Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junzhe Zhao | -- | 2393 | 2023-04-25 07:45:51 | | | |
| 2 | Beatrix Zheng | + 10 word(s) | 2403 | 2023-04-25 08:39:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhao, J.; Fong, A.; Seow, S.V.; Toh, H.C. Translational Immuno-Oncology Research with Organoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/43430 (accessed on 08 February 2026).
Zhao J, Fong A, Seow SV, Toh HC. Translational Immuno-Oncology Research with Organoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/43430. Accessed February 08, 2026.
Zhao, Junzhe, Antoinette Fong, See Voon Seow, Han Chong Toh. "Translational Immuno-Oncology Research with Organoids" Encyclopedia, https://encyclopedia.pub/entry/43430 (accessed February 08, 2026).
Zhao, J., Fong, A., Seow, S.V., & Toh, H.C. (2023, April 25). Translational Immuno-Oncology Research with Organoids. In Encyclopedia. https://encyclopedia.pub/entry/43430
Zhao, Junzhe, et al. "Translational Immuno-Oncology Research with Organoids." Encyclopedia. Web. 25 April, 2023.
Copy Citation
As the complexity of tumour microenvironment (TME) has called for more sophisticated human-based tumour models, organoids have allowed the dynamic study of spatiotemporal interactions between tumour cells and individual TME cell types. Here, the researchers discuss how organoids can study the TME across cancers and how these features may improve precision I/O.
organoid
cancer
immunotherapy
tumour microenvironment
1. Investigating T Cell and ICI Responses
Although unprecedented strides have been made in the role of ICIs across multiple cancers since Allison [1] first uncovered the immunoregulatory role of CTLA-4 and Honjo [2] of PD-1 in the 1990s, a significant proportion of cancer patients still do not benefit. Amongst the responders, intrinsic [3], adaptive, and acquired [4] resistance remains an ongoing challenge. Tumour organoids are excellent platforms for studying T cells and their response to ICIs. The co-culture of dissociated NSCLC/CRC PDOs with autologous T cells from peripheral blood mononuclear cells (PBMCs) enriches the tumour-specific population that, in turn, kills the tumouroids [5][6]. A recent study shows alloreactivity-depleted engineered T cells engage with breast cancer, HNSCC, and glioma PDOs in a manner associated with type I interferon signalling [7]. MOSs [8], PDOTS [9], ALI [10], and TSCs [11][12] have all recapitulated the anti-PD-1 response ex vivo, with the potential to test novel therapeutic combinations such as ICIs + CDK4/6 inhibitors [13]. In another study, PDOs were enriched with matched immune components and treated with pembrolizumab, ipilimumab, or nivolumab for seven days before viability assays [14], which may identify responders. Interestingly, CRC organoids show that the gut microbiota may affect ICI response [15], suggesting its role in immune regulation in addition to tumourigenesis, as examined in other studies [16][17] where organoids are infected with specific bacteria with no or low-dose antibiotics in the media. The pioneering Nobel discoveries of MHC and its restriction (1980, 1996) have laid the foundation for later findings on defective antigen presentation in cancer, which facilitate the evasion of T-cell-mediated killing. Indeed, in CRC [18] and breast cancer [19] organoids, drugs that stimulate MHC-I antigen presentation on the tumour cells can increase T cell cytotoxicity and ICI effectiveness.
Future translational research on tumour organoids and T cell/ICI response may focus on two key questions. First, can ex vivo organoids predict the response of neoadjuvant, adjuvant or upfront systemic ICI, based on tumour biopsies or resected tumours [20]? It is encouraging that there are multiple clinical trials on organoid-guided therapy—such as NCT04777604 for the neoadjuvant space, NCT04736043 for the adjuvant space, and even NCT04931381 in advanced inoperable cancer (Supplementary Table S1 has a complete list)—all of which have offered great promise in validating organoid-guided I/O. Matrigel-based organoids may not serve this purpose well since the cultures may take weeks to stabilise, while PDOTS, ALI, and TSC may establish viable, TME-preserving cultures rapidly. Second, can organoids capture the spatiotemporal dynamics of checkpoint expression [21] and the changes ICI causes in the tumour cells, the TME, and the adjacent normal? This will offer insight into the characteristics of responders versus non-responders and the tumour evolution towards ICI resistance.
