electrolyte to increase the supercapacitor performance [65].
3.3.4. Polypyrrole (PPy)-Based Hydrogels
Polypyrrole (PPy) is a similar kind of intrinsically conducting polymer with excellent electrical properties. However, since it is a п-electron conjugated polymer type, it is brittle and limits its practical use, especially for flexible electronics [66]. Therefore, the common way of preparing the PPy-based hydrogels is making composites to compensate its poor mechanical properties. For instance, Das, D. and S. Kurungot, [67] reported the gelation initiated by the cross-linking of the dopant 5,10,15,20-tetrakis (4-sulfonatophenyl)-21H,23H-porphine manganese (III) chloride (MnTSPP) anion in the PPy chains, that is able to produce flexible supercapacitors that can be further used in the production of wearable clothing for various health-related applications. Another means to overcome such a poor mechanical property is wrapping PPy onto graphene-based hydrogels [68]. The hydrogel based on PPy-graphene achieved high performance supercapacitors with large specific capacitance, good rate capability and cycle stability. This is because graphene is a 3D architecture with high surface area, which helped to improve the poor mechanical property of PPy. When PPy is presented in a hydrogel form, poor mechanical properties can be improved. For instance, the solid-state high performance and flexible supercapacitor was prepared using the PPy hydrogel [69]. The high mechanical property with excellent elongation and flexibility makes easier use of PPy-based hydrogels for the production of wearable clothing.
The environmentally friendly process, low manufacturing cost, flexibility, lightweightdness and ultrahigh porous structure are required and preferred for the manufacturing of energy storage supercapacitors. This issues are currently realized by the combination of bio-based materials suing the lignosulfonate/polypyrrole (Lig/PPy) hydrogel (LP54) [70]. However, electromechanical performances are weak, excepting some enhancement mechanisms that are carried out. Sometimes, without the additions of any cross-linkers, it was possible to create supercapactive PPy-based hydrogels by controlling its morphology [71].
3.4. Cellulose-Based Hydrogels for Supercapacitor Applications
The major commercial materials for the production of supercapacitors mainly depend on carbon and carbon-based compounds in the activated carbon form because of their high surface area due to its porous structure [72]. Most recently, cellulose-based hydrogels have also been paid some attention [73]. Even though the base material is cellulose (bamboo), the basic principle behind it is changing the cellulose into activated carbon [74]. Recent advances in the field of hydrogel preparation for supercapacitor applications have been focused on the use of cellulose-based hydrogels due to their outstanding properties.
The contribution of cellulose-based materials for the sustainable environment is very important. Attributed to this fact, the lignin-based biodegradable material based on electroconductive hydrogels is among them [75]. Lignin has demonstrated a high specific capacitance of 298.6 F/g at 10 mV/s) and excellent rate performance, bringing a high energy density of 13.7 Wh k/g and outstanding capacitance retention of 89.9% after 104 cycles [76]. The report in Ref. [77] demonstrated an excellent electrochemical performance when other materials make composites with cellulose. Converting the cellulose materials into carbon materials is an important step to obtain the best conductivity [78] and hence apply as an electrode in the supercapacitor device fabrication.
3.5. Other Hydrogels for Supercapacitor Production
There are also other hydrogels that have been used for the production of supercapacitors and in the assembly of wearable clothes. In this section, some other hydrogel materials that have been used in the production of supercapacitors for wearable clothes are discussed.
