
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nezih Pala | -- | 4864 | 2023-04-26 22:23:58 | | | |
| 2 | Rita Xu | -10 word(s) | 4854 | 2023-04-27 03:41:32 | | |
Video Upload Options
Climate change primarily caused by the greenhouse gases emitted as a result of the consumption of carbon-based fossil fuels is considered one of the biggest challenges that humanity has ever faced. Moreover, the Ukrainian crisis in 2022 has complicated the global energy and food status quo more than ever. The permanency of this multifaceted fragility implies the need for increased efforts to have energy independence and requires long-term solutions without fossil fuels through the use of clean, zero-carbon renewables energies. Hydrogen technologies have a strong potential to emerge as an energy eco-system in its production-storage-distribution-utilization stages, with its synergistic integration with solar-wind-hydraulic-nuclear and other zero-carbon, clean renewable energy resources, and with the existing energy infrastructure.
1. Introduction
2. Overview of the Global Energy Landscape
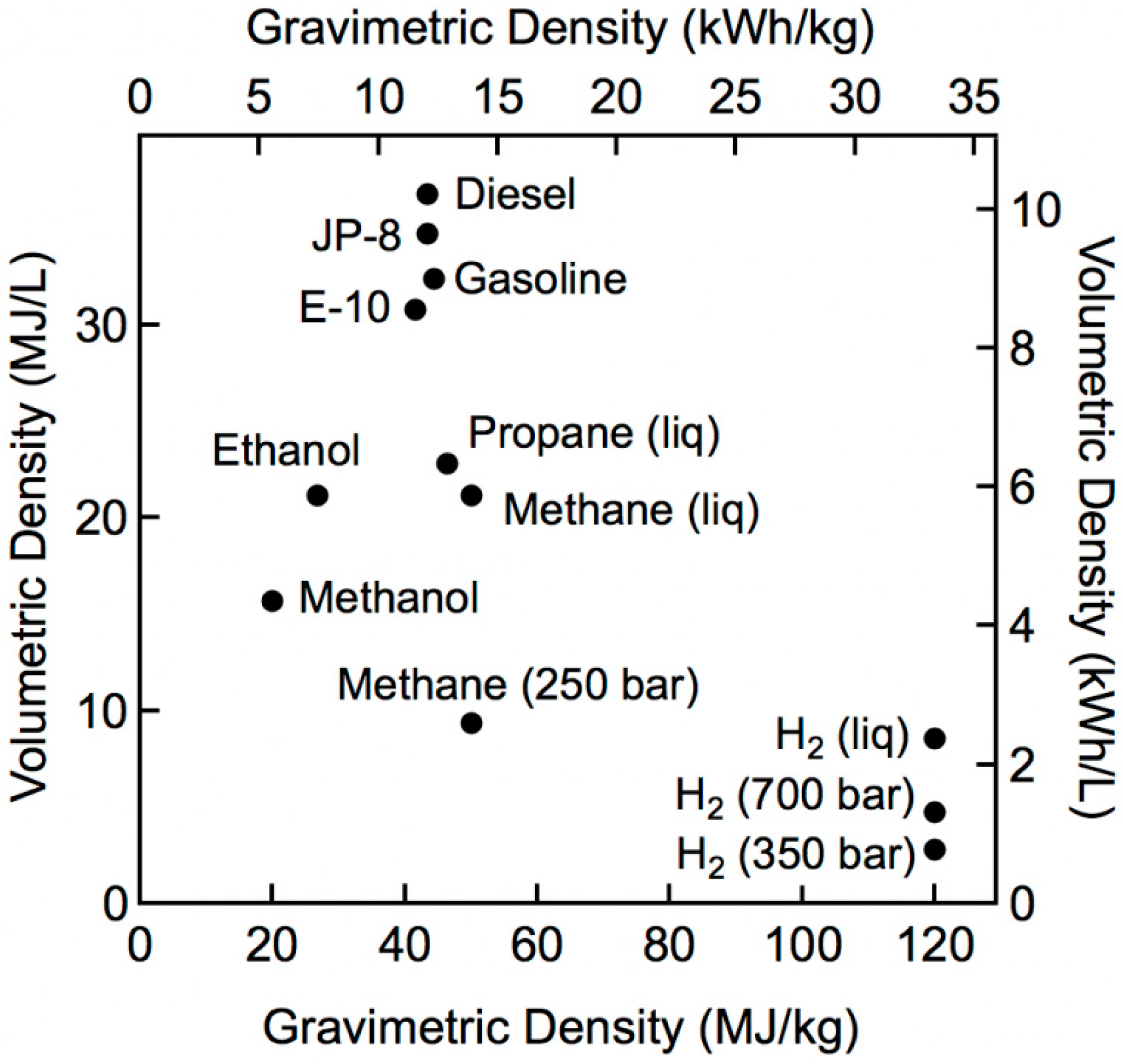

3. Renewable Energies
