
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rahman Md Moshikur | -- | 1757 | 2023-04-27 07:29:13 | | | |
| 2 | Catherine Yang | Meta information modification | 1757 | 2023-04-27 10:01:13 | | |
Video Upload Options
The development of effective drug formulations and delivery systems for newly developed or marketed drug molecules remains a significant challenge. These drugs can exhibit polymorphic conversion, poor bioavailability, and systemic toxicity, and can be difficult to formulate with traditional organic solvents due to acute toxicity. Ionic liquids (ILs) are recognized as solvents that can improve the pharmacokinetic and pharmacodynamic properties of drugs. ILs can address the operational/functional challenges associated with traditional organic solvents. Biocompatible ILs comprising biocompatible cations and anions mainly derived from bio-renewable sources are considered a green alternative to both conventional ILs and organic/inorganic solvents.
1. Bio-ILs in Oral Formulation and Delivery
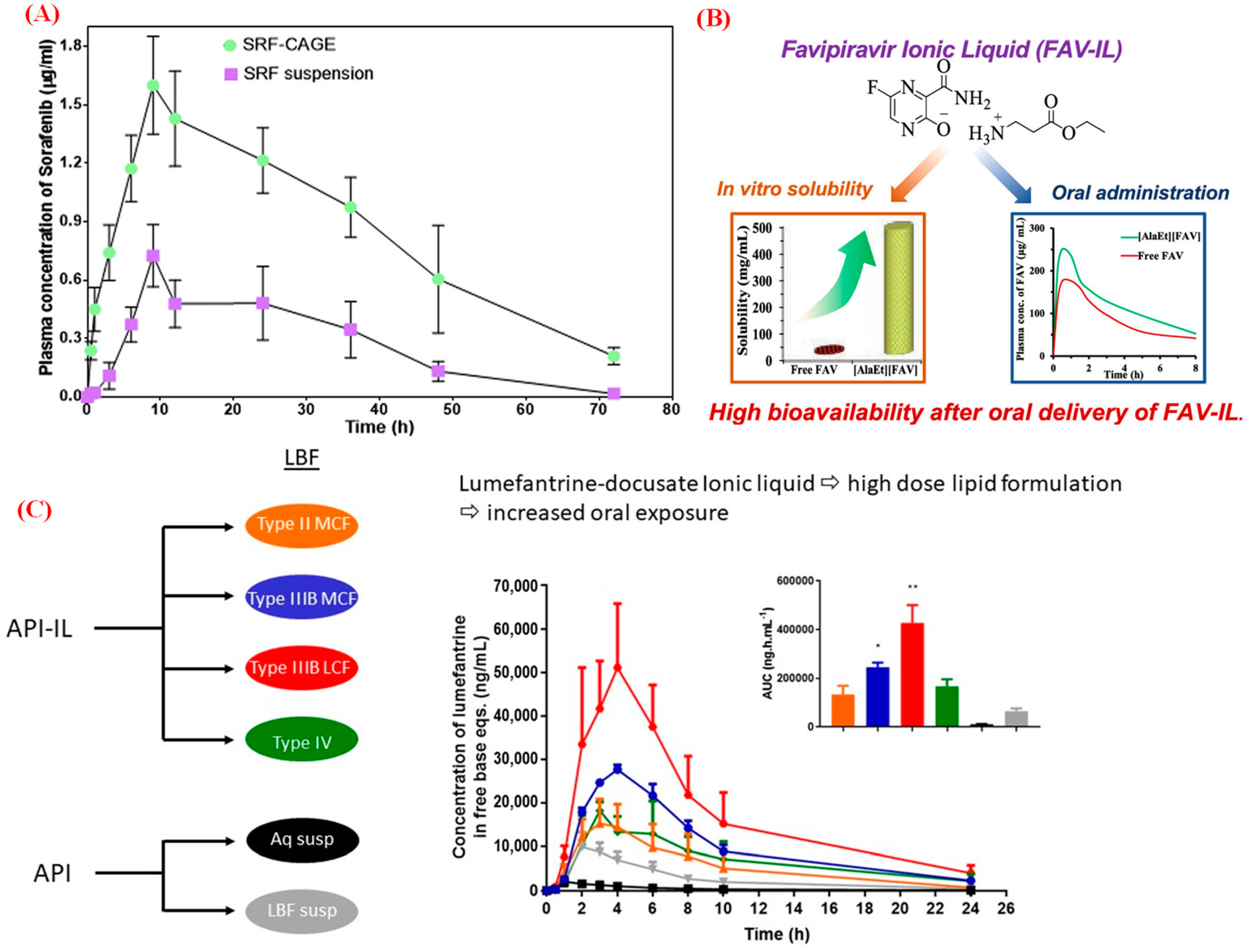
2. Bio-ILs in Injection Formulation and Delivery
3. Bio-ILs in Topical and Transdermal Delivery

4. Bio-ILs in Vaccine Formulation and Delivery
References
- Li, X.; Ma, N.; Zhang, L.; Ling, G.; Zhang, P. Applications of Choline-Based Ionic Liquids in Drug Delivery. Int. J. Pharm. 2022, 612, 121366.
