Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | He Yang | -- | 2325 | 2023-04-25 13:32:44 | | | |
| 2 | Lindsay Dong | Meta information modification | 2325 | 2023-04-26 07:55:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, H.; Fang, B.; Wang, Z.; Chen, Y.; Dong, Y. Timing Sequence and Mechanism of Endocrine Organs Aging. Encyclopedia. Available online: https://encyclopedia.pub/entry/43456 (accessed on 02 March 2026).
Yang H, Fang B, Wang Z, Chen Y, Dong Y. Timing Sequence and Mechanism of Endocrine Organs Aging. Encyclopedia. Available at: https://encyclopedia.pub/entry/43456. Accessed March 02, 2026.
Yang, He, Bing Fang, Zixu Wang, Yaoxing Chen, Yulan Dong. "Timing Sequence and Mechanism of Endocrine Organs Aging" Encyclopedia, https://encyclopedia.pub/entry/43456 (accessed March 02, 2026).
Yang, H., Fang, B., Wang, Z., Chen, Y., & Dong, Y. (2023, April 25). Timing Sequence and Mechanism of Endocrine Organs Aging. In Encyclopedia. https://encyclopedia.pub/entry/43456
Yang, He, et al. "Timing Sequence and Mechanism of Endocrine Organs Aging." Encyclopedia. Web. 25 April, 2023.
Copy Citation
The endocrine glands are one of the most important organs in the context of aging. Failure of the endocrine glands lead to an abnormal hormonal environment, which in turn leads to many age-related diseases. The aging of endocrine glands is closely linked to oxidative stress, cellular autophagy, genetic damage, and hormone secretion. The first endocrine organ to undergo aging is the pineal gland, at around 6 years old. This is followed in order by the hypothalamus, pituitary gland, adrenal glands, gonads, pancreatic islets, and thyroid gland.
endocrine
aging
mechanisms
1. Changes in Structure and Function in Endocrine Glands during Aging
The most significant sign of aging is the aging of the endocrine glands [1]; the timing sequence of aging is different (Figure 1) [2][3][4][5][6][7][8][9]. With the decline of endocrine glands, the levels of endocrine hormones are affected by each other (Figure 2).
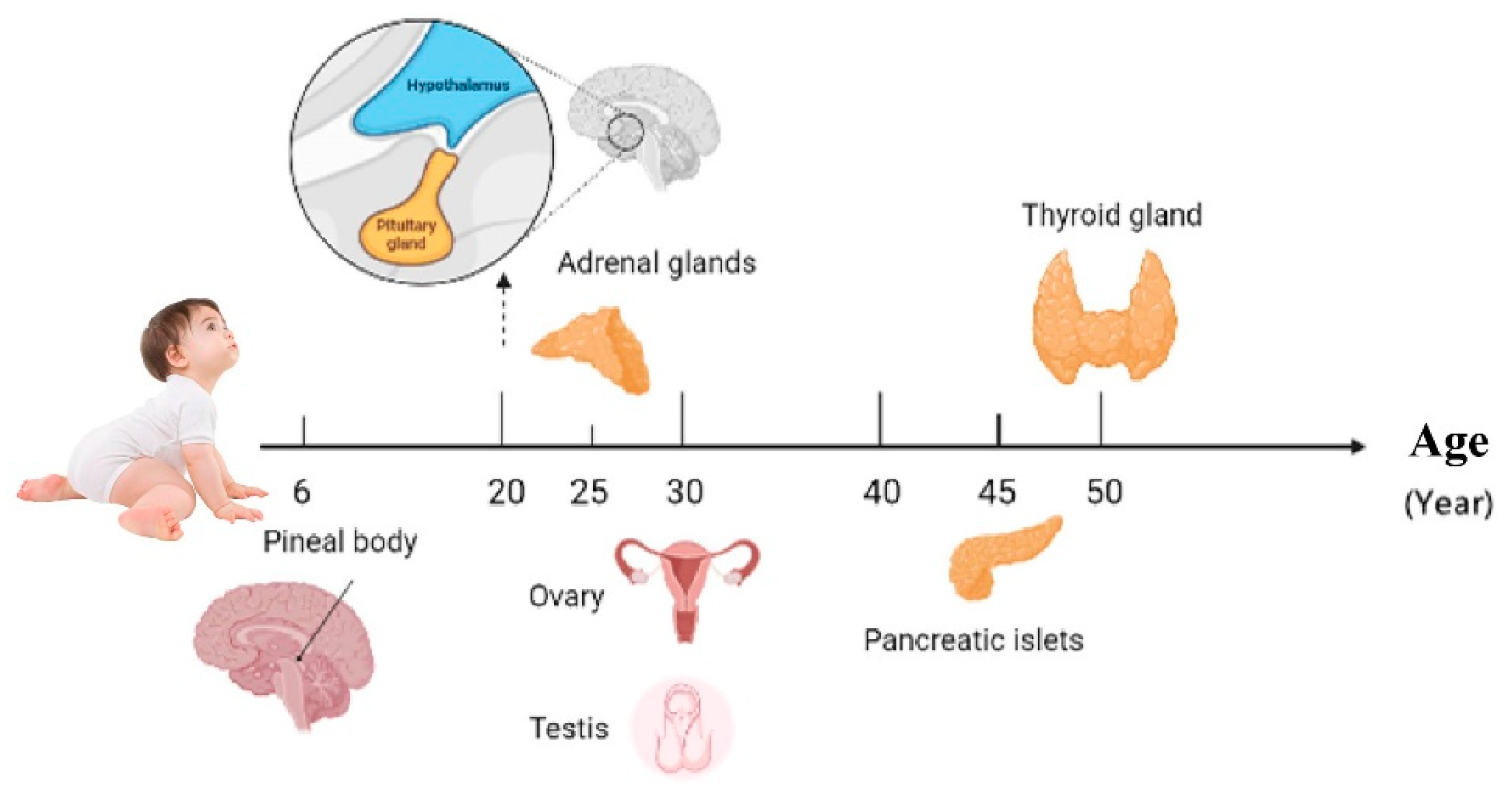
Figure 1. Endocrine glands aging sequence.