4. Clean RE for an Integrated H2 Energy System
5. H2 Production
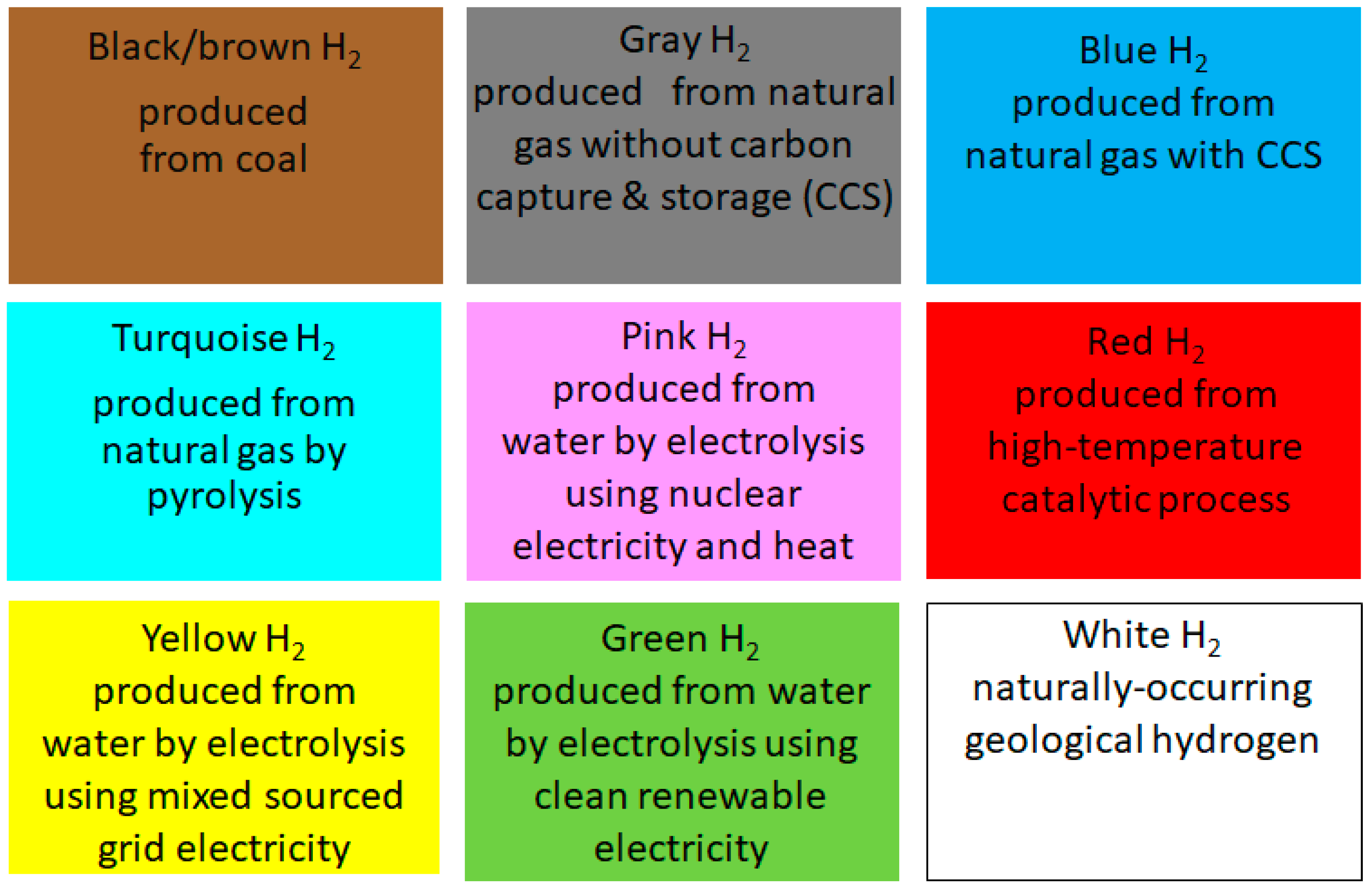
5.1. Black-Brown and Gray H2 Production
5.2. Blue H2 Production
5.3. Turquoise H2 Production
5.4. Pink H2 Production
5.5. Green H2 Production
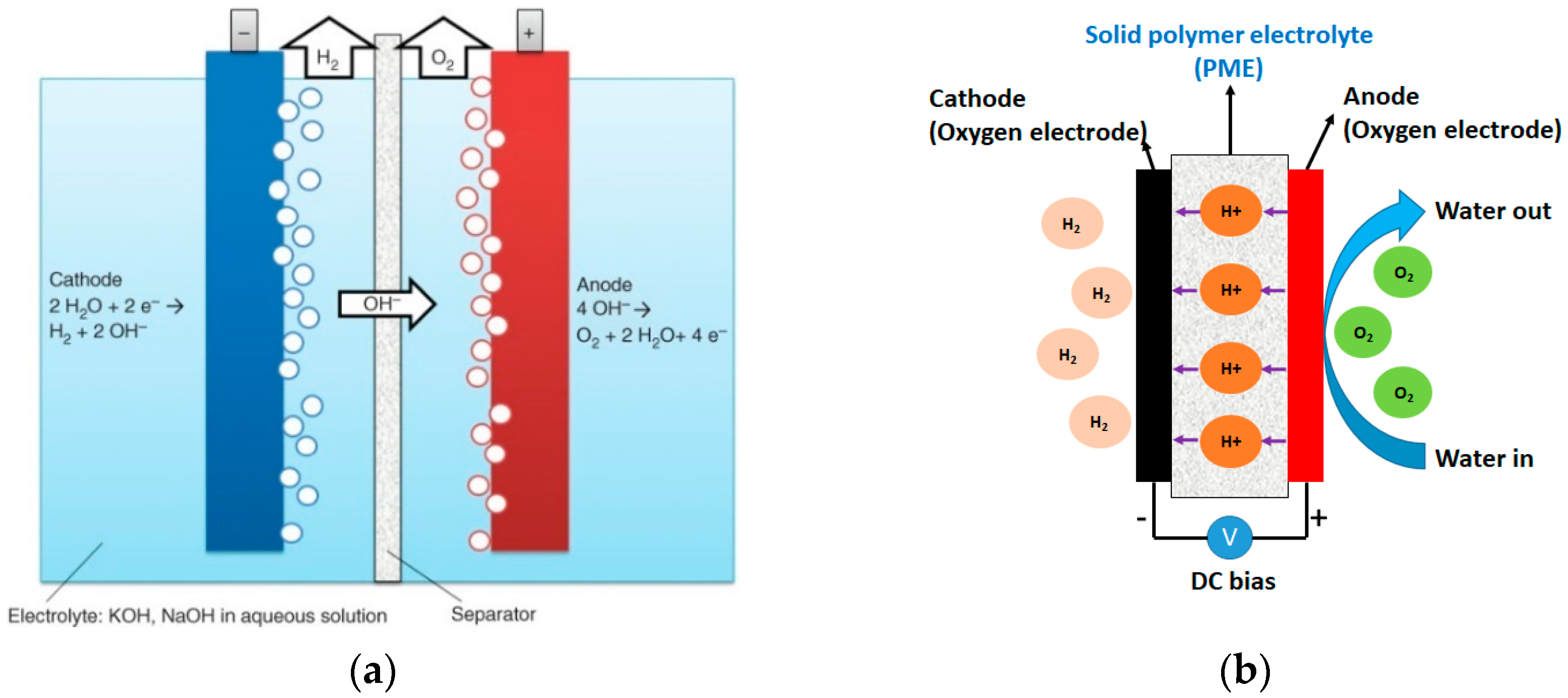
References
- Crippa, M.; Guizzardi, D.; Banja, M.; Solazzo, E.; Muntean, M.; Schaaf, E.; Pagani, F.; Monforti-Ferrario, F.; Olivier, J.; Quadrelli, R.; et al. CO2 Emissions of All World Countries—2022 Report; EUR 31182 EN; Publications Office of the European Union: Luxembourg, 2022.