3.5.1. Chitosan-Based Hydrogels
Chitosan is a biocompatible, biodegradable and hydrophilic natural cationic copolymer [79]. Chitosan is a unique bio-polymer, and it is possible to obtain gels out of it using different means such as the physical/chemical reticulation process (bond formation) and gels without any crosslinkers (such as the acetylation process) [80], which helps to prepare hydrogels with various functions. Even though the best applications for chitosan are potentially the engineering scaffolds that obtain tissue repair achievements, nowadays, the use of chitosan as an active electrode for the preparation of a supercapacitor is also obtained as an equivalent attention. For instance, the authors in Ref. [81] prepared chitosan-based hydrogels in combination with Li+/Ag+ and achieved an aerial capacity of 1 mF cm−2 at a 1.8 mA cm−2 current density, which can survive for more than 10,000 cycles without losing its capacitive performance. Efficient supercapacitors can be produced using chitosan hydrogels [82]
As stated above, the chitosan-based hydrogels can be formed either by the use of external crosslinkers or without using such crosslinkers. Yang, H. et al., [83] reported the crosslinking of chitosan using HCl and obtained a capacitance of 45.9 F g−1, energy density of 5.2 Wh kg−1 and power density of 226.6 W kg−1, and realized the solid-state supercapacitor using a phase separation means. The electrolyte uptake and conductivity of the transparent film was super high. In addition to crosslinking with other biopolymers, the modification of the chitosan to form hydrogels was also achieved [84]. Yang, H. et al., prepared a modified carboxylated chitosan synthesized through a free radical graft copolymerization of acrylamide monomers and chemical crosslinking principles to obtain higher ionic conductivity, and produced an electric double layer capacitor (EDLC) with excellent performance.
For wearable applications, flexible supercapacitors are required. Flexible supercapacitors with a lightweight and simple film structure have been reported elsewhere [85]. The flexible supercapacitors were prepared by using a blend of the chitosan/graphene@ (manganese carbonate nanoparticles) MnCO3 polymer hydrogel and obtained a specific capacitance value of 312.5 F g−1. In addition, the PVA/quaternary ammonium–chitosan hydrogel electrolyte was prepared for detection supercapacitors with brilliant performance [86], which can be attached to the wearable clothes for sensing applications. The chitosan-based oxygen-doped activated carbon/graphene composite for flexible supercapacitors was also reported [87]. This kind of supercapacitor for portable energy storage purposes can be integrated with wearable clothes for energy sources. Overall, the chitosan-based hydrogels are gaining recent attention for the making of flexible, portable and high-geared supercapacitors. These kinds of supercapacitors have the capability to be integrated with wearable clothing for smart textile applications. The other reason why chitosan-based hydrogels are gaining attention is due to its biodegradability, biocompatibility, eco-friendly nature and easy accessibility from natural resources such as plants, animals and other natural resources [88]. Therefore, it is highly recommendable to invest in and utilize chitosan-based hydrogels for supercapacitor production and other similar purposes.
3.5.2. Sodium Alginate
Hydrogels with a electrically conductive nature are being highly explored in the fabrication of flexible and portable energy storage devices due to their excellent electrochemical properties. Sodium alginate hydrogels provide such an excellent electrochemical conductivity when doped with conductive polymers such as polypyrrole [89], reduced graphene oxide (rGO) [90] and Ag nanoparticles [91], which exhibited an excellent electrochemical performance and are able to prepare high performance supercapacitors. It was possible to obtain a high capacitance, excellent rate capability and good cycling ability with the sodium alginate-based hydrogels. Furthermore, flexible supercapacitors using chitosan/sodium alginate composite hydrogels has been reported [92], which are likely to be used for wearable clothing applications. Sometimes, the binder may not be required, and using the physical crosslinking can help in obtaining highly conductive sodium alginate hydrogels [93]. Strain sensors with flexible supercapacitor production approach was claimed in Ref. [94]. Such kinds of supercapacitors are highly stretchable and self-healable, which can be an excellent potential for wearable clothing. All the other information guided to understand that highly conductive sodium alginate hydrogels are capable of producing highly geared supercapacitors for wearable clothing applications. Composites of sodium alginate and rGO are able to provide a capacitance of 753 F. g−1 at 1 A. g−1 with a good rate capability and cycling stability up to 5000 cycles [90].