Figure 2. Changes in the function of endocrine glands. (A) Endocrine glands in the body. (B) Changes in hormone secretion and the action of endocrine organs. The same color curve represents the same endocrine gland, and the arrow is typically used for a particular organ.Abbreviations: 5-hydroxytryptamine (5-HT), melatonin (MT), gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), prolactin (PRL), growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), triiodothyronine (T3), tetraiodothyronine (T4), dehydroepiandrosterone (DHEA), glucocorticoids (GC), noradrenalin (NE), adrenalin (Ad), insulin (INS), glucagon (GCG), estrogen (E), and testosterone (T).
2. Central Endocrine Glands
2.1. Hypothalamus
There are a group of special neuroendocrine cells in the hypothalamus that play an important role in regulating growth, development, metabolism, reproduction, and the homeostasis of the internal environment [10]. The suprachiasmatic nucleus (SCN) is located in the hypothalamus. The hypothalamus operates according to the photoperiod in order to stimulate internal functioning [11]. With age, abnormalities in the circadian rhythm occur, which in turn is accompanied by a failure of the hypothalamus in the first place [12]. Goyal and his colleagues revealed that the brain declined around the age of 20 [2]. The hypothalamus controls most of the brain’s operations. The hypothalamus is thus referred to as the “aging clock”.
When the hypothalamus begins to age, the blood supply is significantly reduced, the connective tissue proliferates, and the cell morphology is irregular [13][14][15] (Figure 3). Its mechanism is found in the aging hypothalamus, which reduces ATG7 and LC3-II levels as well as the autophagic flow rates. Furthermore, p62 significantly accumulates in the POMC neurons, resulting in increased levels of POMC precursor protein and decreased α-MSH in the hypothalamus of aged mice [16]. In addition, Aβ activates NF-κB by selectively inducing the nuclear translocation of the p65 and p50 subunits, thereby repressing the gonadotropin-releasing hormone Ⅰ (GnRHⅠ) gene [17], thus resulting in a decreased GnRH. Due to positive and negative feedback regulation, growth hormone-releasing hormones (GHRHs) and thyroid-stimulating hormone-releasing hormones (TRHs) both decrease. According to the relevant literature research, by giving the NAL-GLU GnRH antagonist to healthy menopausal women between the ages of 48–57 and 70–77 years, the results demonstrated that the levels of GnRH in elderly women aged 70–77 years, were approximately 42% lower than those aged 48–57 years old [18]. The GHRH levels in 80 year olds were 70% lower than those found in 20 year olds [19]. The regulation of these hormone levels was inseparable from the secretion of monoamine transmitters by the hypothalamic monoaminergic neurons. However, with respect to 60-year-old women, when compared to the ones who were 20, the dopamine levels were decreased by 41%, serotonin by 43%, and norepinephrine by 30% [20].