- IEA. The Future of H2: Report prepared by the IEA for the G20, Japan; IEA: Paris, France, 2019.
- Ari, A.; Arregui, N.; Black, S.; Celasun, O.; Iakova, D.; Mineshima, A.; Mylonas, V.; Parry, I.; Teodoru, I.; Zhunussova, K. Surging Energy Prices in Europe in the Aftermath of the War: How to Support the Vulnerable and Speed up the Transition Away from Fossil Fuels; International Monetary Fund: Washington, DC, USA, 2022.
- Öztürk, Y. Reflections of the Russia-Ukraine War on the Energy Sector. Synergy 2022. Available online: https://www.bilkenteprc.com/post/reflections-of-the-russia-ukraine-war-on-the-energy-sector-yaren-%C3%B6zt%C3%BCrk (accessed on 29 August 2022).
- Resource Trade Earth Dashboard. Available online: https://resourcetrade.earth/ (accessed on 21 November 2022).
- Carroll, S.G. Biofuels’ impact on food security debate resurfaces amid Ukraine war. Euractiv, 8 April 2022.
- Boucher, H. Guest view: Global hunger fight means no biofuel. Reuters, 6 June 2022.
- Hosseini, S.E. Transition away from fossil fuels toward renewables: Lessons from Russia-Ukraine crisis. Future Energy 2022, 1, 2–5.
- Taghizadeh-Hesary, F.; Li, Y.; Rasoulinezhad, E.; Mortha, A.; Long, Y.; Lan, Y.; Zhang, Z.; Li, N.; Zhao, X.; Wang, Y. Green finance and the economic feasibility of hydrogen projects. Int. J. Hydrogen Energy 2022, 47, 24511–24522.
- 14th Five-year Plan for Renewable Energy Development. Available online: https://chinaenergyportal.org/en/14th-five-year-plan-for-renewable-energy-development/ (accessed on 25 December 2022).
- Samsun, R.C.; Rex, M.; Antoni, L.; Stolten, D. Deployment of fuel cell vehicles and hydrogen refueling station infrastructure: A global overview and perspectives. Energies 2022, 15, 4975.
- Wood, J. Which Countries Could Become the World’s Hydrogen Superpowers? Available online: https://www.weforum.org/agenda/2022/02/clean-hydrogen-energy-low-carbon-superpowers/ (accessed on 21 November 2022).
- Hosseini, S.E. Hydrogen has found its way to become the fuel of the future. Future Energy 2022, 1, 11–12.
- Office of Energy Efficiency & Renewable Energy: Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 25 November 2022).
- Dong, Z.Y.; Yang, J.; Yu, L.; Daiyan, R.; Amal, R. A green hydrogen credit framework for international green hydrogen trading towards a carbon neutral future. Int. J. Hydrogen Energy 2022, 47, 728–734.
- IRENA. Renewable Power Generation Costs in 2021; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2022.