3.5.3. MXene-Based Hydrogels
MXene is a group of two-dimensional inorganic substance with high electron density, which can be predicted to be metal except for its hydrophilic properties due to the hydroxyl- or oxygen-terminated surfaces [95]. Its high conductivity might be used where a high electrochemical property is required. MXene-based hydrogels have been used in the preparation of flexible supercapacitors and possess excellent electrochemical performance [96].
To increase the conductivity of the hydrogel, MXene has been combined with graphene as a gelation element and obtained a superhigh gravimetric and volumetric capacitance [97]. For wearable clothing applications, high conductivity is not sufficient, rather flexibility and light delightedness are equally important. Flexible supercapacitors based on MXene-based hydrogels composites were made possible [98].
References
- Peppas, N.A. Hydrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 1997, 2, 531–537.
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314.
- Gupta, B.; Agarwal, R.; Alam, M. Hydrogels for Wound Healing Applications. In Biomedical Hydrogels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 184–227.
- Zohuriaan-Mehr, M.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. JMatS 2010, 45, 5711–5735.
- Tien, H.N.; Hien, N.T.M.; Oh, E.-S.; Chung, J.; Kim, E.J.; Choi, W.M.; Kong, B.-S.; Hur, S.H. Synthesis of a highly conductive and large surface area graphene oxide hydrogel and its use in a supercapacitor. J. Mater. Chem. A 2013, 1, 208–211.
- Tadesse, M.G.; Kasaw, E.; Fentahun, B.; Loghin, E.; Lübben, J.F. Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage. Energies 2022, 15, 2471.
- Tadesse, M.G.; Loghin, C.; Dulgheriu, I.; Loghin, E. Comfort Evaluation of Wearable Functional Textiles. Materials 2021, 14, 6466.
- Blažic, R.; Kučić Grgić, D.; Kraljić Roković, M.; Vidović, E. Cellulose-g-poly (2-(dimethylamino) ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels 2022, 8, 636.
- Zhang, X. Heat-storage and thermo-regulated textiles and clothing. Smart Fibres Fabr. Cloth. 2001, 2001, 34–57.
- Park, S.; Jayaraman, S. Smart textiles: Wearable electronic systems. MRS Bull. 2003, 28, 585–591.
- Hossain, I.Z.; Khan, A.; Hossain, G. A Piezoelectric Smart Textile for Energy Harvesting and Wearable Self-Powered Sensors. Energies 2022, 15, 5541.
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT: PSS and polyurethane. JMatS 2019, 54, 9591–9602.
- Tadesse, M.G.; Dumitrescu, D.; Loghin, C.; Chen, Y.; Wang, L.; Nierstrasz, V. 3D printing of NinjaFlex filament onto PEDOT: PSS-coated textile fabrics for electroluminescence applications. J. Electron. Mater. 2018, 47, 2082–2092.
- Jost, K.; Perez, C.R.; McDonough, J.K.; Presser, V.; Heon, M.; Dion, G.; Gogotsi, Y. Carbon coated textiles for flexible energy storage. Energy Environ. Sci. 2011, 4, 5060–5067.
- Fang, Y.; Chen, G.; Bick, M.; Chen, J. Smart textiles for personalized thermoregulation. Chem. Soc. Rev. 2021, 50, 9357–9374.
- Niu, Z.; Qi, S.; Shuaib, S.S.A.; Yuan, W. Flexible, stimuli-responsive and self-cleaning phase change fiber for thermal energy storage and smart textiles. Compos. Part B 2022, 228, 109431.
- Lee, S.-Y.; Choi, K.-H.; Choi, W.-S.; Kwon, Y.H.; Jung, H.-R.; Shin, H.-C.; Kim, J.Y. Progress in flexible energy storage and conversion systems, with a focus on cable-type lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2414–2423.
- Tebyetekerwa, M.; Marriam, I.; Xu, Z.; Yang, S.; Zhang, H.; Zabihi, F.; Jose, R.; Peng, S.; Zhu, M.; Ramakrishna, S. Critical insight: Challenges and requirements of fibre electrodes for wearable electrochemical energy storage. Energy Environ. Sci. 2019, 12, 2148–2160.