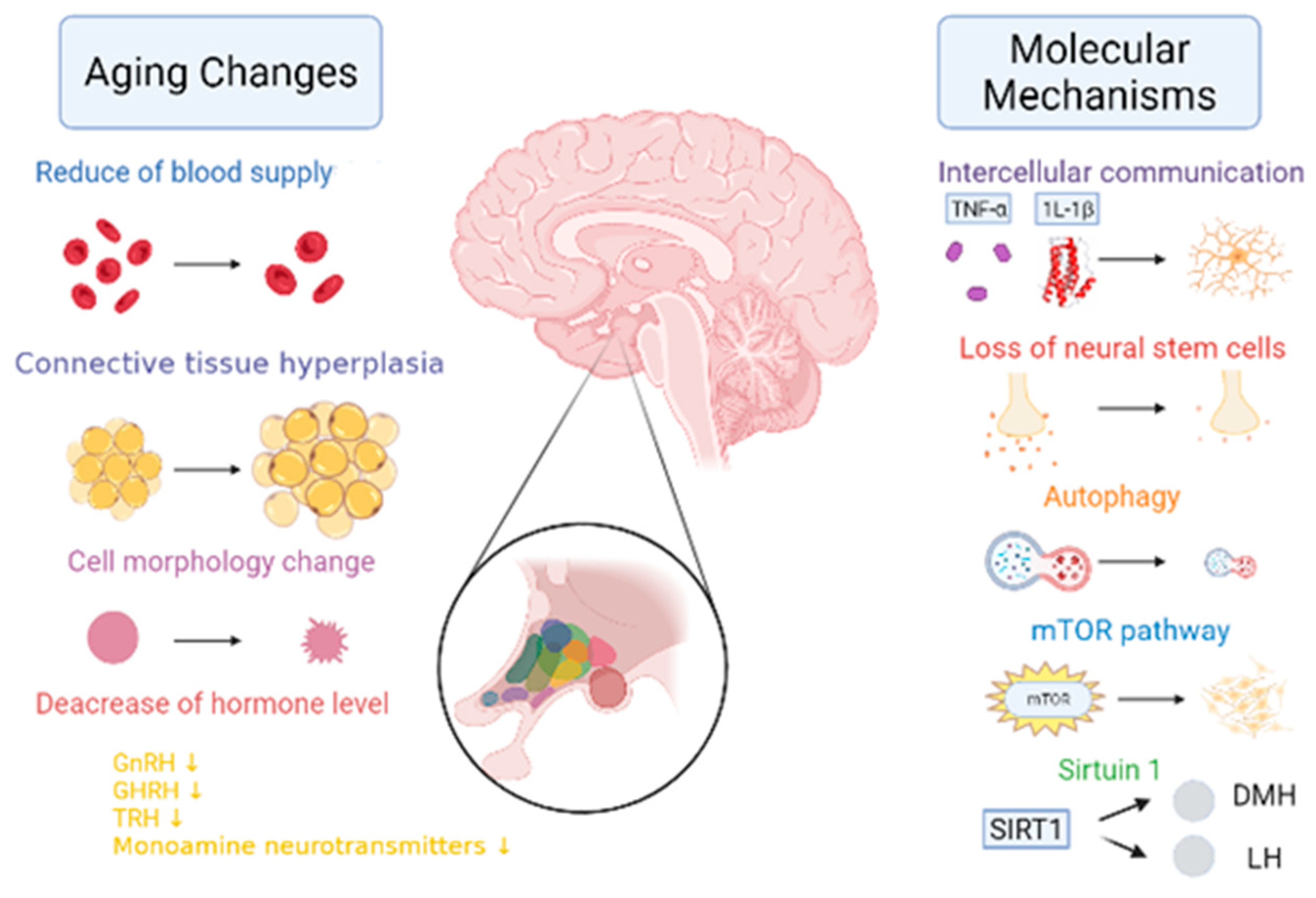
Figure 3. The changes and molecular mechanisms of hypothalamic aging. With aging, the hypothalamus undergoes the following changes in aging: a significant reduction in blood supply [13]; the proliferation of connective tissue [14]; a change in cell morphology [15]; a reduction in the gonadotropin-releasing hormone (GnRH) [17], the growth hormone releasing hormone (GHRH) [17], the thyrotropin-releasing hormone (TRH) [17], and in the monoamine neurotransmitter levels [20]. Molecular mechanisms of aging include the following: a blocked TNF-α and IL-1β signaling; the loss of neural stem cells [21]; reduced autophagy [22]; enhanced mTOR activity [23]; and reduced SIRT1 expression in SCN, thereby resulting in reduced dorsal medial hypothalamic (DMH) and lateral hypothalamic (LH) activity [24][25].
2.2. Pituitary
The pituitary gland secretes five main hormones, namely the growth hormone (GH), thyroid-stimulating hormone (TSH), and gonadotropins—including the luteinizing hormone (LH) and the follicle-stimulating hormone (FSH)—as well as the adrenocorticotropic hormone (ACTH) and prolactin (PRL) (Figure 4). GH dropped by 50% in the 60-year-old women [26]. Before menopause, elderly women had an increase in their FSH secretion by 76% compared with 30-year-old middle-aged women [27]. Furthermore, the FSH secretion of 65-year-old women increased by 50% after menopause [28]. The release of ACTH and TSH hardly changes with aging [29]. In fact, for the 20-year-old women, the pituitary stem cells show degenerative proliferative activation and up-regulated IL-6 injury [3]. Thus, pituitary stem cell capacity is activated. However, with aging, the inflammatory properties of senile glands increase, and the up-regulation ability of IL-6 damage is weakened, such that only organoids can be relied on to restore the activation ability of pituitary stem cells [30]. IGFBP7, TGFβ, IL-8, and IL-6 induce pituitary senescence due to an up-regulation of DNA damage as well as an activation of the p53 pathway [31][32][33]. Cells undergoing oncogene-induced senescence communicate with an inflammatory cytokine-producing environment. In addition, the transcription factor, C/EBPβ, cooperates with IL-6 in order to aggravate the inflammatory response [34]. Ultimately, the aging pituitary displays a notable reduction in mass, a reduction in blood supply, and an increase in connective tissue [35]. Eosinophils and basophils decrease due to a decrease in the number of effective divisions of nerve cells, and the pituitary gland displays more pronounced diffuse fibrosis and increases iron accumulation [36].