- Baldino, C.; O’Malley, J.; Searle, S.; Zhou, Y.; Christensen, A. Hydrogen for heating? Decarbonization options for households in the United Kingdom in 2050. Int. Counc. Clean Transp. 2020. Available online: https://theicct.org/publication/hydrogen-for-heating-decarbonization-options-for-households-in-the-united-kingdom-in-2050/ (accessed on 25 December 2022).
- Longden, T.; Beck, F.J.; Jotzo, F.; Andrews, R.; Prasad, M. ‘Clean’ hydrogen?—Comparing the emissions and costs of fossil fuel versus renewable electricity based hydrogen. Appl. Energy 2022, 306, 118145.
- Agency, I.R.E. Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020.
- Stöckl, F.; Schill, W.-P.; Zerrahn, A. Optimal supply chains and power sector benefits of green hydrogen. Sci. Rep. 2021, 11, 14191.
- Mao, S.; Basma, H.; Ragon, P.-L.; Zhou, Y.; Rodríguez, F. Total Cost of Ownership for Heavy Trucks in China: Battery Electric, Fuel Cell, and Diesel Trucks; National Academy of Sciences: Washington, DC, USA, 2021.
- Zhou, Y.; Searle, S.; Baldino, C. Cost of Renewable Hydrogen Produced Onsite at Hydrogen Refueling Station in Europe; The International Council on Clean Transportation: San Francisco, CA, USA, 2022.
- Siddiqui, O.; Dincer, I. Optimization of a new renewable energy system for producing electricity, hydrogen and ammonia. Sustain. Energy Technol. Assess. 2021, 44, 101023.
- Abdin, Z.; Webb, C.; Gray, E. Solar hydrogen hybrid energy systems for off-grid electricity supply: A critical review. Renew. Sustain. Energy Rev. 2015, 52, 1791–1808.
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180.
- Avargani, V.M.; Zendehboudi, S.; Saady, N.M.C.; Dusseault, M.B. A comprehensive review on hydrogen production and utilization in North America: Prospects and challenges. Energy Convers. Manag. 2022, 269, 115927.
- Sorensen, B. Hydrogen and Fuel Cells: Emerging Technologies and Applications; Academic Press: New York, NY, USA, 2011.
- Demirbas, M.F. Hydrogen from various biomass species via pyrolysis and steam gasification processes. Energy Sources Part A 2006, 28, 245–252.
- Singh, V.; Buelens, L.C.; Poelman, H.; Saeys, M.; Marin, G.B.; Galvita, V.V. Intensifying blue hydrogen production by in situ CO2 utilisation. J. CO2 Util. 2022, 61, 102014.
- Asadullah, M.; Ito, S.-I.; Kunimori, K.; Yamada, M.; Tomishige, K. Energy efficient production of hydrogen and syngas from biomass: Development of low-temperature catalytic process for cellulose gasification. Environ. Sci. Technol. 2002, 36, 4476–4481.
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260.
- Birol, F. The future of hydrogen: Seizing today’s opportunities. IEA Rep. Prep. G 2019, 20. Available online: https://read.oecd-ilibrary.org/energy/the-future-of-hydrogen_1e0514c4-en (accessed on 25 December 2022).
- Watson, N.; Donovan, J. IAEA Modelling Shows High NG Prices Shift Optimal H2 Production to Nuclear Energy. IAEA Department of Nuclear Energy, 28 October 2021.
- Sönnichsen, N. Global hydrogen production outlook by type 2015–2050. Available online: https://www.statista.com/statistics/859104/hydrogen-production-outlook-worldwide-by-type/ (accessed on 29 August 2022).
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R. On the climate impacts of blue hydrogen production. Sustain. Energy Fuels 2022, 6, 66–75.
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687.
- Mac Dowell, N.; Sunny, N.; Brandon, N.; Herzog, H.; Ku, A.Y.; Maas, W.; Ramirez, A.; Reiner, D.M.; Sant, G.N.; Shah, N. The hydrogen economy: A pragmatic path forward. Joule 2021, 5, 2524–2529.
- Cloete, S.; del Pozo, C.A.; Álvaro, Á.J. System-friendly process design: Optimizing blue hydrogen production for future energy systems. Energy 2022, 259, 124954.