- Shi, Y.; Zhao, Z.; Gao, Y.; Pan, D.C.; Salinas, A.K.; Tanner, E.E.L.; Guo, J.; Mitragotri, S. Oral Delivery of Sorafenib through Spontaneous Formation of Ionic Liquid Nanocomplexes. J. Control. Release 2020, 322, 602–609.
- Zhang, W.X.; Gao, Y.R.; Xue, R.; Nguyen, W.; Chen, W.; Wang, J.H.; Shu, Y. Liquid Formulations Based on Ionic Liquids in Biomedicine. Mater. Today Phys. 2023, 30, 100925.
- Peng, K.; Gao, Y.; Angsantikul, P.; LaBarbiera, A.; Goetz, M.; Curreri, A.M.; Rodrigues, D.; Tanner, E.E.L.; Mitragotri, S. Modulation of Gastrointestinal Mucus Properties with Ionic Liquids for Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2002192.
- Angsantikul, P.; Peng, K.; Curreri, A.M.; Chua, Y.; Chen, K.Z.; Ehondor, J.; Mitragotri, S. Ionic Liquids and Deep Eutectic Solvents for Enhanced Delivery of Antibodies in the Gastrointestinal Tract. Adv. Funct. Mater. 2021, 31, 2002912.
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic Liquids for Oral Insulin Delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296–7301.
- Zheng, X.; Fang, Z.; Huang, W.; Qi, J.; Dong, X.; Zhao, W.; Wu, W.; Lu, Y. Ionic Co-Aggregates (ICAs) Based Oral Drug Delivery: Solubilization and Permeability Improvement. Acta Pharm. Sin. B 2022, 12, 3972–3985.
- Moshikur, R.M.; Ali, M.K.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Methotrexate-Based Ionic Liquid as a Potent Anticancer Drug for Oral Delivery: In Vivo Pharmacokinetics, Biodistribution, and Antitumor Efficacy. Int. J. Pharm. 2021, 608, 121129.
- Moshikur, R.M.; Ali, M.K.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Favipiravir-Based Ionic Liquids as Potent Antiviral Drugs for Oral Delivery: Synthesis, Solubility, and Pharmacokinetic Evaluation. Mol. Pharm. 2021, 18, 3108–3115.
- Tay, E.; Nguyen, T.H.; Ford, L.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. Ionic Liquid Forms of the Antimalarial Lumefantrine in Combination with LFCS Type IIIB Lipid-Based Formulations Preferentially Increase Lipid Solubility, in Vitro Solubilization Behavior and in Vivo Exposure. Pharmaceutics 2020, 12, 17.
- Albadawi, H.; Zhang, Z.; Altun, I.; Hu, J.; Jamal, L.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S.; Oklu, R. Percutaneous Liquid Ablation Agent Fortumor Treatment and Drug Delivery. Sci. Transl. Med. 2021, 13, eabe3889.
- Tang, W.; Liu, B.; Wang, S.; Liu, T.; Fu, C.; Ren, X.; Tan, L.; Duan, W.; Meng, X. Doxorubicin-Loaded Ionic Liquid-Polydopamine Nanoparticles for Combined Chemotherapy and Microwave Thermal Therapy of Cancer. RSC Adv. 2016, 6, 32434–32440.
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. In Vivo Biocompatibility, Pharmacokinetics, Antitumor Efficacy, and Hypersensitivity Evaluation of Ionic Liquid-Mediated Paclitaxel Formulations. Int. J. Pharm. 2019, 565, 219–226.
- Curreri, A.M.; Kim, J.; Dunne, M.; Angsantikul, P.; Goetz, M.; Gao, Y.; Mitragotri, S. Deep Eutectic Solvents for Subcutaneous Delivery of Protein Therapeutics. Adv. Sci. 2023, 2205389, 2205389.