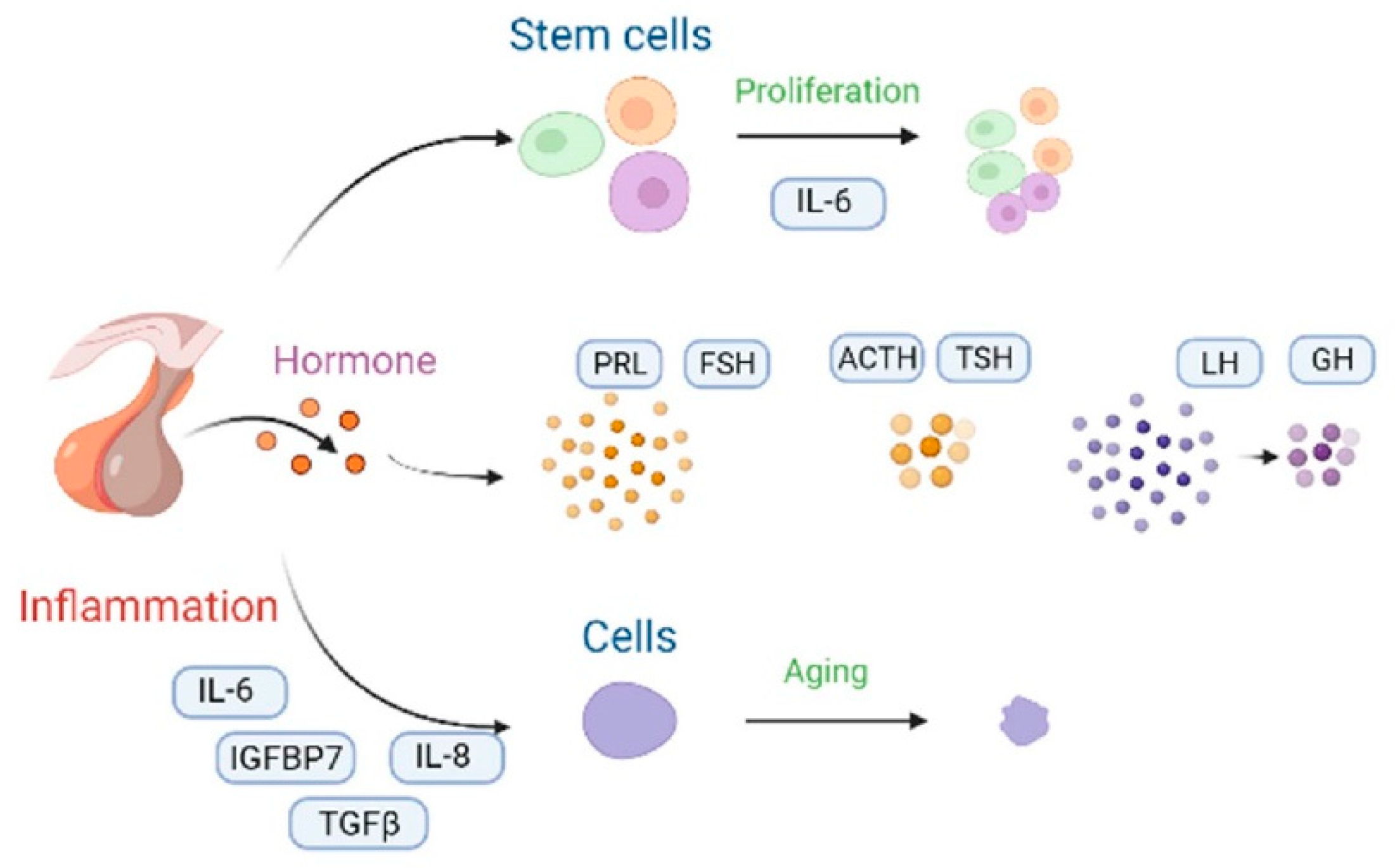
Figure 4. The changes and molecular mechanisms of the pituitary aging hormone. Proliferation of the pituitary stem cells can be enhanced by IL-6 supplementation, and they can also achieve anti-aging purposes [25]. When the levels of the pituitary aging hormones changed, the levels of prolactin (PRL) and the follicle-stimulating hormone (FSH) increased [27], whereas the adrenocorticotropic hormone (ACTH) and the thyroid-stimulating hormone (TSH) did not change significantly [29]. Moreover, the luteinizing hormone (LH) and the growth hormone (GH) levels decreased [26]. The inflammatory factors, including IL-6, IGHBP7, IL-8, and TGFβ activated the cellular senescence inflammatory network [31][32][33]. The black curved arrow represents the transformation method.
2.3. Pineal Body
The pineal gland regulates the neuroendocrine and reproductive systems. Melatonin (MT), which is secreted by the pineal gland, aids with the regulation of the circadian rhythm and inhibits the secretion of gonadotropin. The pineal gland begins to calcify at birth, and melatonin synthesis starts to decline at age six [4]. The aging pineal gland is characterized by vascular narrowing, hardening, weight loss, decreased cell number, and lower melatonin levels. Reduced MT secretion is due to a reduced density of beta-adrenergic receptors in the pineal gland as well as the down-regulation of the AANAT/SNAT gene expression or phosphorylation [37]. Another reason is the increased consumption of MT due to metabolic reasons. For example, senescent cells produce more ROS than younger cells, and MT is used as a regenerative antioxidant to neutralize excessive ROS production in older organisms [38]. MT is a direct free radical scavenger with more powerful antioxidant potential than traditional antioxidants, such as vitamins C and E, as well as mannitol and glutathione [39]. It can quickly remove damaging free radicals in the aging body, and then play a role in delaying aging.
3. Peripheral Endocrine Glands
3.1. Thyroid
The thyroid gland begins to age more quickly around age 50 [5]. It begins to undergo fibrosis and atrophy, resulting in a significant reduction in its size and weight by 40% to 60% [5]. The number, size, and secreted particles of the follicles are reduced, and the glial staining in the follicles is abnormal. The overall level of thyroid hormones decrease. For women who are 60 years old, T3 decreases by 25% to 40%. However, there was no significant change in the T4. That may be due to the TH clearance rate and the decreased secretion [40]. However, T3 decrease may be due to a decreased basal metabolic rate, thereby resulting in a decrease in the peripheral conversion rate of T4 to T3. Up to 6% of subjects over 65-years-old suffer from significant hypothyroidism, and the prevalence of women was higher than that of men [41].