- Lu, Z.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Carbon nanotube based fiber supercapacitor as wearable energy storage. Front. Mater. 2019, 6, 138.
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage–A review. J. Energy Storage 2019, 25, 100852.
- Wen, Z.; Yeh, M.-H.; Guo, H.; Wang, J.; Zi, Y.; Xu, W.; Deng, J.; Zhu, L.; Wang, X.; Hu, C. Self-powered textile for wearable electronics by hybridizing fiber-shaped nanogenerators, solar cells, and supercapacitors. Sci. Adva. 2016, 2, e1600097.
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196.
- Zhao, C.; Wan, T.; Yuan, W.; Zheng, Z.; Jia, X.; Shu, K.; Feng, L.; Min, Y. Re-Stickable Yarn Supercapacitors with Vaper Phase Polymerized Multi-Layered Polypyrrole Electrodes for Smart Garments. Macromol. Rapid Commun. 2022, 43, 2200347.
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A wearable supercapacitor based on conductive PEDOT: PSS-coated cloth and a sweat electrolyte. Adv. Mater. 2020, 32, 1907254.
- Jiang, L.; Lu, X. Functional hydrogel-based supercapacitors for wearable bioelectronic devices. Mater. Chem. Front. 2021, 5, 7479–7498.
- Hasan, K.; Bashir, S.; Subramaniam, R.; Kasi, R.; Kamran, K.; Iqbal, J.; Algharni, H.; Al-Sehemi, A.G.; Wageh, S.; Pershaanaa, M. Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes. Polymers 2022, 14, 4784.
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen-and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641.
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic plant-derived polysaccharide-based hydrogels. Carbohydr. Polym. 2020, 231, 115743.
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-forming algae polysaccharides: From seaweed to biomedical applications. Biomacromolecules 2021, 22, 1027–1052.
- Kabir, S.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.; Ali, A.; Islam, M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Majee, S.B. Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: Rijeka, Croatia, 2016; pp. 232–248.
- Meng, X.; Sun, H.; Zhu, J.; Bi, H.; Han, Q.; Liu, X.; Wang, X. Graphene-based cobalt sulfide composite hydrogel with enhanced electrochemical properties for supercapacitors. New J. Chem. 2016, 40, 2843–2849.
- Khan, Y.; Bashir, S.; Hina, M.; Ramesh, S.; Ramesh, K.; Mujtaba, M.; Lahiri, I. Effect of charge density on the mechanical and electrochemical properties of poly (acrylic acid) hydrogel electrolytes based flexible supercapacitors. Mater. Today Commun. 2020, 25, 101558.
- Tadesse, M.G.; Loghin, C.; Chen, Y.; Wang, L.; Catalin, D.; Nierstrasz, V. Effect of liquid immersion of PEDOT: PSS-coated polyester fabric on surface resistance and wettability. Smart Mater. Struct. 2017, 26, 065016.
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518.
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-performance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862.
- Beaucamp, A.; Muddasar, M.; Crawford, T.; Collins, M.N.; Culebras, M. Sustainable lignin precursors for tailored porous carbon-based supercapacitor electrodes. Int. J. Biol. Macromol. 2022, 221, 1142–1149.
- Ju, H.; Xu, W.; Fang, L.; Duan, J. High performance of functionalized graphene hydrogels using ethylenediamine for supercapacitor applications. Front. Chem. 2022, 10, 377.
- Yang, Z.; Chabi, S.; Xia, Y.; Zhu, Y. Preparation of 3D graphene-based architectures and their applications in supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 554–562.
- Chen, S.; Duan, J.; Tang, Y.; Zhang Qiao, S. Hybrid hydrogels of porous graphene and nickel hydroxide as advanced supercapacitor materials. Chem. A Eur. J. 2013, 19, 7118–7124.
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860.
- Pour, G.B.; Aval, L.F.; Mirzaee, M. CNTs Supercapacitor Based on the PVDF/PVA Gel Electrolytes. Recent Pat. Nanotechnol. 2020, 14, 163–170.