- Ali, M.K.; Moshikur, R.M.; Goto, M.; Moniruzzaman, M. Recent Developments in Ionic Liquid-Assisted Topical and Transdermal Drug Delivery. Pharm. Res. 2022, 39, 2335–2351.
- Moshikur, R.M.; Ali, M.K.; Moniruzzaman, M.; Goto, M. Recent Advances in Surface-Active Ionic Liquid-Assisted Self-Assembly Systems for Drug Delivery. Curr. Opin. Colloid Interface Sci. 2021, 56, 101515.
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485.
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10.
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P.; Zelinsky, N.D. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189.
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Choline and Amino Acid Based Biocompatible Ionic Liquid Mediated Transdermal Delivery of the Sparingly Soluble Drug Acyclovir. Int. J. Pharm. 2020, 582, 119335.
- Huda, M.N.; Deaguro, I.G.; Borrego, E.A.; Kumar, R.; Islam, T.; Afrin, H.; Varela-Ramirez, A.; Aguilera, R.J.; Tanner, E.E.L.; Nurunnabi, M. Ionic Liquid-Mediated Delivery of a BCL-2 Inhibitor for Topical Treatment of Skin Melanoma. J. Control. Release 2022, 349, 783–795.
- Tanner, E.E.L.; Wiraja, C.; Curreri, C.A.; Xu, C. Stabilization and Topical Skin Delivery of Framework Nucleic Acids Using Ionic Liquids. Adv. Therap. 2020, 3, 2000041.
- Qi, Q.M.; Mitragotri, S. Mechanistic Study of Transdermal Delivery of Macromolecules Assisted by Ionic Liquids. J. Control. Release 2019, 311–312, 162–169.
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.V.L.B.; Mitragotri, S. Topical Delivery of SiRNA into Skin Using Ionic Liquids. J. Control. Release 2020, 323, 475–482.
- Bekdemir, A.; Tanner, E.E.L.; Kirkpatrick, J.; Soleimany, A.P.; Mitragotri, S.; Bhatia, S.N. Ionic Liquid-Mediated Transdermal Delivery of Thrombosis-Detecting Nanosensors. Adv. Healthc. Mater. 2022, 11, 2102685.
- Vaidya, A.; Mitragotri, S. Ionic Liquid-Mediated Delivery of Insulin to Buccal Mucosa. J. Control. Release 2020, 327, 26–34.
- Goto, M.; Tahara, Y.; Morita, K.; Wakabayashi, R.; Kamiya, N. Biocompatible Ionic Liquid Enhances Transdermal Antigen Peptide Delivery and Preventive Vaccination Effect. Mol. Pharm. 2020, 17, 3845–3856.
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustain. Chem. Eng. 2020, 8, 6263–6272.
- Islam, M.R.; Uddin, S.; Chowdhury, M.R.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Insulin Transdermal Delivery System for Diabetes Treatment Using a Biocompatible Ionic Liquid-Based Microemulsion. ACS Appl. Mater. Interfaces 2021, 13, 42461–42472.
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larrañeta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and Characterisation of Novel Poly (Vinyl Alcohol)/Poly (Vinyl Pyrrolidone)-Based Hydrogel-Forming Microneedle Arrays for Enhanced and Sustained Transdermal Delivery of Methotrexate. Int. J. Pharm. 2020, 586, 119580.
- Lin, X.; Yang, Y.; Li, S.; Li, Z.; Sheng, Y.; Su, Z.; Zhang, S. Oil-in-Ionic Liquid Nanoemulsion-Based Adjuvant Simultaneously Enhances the Stability and Immune Responses of Inactivated Foot-and-Mouth Disease Virus. Int. J. Pharm. 2022, 625, 122083.
- Lin, X.; Sheng, Y.; Zhang, X.; Li, Z.; Yang, Y.; Wu, J.; Su, Z.; Ma, G.; Zhang, S. Oil-in-Ionic Liquid Nanoemulsion-Based Intranasal Delivery System for Influenza Split-Virus Vaccine. J. Control. Release 2022, 346, 380–391.
- Ukidve, A.; Cu, K.; Goetz, M.; Angsantikul, P.; Curreri, A.; Tanner, E.E.L.; Lahann, J.; Mitragotri, S. Ionic-Liquid-Based Safe Adjuvants. Adv. Mater. 2020, 32, 2002990.
- Araki, S.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic Liquid-Mediated Transcutaneous Protein Delivery with Solid-in-Oil Nanodispersions. Medchemcomm 2015, 6, 2124–2128.