An aging thyroid gland and hormonal abnormalities will cause significant adverse effects on the body. For example, thyroid hormones have a role in regulating heartbeat, pulse, blood circulation, heart contraction, and the rate of oxygen consumption [42]. When thyroid hormone levels are abnormal, primarily T3, there will be adverse effects on the heart and cardiovascular system. Patients may initially experience low blood pressure with thyroid hormone deficiency. After a while, blood pressure rises, resulting in atherosclerosis as well as heart attack and stroke. The rationale boils down to the fact that thyroid hormones are closely linked to genes that regulate the contractile function of the heart, including sarcoplasmic reticulum (SR) Ca2+ ATPase (SERCA2), phospholamban (PLB), and the myosin heavy chains (MHC), α and β [42]. Some of these genes are positively regulated (SERCA2, α-MHC) whereas others are negatively regulated (PLB, β-MHC) by T3 [42][43].
3.2. Adrenal Glands
After age 20, fibrosis, weight loss, cortical nodules, cortical and medullary cells, and connective tissue hyperplasia are evident in adrenal glands [6]. The reticular zone (ZR) thickness reduces, but the total thickness of the adrenal cortex does not alter significantly [44]. The cortical globular zone shrinks, the levels of glucocorticoids decrease, renin activity decreases, and the production of renin-angiotensin II decreases, thereby leading to a decrease in aldosterone content. Catecholamine levels decrease due to lower norepinephrine and epinephrine levels. In a study of aging mice, the ratio of dopamine to norepinephrine was lower in 24-month-old mice than those found in 12-month-old mice. In addition, the ratio of norepinephrine to epinephrine was significantly lower at 24 months of age than at 6 months of age. Adrenal levels were 17% and 37% lower at 12 and 24 months of age, respectively, than at 6 months of age [45]. In addition, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) reached secreted peaks in 20-month-old mice and then began to plummet in 25-month-old mice [45].
4. Endocrine Tissue
4.1. Ovary
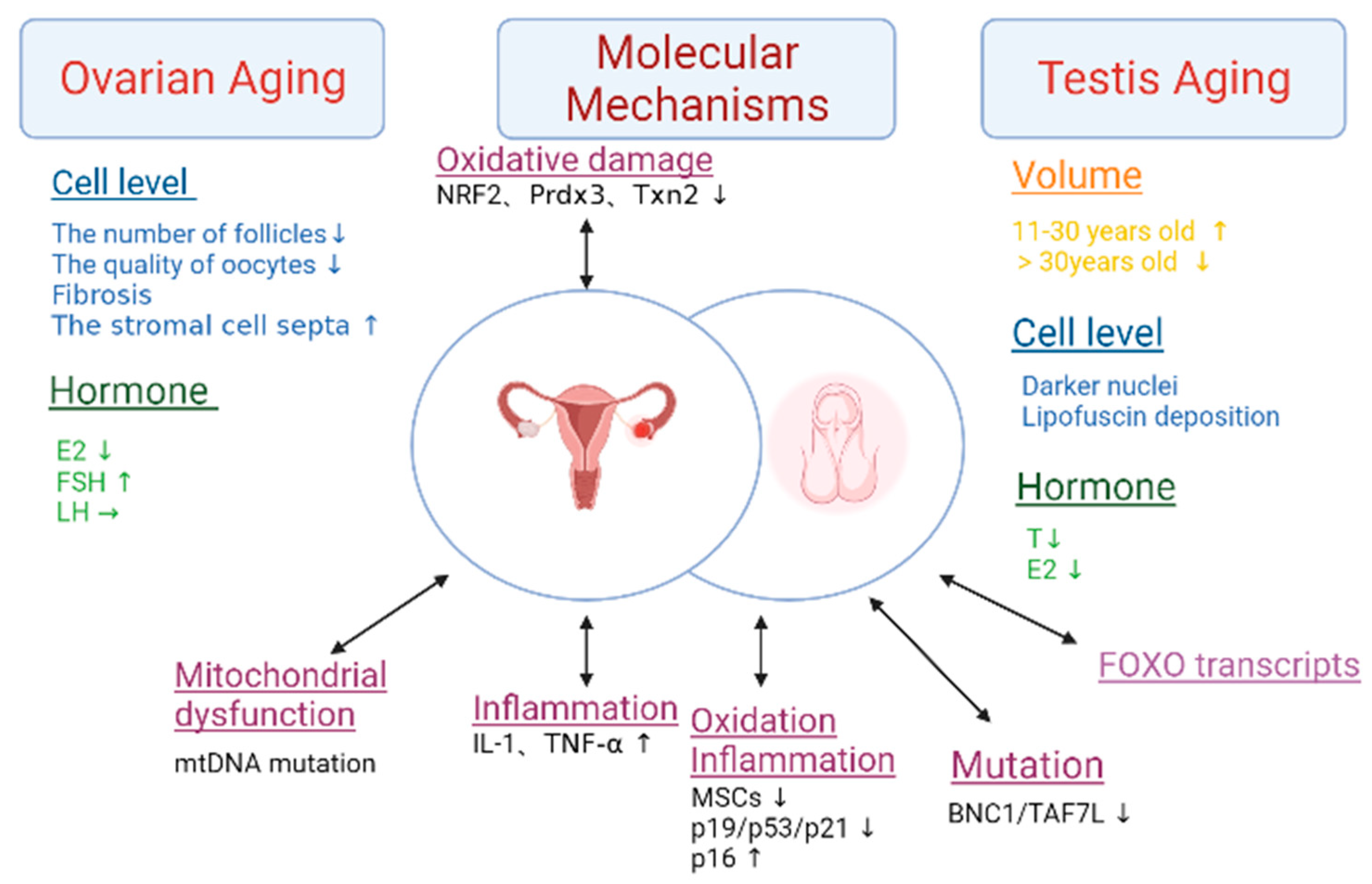
After the age of 30, ovarian function declines significantly, resulting in a decrease in follicle number and oocyte quality [7]. This results in the down-regulation of the Prdx3 and Txn2 genes, which ultimately leads to a decline in female fertility [7] (Figure 5). When women reach 45, fibrosis begins to occur, the uterus and vagina atrophy, and they also lose weight and shrink into smaller pieces of connective tissue. In addition, the stromal cell septa expand. In addition, the medulla and cortex decrease in volume. The swellings on the surface of the ovaries disappear and are flat, with no follicles on the surface, and they also occasionally lose vesicles. The cortex has only a few atresia or cystic follicles, which are rich in connective tissue. Calcinosis or old hemorrhage can be seen, and the blood vessels show signs of sclerosis. Estrogen and progesterone are rapidly reduced. The menstrual cycle gradually develops from disorder to menopause. Under normal circumstances, women enter the menopausal stage around the age of 60. At the age of 40, the frequency of ovulation decreases, and ovarian function ceases within 15 years [46]. During this period, the function of the follicles is extremely poor, and the ovaries no longer secrete estrogen and estradiol (E2). These hormones are only supplied by androstenedione and interstitial ovarian cells in the adrenal cortex, but the total amount is significantly reduced. The concentration of FSH is higher than that of young women, and there was almost no change in LH.