- Guo, X.; Li, J.; Wang, F.; Zhang, J.H.; Zhang, J.; Shi, Y.; Pan, L. Application of conductive polymer hydrogels in flexible electronics. J. Polym. Sci. 2022, 60, 2635–2662.
- Wang, X.; Wang, X.; Pi, M.; Ran, R. High Performance Double Conductive Network Hydrogel Based on Soaking Strategy for Supercapacitors. Macromol. Mater. Eng. 2022, 307, 2100652.
- Cevik, E.; Bozkurt, A.; Hassan, M.; Gondal, M.A.; Qahtan, T.F. Redox-mediated poly (2-acrylamido-2-methyl-1-propanesulfonic acid)/ammonium molybdate hydrogels for highly effective flexible supercapacitors. ChemElectroChem 2019, 6, 2876–2882.
- Afrifah, V.A.; Phiri, I.; Hamenu, L.; Madzvamuse, A.; Lee, K.S.; Ko, J.M. Electrochemical properties of poly (2-acrylamido-2-methylpropane sulfonic acid) polyelectrolyte containing zwitterionic silica sulfobetaine for supercapacitors. J. Power Source 2020, 479, 228657.
- Bozkurt, A.; Cevik, E. Supercapacitor Based on Polymer Electrolyte Containing MO (iv) Doped Hydrogel. U.S. Patent 16/660,267, 22 April 2021.
- Yazar, S.; Atun, G. Electrochemical synthesis of tunable polypyrrole-based composites on carbon fabric for wide potential window aqueous supercapacitor. IJER 2022, 46, 14408–14423.
- Kalam, A.A.; Yoo, J.E.; Bae, J. Novel application of water-dispersible polyaniline-poly (2-acrylamido-2-methyl-1-propanesulfonic acid) for all-solution-based electrochemical capacitors. Polym. Test. 2015, 44, 49–56.
- Jung, G.; Lee, H.; Park, H.; Kim, J.; Kim, J.W.; Kim, D.S.; Keum, K.; Lee, Y.H.; Ha, J.S. Temperature-tolerant flexible supercapacitor integrated with a strain sensor using an organohydrogel for wearable electronics. Chem. Eng. J. 2022, 450, 138379.
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484.
- Alesary, H.F.; Ismail, H.K.; Khudhair, A.F.; Mohammed, M.Q. Effects of dopant ions on the properties of polyaniline conducting polymer. Orient. J. Chem. 2018, 34, 2525.
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292.
- Zhao, J.; Cao, L.; Lai, F.; Wang, X.; Huang, S.; Du, X.; Li, W.; Lin, Z.; Zhang, P. Double-cross-linked polyaniline hydrogel and its application in supercapacitors. Ionics 2022, 28, 423–432.
- Joo, H.; Han, H.; Cho, S. Fabrication of poly (vinyl alcohol)-polyaniline nanofiber/graphene hydrogel for high-performance coin cell supercapacitor. Polymers 2020, 12, 928.
- Dou, P.; Liu, Z.; Cao, Z.; Zheng, J.; Wang, C.; Xu, X. Rapid synthesis of hierarchical nanostructured Polyaniline hydrogel for high power density energy storage application and three-dimensional multilayers printing. JMatS 2016, 51, 4274–4282.
- Suganya, N.; Jaisankar, V.; Sivakumar, E. Conducting polymeric hydrogel electrolyte based on carboxymethylcellulose and polyacrylamide/polyaniline for supercapacitor applications. IJN 2018, 17, 1760003.
- Li, S.; Tao, Y.; Maryum, P.; Wang, Q.; Zhu, J.; Min, F.; Cheng, H.; Zhao, S.; Wang, C. Bifunctional polyaniline electroconductive hydrogels with applications in supercapacitor and wearable strain sensors. J. Biomater. Sci. Polym. Ed. 2020, 31, 938–953.