- Schneider, S.; Bajohr, S.; Graf, F.; Kolb, T. State of the art of hydrogen production via pyrolysis of natural gas. ChemBioEng Rev. 2020, 7, 150–158.
- Ashton, L.; Fast-Tracking Nuclear Hydrogen: IAEA to Develop Roadmap for Commercial Deployment. International Atomic Energy Agency (IAEA) 5 May 2022. Available online: https://www.iaea.org/newscenter/news/fast-tracking-nuclear-hydrogen-iaea-to-develop-roadmap-for-commercial-deployment (accessed on 29 August 2022).
- IEA. H2 Production and Storage, R&D Priorities and Gaps; International Energy Agency (IEA): Paris, France, 2006.
- Ashton, L. IAEA Ministerial Conference to Highlight Nuclear Power’s Role in Achieving Net Zero and Energy Security; International Atomic Energy Agency (IAEA): Vienna, Austria, 2022.
- US Department of Energy. Energy Department Announces up to $3.5M for Nuclear-Compatible Hydrogen Production Office of Energy Efficiency & Renewable Energy; US Department of Energy: Washington, DC, USA, 2018.
- Watson, N.; Constantin, A. IAEA Event Showcases Progress, Innovations in Nuclear Hydrogen for a Clean Energy Transition; International Atomic Energy Agency (IAEA): Vienna, Austria, 2021.
- BloombergNEF. H2 Economy Outlook; Bloomberg Finance L.P. 2020. Available online: https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 29 August 2022).
- European Commission. H2 Energy and FCSs: A Vision of Our Future; European Commission Directorate-General for Research: Brussels, Belgium, 2003; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/37/121/37121708.pdf (accessed on 29 August 2022).
- Clean H2 Joint Undertaking, E.C. Strategic Research and Innovation Agenda 2021–2027, Annex to GB decision no. CleanHydrogen-GB-2022-02. 25 February 2022. Available online: https://www.clean-hydrogen.europa.eu/system/files/2022-02/Clean%20Hydrogen%20JU%20SRIA%20-%20approved%20by%20GB%20-%20clean%20for%20publication%20%28ID%2013246486%29.pdf (accessed on 29 August 2022).
- Zorpette, G. 2022—The Year the Hydrogen Economy Launched? IEEE Spectr. 17 August 2022. Available online: https://spectrum.ieee.org/hydrogen-economy-inflation-reduction-act (accessed on 29 August 2022).
- Huang, Y.-S.; Liu, S.-J. Chinese green hydrogen production potential development: A provincial case study. IEEE Access 2020, 8, 171968–171976.
- Tang, O.; Rehme, J.; Cerin, P. Levelized cost of hydrogen for refueling stations with solar PV and wind in Sweden: On-grid or off-grid? Energy 2022, 241, 122906.
- Ni, N.; Qie, B.; Du, S.; Sang, Z.; Wang, Q.; Meng, C.; Tong, Y. A novel all-solid-state S-scheme in CdS/ZnTHPP binary nanosystem for hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 13044–13053.
- Dutton, G. Hydrogen Energy Technology; Tyndall Centre for Climate Change Research: Norwich, UK, 2002; Volume 31.
- Ewan, B.; Allen, R. A figure of merit assessment of the routes to hydrogen. Int. J. Hydrogen Energy 2005, 30, 809–819.
- Noyan, Ö.F. Some approach to possible atmospheric impacts of a hydrogen energy system in the light of the geological past and present-day. Int. J. Hydrogen Energy 2011, 36, 11216–11228.
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454.
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317.
- Naito, T.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Gas Crossover Regulation by Porosity—Controlled Glass Sheet Achieves Pure Hydrogen Production by Buffered Water Electrolysis at Neutral pH. ChemSusChem 2022, 15, e202102294.
- Bodner, M.; Hofer, A.; Hacker, V. H2 generation from alkaline electrolyzer. Wiley Interdiscip. Rev. Energy Environ. 2015, 4, 365–381.