Figure 5. The characteristics and molecular mechanisms of gonads aging. This figure summarizes the changes in volume, cellular levels, and hormone levels in the aging ovary and testis. The mechanisms of aging in the ovary include oxidative damage, mitochondrial dysfunction, and inflammation. The mechanisms of aging in the testis include oxidation, inflammation, mutation and FOXO transcripts. Abbreviations: estradiol (E2), the follicle-stimulating hormone (FSH), the luteinizing hormone (LH), testosterone (T), interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-a), and mesenchymal stem cells (MSCs). The double arrows represent the two-way mode of action.
4.2. Testis
The average testis volume increases between men who are 11–30 years old and only decreases in those who are 30–60 years old, specifically around 2.8%. After reaching 60 years of age, the average testis volume decreases at a rate of 0.42 cm3/y [47]. After 30 years, the testis will see the thickening of the seminiferous tubules and basement membrane, a decrease in seminiferous epithelial cells, and the narrowing and hardening of the lumen [8]. The number of testicular stromal cells decreases and become steroidogenically hypofunctional [48]. Compared with normal cells, the testicular stromal cells of elderly men have darker nuclei and more irregular shapes, and may also contain an underdeveloped endoplasmic reticulum, with many lipofuscin particles attached to the surface [49]. Leydig cell counts are maintained, but these cells may have less testosterone synthesis capacity, which would lower blood testosterone levels. Additionally, there is a reduction in the amount of androgen secreted and a weakening of the testicular response to gonadotropin stimulation. Men begin to experience a decrease in serum total testosterone and free testosterone levels from the age of 50–59. Compared with those who are 20-years-old, when they men are 80, the total testosterone level in the serum drops by 35% and the free testosterone level drops by 50% [50].
4.3. Pancreatic Islets
Pancreatic islets mainly play the role of secreting insulin and glucagon to regulate the body’s blood sugar balance. With aging, the pancreatic islets shrink and the islet cells degenerate. The secretion of glucagon and the responsiveness of stimulation hardly change. The number of β-cells decreases and atrophy occurs. In addition, the process also includes lipofuscin deposits and insulin content decreases. There is another argument that insulin content is not affected by age and that endogenous insulin sensitivity is reduced by 40% [51].
Type 2 diabetes (T2D) is the most typical age-related disease that is caused by the aging of pancreatic islets. Insulin resistance and β-cells dysfunction are two major causes of T2D pathology. The pathological features of pancreatic islets in insulin-dependent T2D mellitus are characterized by acinar cell atrophy, stromal fibrosis, atherosclerosis, and obstructive ductal lesions with epithelial hyperplasia or dysplasia [52]. In addition, T2D patients showed significant histological features of inflammation in the islets, including the infiltration of immune cells [53], amyloid deposits [54], cell death, and fibrosis [55].
References
- Salas, I.H.; Burgado, J.; Allen, N.J. Glia: Victims or villains of the aging brain? Neurobiol. Dis. 2020, 143, 105008.
- Goyal, M.S.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.S.; Morris, J.C.; Raichle, M.E.; Vlassenko, A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA 2019, 116, 3251–3255.
- Seeman, T.E.; Robbins, R.J. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr. Rev. 1994, 15, 233–260.
- Winkler, P.; Helmke, K. Age-related incidence of pineal gland calcification in children: A roentgenological study of 1044 skull films and a review of the literature. J. Pineal Res. 1987, 4, 247–252.
- Freilinger, A.; Kaserer, K.; Zettinig, G.; Pruidze, P.; Reissig, L.F.; Rossmann, T.; Weninger, W.J.; Meng, S. Ultrasound for the detection of the pyramidal lobe of the thyroid gland. J. Endocrinol. Investig. 2022, 45, 1201–1208.