- Alhashmi Alamer, F.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y. Review on PEDOT: PSS-Based Conductive Fabric. ACS Omega 2022, 7, 35371–35386.
- Zhou, Q.; Teng, W.; Jin, Y.; Sun, L.; Hu, P.; Li, H.; Wang, L.; Wang, J. Highly-conductive PEDOT: PSS hydrogel framework based hybrid fiber with high volumetric capacitance and excellent rate capability. Electrochim. Acta 2020, 334, 135530.
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.; Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. J. Energy Storage 2021, 45, 103510.
- Cheng, T.; Zhang, Y.Z.; Wang, S.; Chen, Y.L.; Gao, S.Y.; Wang, F.; Lai, W.Y.; Huang, W. Conductive hydrogel-based electrodes and electrolytes for stretchable and self-healable supercapacitors. Adv. Funct. Mater. 2021, 31, 2101303.
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. A Eur. J. 2018, 24, 16930–16943.
- Mirzaee, M.; Pour, G.B. Design and fabrication of ultracapacitor based on paper substrate and BaTiO3/PEDOT: PSS separator film. Recent Pat. Nanotechnol. 2018, 12, 192–199.
- Murray, P.; Spinks, G.; Wallace, G.; Burford, R. In-situ mechanical properties of tosylate doped (pTS) polypyrrole. Synth. Met. 1997, 84, 847–848.
- Das, D.; Kurungot, S. Porphyrin-Based Conducting Polymer Hydrogel for Supercapacitor Application. Energy Technol. 2020, 8, 2000061.
- Zhang, F.; Xiao, F.; Dong, Z.H.; Shi, W. Synthesis of polypyrrole wrapped graphene hydrogels composites as supercapacitor electrodes. Electrochim. Acta 2013, 114, 125–132.
- Zang, L.; Liu, Q.; Qiu, J.; Yang, C.; Wei, C.; Liu, C.; Lao, L. Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947.
- Peng, Z.; Wang, C.; Zhang, Z.; Zhong, W. Synthesis and Enhancement of Electroactive Biomass/Polypyrrole Hydrogels for High Performance Flexible All-Solid-State Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1901393.
- Bo, J.; Luo, X.; Huang, H.; Li, L.; Lai, W.; Yu, X. Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor. J. Power Source 2018, 407, 105–111.
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Source 2001, 101, 109–116.
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. CAP 2014, 14, 1616–1620.
- Gebeyehu, E.K.; Sui, X.; Adamu, B.F.; Beyene, K.A.; Tadesse, M.G. Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary. Gels 2022, 8, 140.
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739.
- Wang, D.; Yang, F.; Cong, L.; Feng, W.; Wang, C.; Chu, F.; Nan, J.; Chen, R. Lignin-containing hydrogel matrices with enhanced adhesion and toughness for all-hydrogel supercapacitors. Chem. Eng. J. 2022, 450, 138025.
- Wan, H.; Qin, C.; Lu, A. A flexible, robust cellulose/phytic acid/polyaniline hydrogel for all-in-one supercapacitors and strain sensors. J. Mater. Chem. A 2022, 10, 17279–17287.
- Kasaw, E.; Haile, A.; Getnet, M. Conductive coatings of cotton fabric consisting of carbonized charcoal for E-Textile. Coatings 2020, 10, 579.
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1.
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67.
- Cao, L.; Yang, M.; Wu, D.; Lyu, F.; Sun, Z.; Zhong, X.; Pan, H.; Liu, H.; Lu, Z. Biopolymer-chitosan based supramolecular hydrogels as solid state electrolytes for electrochemical energy storage. Chem. Commun. 2017, 53, 1615–1618.
- Xue, X.; Wan, L.; Li, W.; Tan, X.; Du, X.; Tong, Y. A Self-Healing Gel Polymer Electrolyte, Based on a Macromolecule Cross-Linked Chitosan for Flexible Supercapacitors. Gels 2023, 9, 8.
- Yang, H.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J. Power Source 2019, 426, 47–54.
- Yang, H.; Ji, X.; Tan, Y.; Liu, Y.; Ran, F. Modified supramolecular carboxylated chitosan as hydrogel electrolyte for quasi-solid-state supercapacitors. J. Power Source 2019, 441, 227174.
- Selvam, S.; Yim, J.-H. Leakage free electrolyte engraved flexible supercapacitors from Chitosan/ MnCO3 polymer hydrogel chelate film under BMIMBF4 ionic liquid assistance. J. Energy Storage 2021, 43, 103300.
- Li, C.; Liu, G.; Wang, S.; Wang, D.; Liu, F.; Cui, Y.; Liang, D.; Wang, X.; Yong, Z.; Chi, Y. Polyvinyl alcohol/quaternary ammonium chitosan hydrogel electrolyte for sensing supercapacitors with excellent performance. J. Energy Storage 2022, 46, 103918.
- Ren, R.; Zhong, Y.; Ren, X.; Fan, Y. Chitosan-based oxygen-doped activated carbon/graphene composite for flexible supercapacitors. RSC Adv. 2022, 12, 25807–25814.
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94.
- Yin, J.; Liu, Q.; Zhou, J.; Zhang, L.; Zhang, Q.; Rao, R.; Liu, S.; Jiao, T. Self-assembled functional components-doped conductive polypyrrole composite hydrogels with enhanced electrochemical performances. RSC Adv. 2020, 10, 10546–10551.
- El-Sayed, N.S.; Al Kiey, S.A.; Darwish, A.; Turky, G.; Kamel, S. High performance hydrogel electrodes based on sodium alginate-g-poly (AM-c o-ECA-co-AMPS for supercapacitor application. Int. J. Biol. Macromol. 2022, 218, 420–430.
- Jovanović, Ž.; Stojkovska, J.; Obradović, B.; Mišković-Stanković, V. Alginate hydrogel microbeads incorporated with Ag nanoparticles obtained by electrochemical method. Mater. Chem. Phys. 2012, 133, 182–189.
- Peng, K.; Wang, W.; Zhang, J.; Ma, Y.; Lin, L.; Gan, Q.; Chen, Y.; Feng, C. Preparation of Chitosan/Sodium alginate conductive hydrogels with high salt contents and their application in flexible supercapacitors. Carbohydr. Polym. 2022, 278, 118927.
- You, Y.; Qu, K.; Shi, C.; Sun, Z.; Huang, Z.; Li, J.; Dong, M.; Guo, Z. Binder-free CuS/ZnS/sodium alginate/rGO nanocomposite hydrogel electrodes for enhanced performance supercapacitors. Int. J. Biol. Macromol. 2020, 162, 310–319.
- Li, Y.; Liu, X.; Gong, Q.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Facile preparation of stretchable and self-healable conductive hydrogels based on sodium alginate/polypyrrole nanofibers for use in flexible supercapacitor and strain sensors. Int. J. Biol. Macromol. 2021, 172, 41–54.
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253.
- Yin, J.; Wei, K.; Zhang, J.; Liu, S.; Wang, X.; Wang, X.; Zhang, Q.; Qin, Z.; Jiao, T. MXene-based film electrode and all-round hydrogel electrolyte for flexible all-solid supercapacitor with extremely low working temperature. Cell Rep. Phys. Sci. 2022, 23, 4248–4253.
- Dutta, P.; Sikdar, A.; Majumdar, A.; Borah, M.; Padma, N.; Ghosh, S.; Maiti, U.N. Graphene aided gelation of MXene with oxidation protected surface for supercapacitor electrodes with excellent gravimetric performance. Carbon 2020, 169, 225–234.
- Ma, C.; Ma, M.G.; Si, C.; Ji, X.X.; Wan, P. Flexible MXene-based composites for wearable devices. Adv. Funct. Mater. 2021, 31, 2009524.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
28 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