- Parker, C.R.; Slayden, S.M.; Azziz, R.; Crabbe, S.L.; Hines, G.A.; Boots, L.R.; Bae, S. Effects of aging on adrenal function in the human: Responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 48–54.
- Tilly, J.L.; Sinclair, D.A. Germline energetics, aging, and female infertility. Cell Metab. 2013, 17, 838–850.
- Santiago, J.; Silva, J.V.; Alves, M.G.; Oliveira, P.F.; Fardilha, M. Testicular Aging: An Overview of Ultrastructural, Cellular, and Molecular Alterations. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 860–871.
- Bakhti, M.; Böttcher, A.; Lickert, H. Modelling the endocrine pancreas in health and disease. Nat. Rev. Endocrinol. 2019, 15, 155–171.
- Burbridge, S.; Stewart, I.; Placzek, M. Development of the Neuroendocrine Hypothalamus. Compr. Physiol. 2016, 6, 623–643.
- Chi, L.; Li, X.; Liu, Q.; Liu, Y. Photoperiod regulate gonad development via kisspeptin/kissr in hypothalamus and saccus vasculosus of Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0169569.
- Deurveilher, S.; Burns, J.; Semba, K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: A dual tract-tracing study in rat. Eur. J. Neurosci. 2002, 16, 1195–1213.
- Daniel, P.M. The blood supply of the hypothalamus and pituitary gland. Br. Med. Bull. 1966, 22, 202–208.
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150.
- Xie, Y.; Dorsky, R.I. Development of the hypothalamus: Conservation, modification and innovation. Development 2017, 144, 1588–1599.
- Biglari, N.; Gaziano, I.; Schumacher, J.; Radermacher, J.; Paeger, L.; Klemm, P.; Chen, W.; Corneliussen, S.; Wunderlich, C.M.; Sue, M.; et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci. 2021, 24, 913–929.
- Shi, C.; Shi, R.; Guo, H.; Shi, Y.; Liu, X. β-amyloid-induced gonadotropin-releasing hormone decline involving Forkhead transcription factor FOXO3a and nuclear factor-κB. Neuroreport 2020, 31, 923–927.
- Shaw, N.D.; Srouji, S.S.; Histed, S.N.; McCurnin, K.E.; Hall, J.E. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J. Clin. Endocrinol. Metab. 2009, 94, 3259–3264.
- Soule, S.G.; Macfarlane, P.; Levitt, N.S.; Millar, R.P. Contribution of growth hormone-releasing hormone and somatostatin to decreased growth hormone secretion in elderly men. S. Afr. Med. J. 2001, 91, 254–260.
- Gilbreath, E.T.; Jaganathan, L.; Subramanian, M.; Balasubramanian, P.; Linning, K.D.; MohanKumar, S.M.J.; MohanKumar, P.S. Chronic estrogen affects TIDA neurons through IL-1beta and NO: Effects of aging. J. Endocrinol. 2019, 240, 157–167.
- André, C.; Guzman-Quevedo, O.; Rey, C.; Rémus-Borel, J.; Clark, S.; Castellanos-Jankiewicz, A.; Ladeveze, E.; Leste-Lasserre, T.; Nadjar, A.; Abrous, D.N.; et al. Inhibiting Microglia Expansion Prevents Diet-Induced Hypothalamic and Peripheral Inflammation. Diabetes 2017, 66, 908–919.
- Timper, K.; Brüning, J. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689.
- Yang, S.-B.; Tien, A.-C.; Boddupalli, G.; Xu, A.W.; Jan, Y.N.; Jan, L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012, 75, 425–436.
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.-I. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430.
- Kukkemane, K.; Jagota, A. Therapeutic effects of curcumin on age-induced alterations in daily rhythms of clock genes and Sirt1 expression in the SCN of male Wistar rats. Biogerontology 2019, 20, 405–419.
- Verhaeghe, J. Does the physiological acromegaly of pregnancy benefit the fetus? Gynecol. Obstet. Investig. 2008, 66, 217–226.
- Sun, N.; Lin, S.-Q.; Lin, H.-J.; He, Z.; Wang, Y.-H.; Zhang, Y.; Chen, F.-L.; Jiang, Y. Comparison of follicle-stimulating hormone, estradiol, ovarian volume, and antral follicle count, based on the Stages of Reproductive aging Workshop system, among community-based women in China. Menopause 2013, 20, 736–741.
- Amsterdam, J.D.; Maislin, G.; Rosenzweig, M.; Halbrecht, U. Gonadotropin (LH and FSH) response after submaximal GnRH stimulation in depressed premenopausal women and healthy controls. Psychoneuroendocrinology 1995, 20, 311–321.
- Lehmann, M.; Knizia, K.; Gastmann, U.; Petersen, K.G.; Khalaf, A.N.; Bauer, S.; Kerp, L.; Keul, J. Influence of 6-week, 6 days per week, training on pituitary function in recreational athletes. Br. J. Sport. Med. 1993, 27, 186–192.
- Vennekens, A.; Laporte, E.; Hermans, F.; Cox, B.; Modave, E.; Janiszewski, A.; Nys, C.; Kobayashi, H.; Malengier-Devlies, B.; Chappell, J.; et al. Interleukin-6 is an activator of pituitary stem cells upon local damage, a competence quenched in the aging gland. Proc. Natl. Acad. Sci. USA 2021, 118, e2100052118.
- Sapochnik, M.; Fuertes, M.; Arzt, E. Programmed cell senescence: Role of IL-6 in the pituitary. J. Mol. Endocrinol. 2017, 58, R241–R253.
- Wajapeyee, N.; Serra, R.W.; Zhu, X.; Mahalingam, M.; Green, M.R. Role for IGFBP7 in senescence induction by BRAF. Cell 2010, 141, 746–747.
- Kumar, R.; Gont, A.; Perkins, T.J.; Hanson, J.E.L.; Lorimer, I.A.J. Induction of senescence in primary glioblastoma cells by serum and TGFβ. Sci. Rep. 2017, 7, 2156.
- Shao, A.W.; Sun, H.; Geng, Y.; Peng, Q.; Wang, P.; Chen, J.; Xiong, T.; Cao, R.; Tang, J. Bclaf1 is an important NF-κB signaling transducer and C/EBPβ regulator in DNA damage-induced senescence. Cell Death Differ. 2016, 23, 865–875.
- Sano, T.; Kovacs, K.T.; Scheithauer, B.W.; Young, W.F. Aging and the human pituitary gland. Mayo Clin. Proc. 1993, 68, 971–977.
- Cónsole, G.; Dumm, C.; Brown, O.A.; Ferese, C.; Goya, R.G. Sexual dimorphism in the age changes of the pituitary lactotrophs in rats. Mech. Ageing Dev. 1997, 95, 157–166.
- Wolfe, M.S.; Lee, N.R.; Zatz, M. Properties of clock-controlled and constitutive N-acetyltransferases from chick pineal cells. Brain Res. 1995, 669, 100–106.
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509.
- Reiter, R.J. Functional pleiotropy of the neurohormone melatonin: Antioxidant protection and neuroendocrine regulation. Front. Neuroendocrinol. 1995, 16, 383–415.
- Jasim, S.; Gharib, H. Thyroid and Aging. Endocr. Pract. 2018, 24, 369–374.
- He, J.; Lai, Y.; Yang, J.; Yao, Y.; Li, Y.; Teng, W.; Shan, Z. The Relationship Between Thyroid Function and Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Chinese Population. Front. Endocrinol. 2021, 12, 661160.
- Klein, I.; Danzi, S. Thyroid Disease and the Heart. Curr. Probl. Cardiol. 2016, 41, 65–92.
- Danzi, S.; Klein, S.; Klein, I. Differential regulation of the myosin heavy chain genes alpha and beta in rat atria and ventricles: Role of antisense RNA. Thyroid 2008, 18, 761–768.
- Díaz-Aguila, Y.; Cuevas-Romero, E.; Castelán, F.; Martínez-Gómez, M.; Rodríguez-Antolín, J.; Nicolás-Toledo, L. Chronic stress and high sucrose intake cause distinctive morphometric effects in the adrenal glands of post-weaned rats. Biotech. Histochem. 2018, 93, 565–574.
- Amano, A.; Tsunoda, M.; Aigaki, T.; Maruyama, N.; Ishigami, A. Age-related changes of dopamine, noradrenaline and adrenaline in adrenal glands of mice. Geriatr. Gerontol. Int. 2013, 13, 490–496.
- Paciuc, J. Hormone Therapy in Menopause. Adv. Exp. Med. Biol. 2020, 1242, 89–120.
- Yang, H.; Chryssikos, T.; Houseni, M.; Alzeair, S.; Sansovini, M.; Iruvuri, S.; Torigian, D.A.; Zhuang, H.; Dadparvar, S.; Basu, S.; et al. The effects of aging on testicular volume and glucose metabolism: An investigation with ultrasonography and FDG-PET. Mol. Imaging Biol. 2011, 13, 391–398.
- Zirkin, B.R.; Chen, H.; Luo, L. Leydig cell steroidogenesis in aging rats. Exp. Gerontol. 1997, 32, 529–537.
- Gunes, S.; Hekim, G.N.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454.
- Li, D.; Meng, L.; Tao, X.; Su, Y.; Wang, X. Necroptosis promotes the Aging of the Male Reproductive System in Mice. bioRxiv 2017.
- Chang, A.M.; Smith, M.J.; Galecki, A.T.; Bloem, C.J.; Halter, J.B. Impaired beta-cell function in human aging: Response to nicotinic acid-induced insulin resistance. J. Clin. Endocrinol. Metab. 2006, 91, 3303–3309.
- Xin, A.; Mizukami, H.; Inaba, W.; Yoshida, T.; Takeuchi, Y.-K.; Yagihashi, S. Pancreas Atrophy and Islet Amyloid Deposition in Patients With Elderly-Onset Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 3162–3171.
- Martino, L.; Masini, M.; Bugliani, M.; Marselli, L.; Suleiman, M.; Boggi, U.; Nogueira, T.C.; Filipponi, F.; Occhipinti, M.; Campani, D.; et al. Mast cells infiltrate pancreatic islets in human type 1 diabetes. Diabetologia 2015, 58, 2554–2562.
- Kamata, K.; Mizukami, H.; Inaba, W.; Tsuboi, K.; Tateishi, Y.; Yoshida, T.; Yagihashi, S. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid 2014, 21, 191–201.
- Hayden, M.R. Islet amyloid and fibrosis in the cardiometabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2007, 2, 70–75.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
2 times
(View History)
Update Date:
26 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