2. Unravelling the Functions of TME Cells
Metchnikoff might not have foreseen a century ago [22] that different macrophage populations possess distinct influences on cancers beyond phagocytosis, either in reducing the efficacy of therapies or limiting tumour growth in general. In 2004, Alberto Mantovani and colleagues proposed an M1–M2 spectrum of macrophage polarisation [23], and they subsequently defined the concept of tumour-associated macrophages (TAMs) [24]. Ovarian cancer organoids have shown that UBR5 from tumour cells drives the recruitment and immunosuppressive reprogramming of TAMs [25]. In intestinal adenoma, co-cultured macrophages promote the growth of organoids in a prostaglandin E2-dependent manner, while macrophages themselves adopt a TAM-like phenotype [26]. This reciprocal interaction is abrogated by celecoxib, a selective COX-2 inhibitor. Another co-culture of pancreatic cancer PDOs, U937 monocytes, and primary pancreatic stellate cells leads to increased PDO invasiveness and an M2-like phenotype of U937 [27], whose depletion significantly increases PDO sensitivity to chemotherapy. Finally, autologous co-culture of CRC PDOs with PBMC-derived CD8+ cytotoxic T lymphocytes (CTLs) and macrophages shows that a high sirtuin 1 (SIRT1) level in CRC cells increases macrophage infiltration and M2 polarisation, contributing to CTL dysfunction [28]. While tumour organoids have offered insight into how TAMs may act as friend or foe, caution must be taken when optimising the culture conditions (especially the external growth factor/cytokine supply), as they might shift the phenotypes of many cell types and mask the true effect of tumour–immune interaction. Future organoid-based TAM research may detail how TAMs cause T cell dysfunction [29], tumour cell inflammation, metabolic derangements [30], and increased angiogenesis [31][32] (discussed below). In fact, an M1–M2 dichotomy may be an oversimplification for TAMs in vivo, which can possess markers for both M1 and M2 at the same time [33]. It's known that macrophages are pro-inflammatory during pre-tumour necroinflammation, which are subsequently polarised towards immunosuppressive cells in the tumour. However, what leads to this cascade of TAM reprogramming is not understood; thus, organoids may offer insight into this profound question.
Many years after Burnet and Medawar’s discovery of the concept and principles of immune tolerance, myeloid-derived suppressor cells (MDSCs) were described as a vital contributor to cancer immunosuppression. Several studies have shown viable MDSCs in PDOs, whose depletion sensitises ICI response [34][35]. However, detailed, longitudinal examination of MDSCs in organoids remains rare. The same holds true for dendritic cells (DCs) [36], neutrophils, and other granulocytes [37]—important TME cell types yet to be further studied in organoids.
While lymphoid organoids have been successfully generated in vitro [38][39], the recapitulation or study of B cells in tumour organoids remains challenging and hence very limited [10]. As more research has uncovered the multifarious roles of B cells in the TME [40], further work on B-cell-containing tumour organoids may be necessary.
Endothelial cells (ECs) are another critical cell type in the TME. While it is well known that angiogenesis, a hallmark of cancer [41], can be driven by tumour cells rather aberrantly [31][42][43], the researchers now understand that the endothelium does much more than the sheer lining of blood vessels. For instance, ECs can mediate metastasis [42][44], present antigens via MHC-II [45], reprogram the myeloid phenotypes [46][47], and regulate tissue fibrosis [48]. Tumour organoids have uncovered new roles of ECs other than vascularisation. ECs utilise the tumour cell secretome and hijack the M2-like TAM polarisation for angiogenesis in HCC [31] and glioblastoma [32] organoids. Through their angiocrine functions, ECs in tumour organoids have been shown to drive a pro-inflammatory environment and M1-like TAM polarisation, antagonising the effect of M2-like TAMs [31]. Organoids have also revealed the importance of angiocrine signalling in the survival of pancreatic cancer cells [49][50] and in promoting ovarian cancer metastasis [51]. Strikingly, ovarian cancer organoids with ECs bear a close resemblance to metastatic tumour spheres in the peritoneum or ascites of patients [51][52]. Careful consideration is needed if HUVECs are used in organoids, as they may behave differently from tumour ECs and tissue-specific ECs, such as liver sinusoidal endothelial cells (LSECs) [53]. Further organoid studies are warranted on the spatiotemporal dynamics between ECs, TAMs and T cells, the vascular normalisation by anti-angiogenic drugs [54][55], and the effect of ECs in pre-cancer [56].
Tumour organoids provide the most physiologically relevant platform ex vivo and in vitro for studying CAFs. Defined as the tumour mesenchymal cells that do not bear epithelial, immune, or endothelial markers [57], CAFs in pancreatic cancer maintain their phenotypes only in organoids but not in mono-culture [50][58]. The distinction between α-SMA-positive myofibroblastic CAFs (myCAFs) and IL-6-positive inflammatory CAFs (iCAFs) [58] is driven by TGF-β and IL-1 signalling, respectively [59]. CAF plasticity in pancreatic cancer leads to distinct TME of a reactive, immune-hot subtype versus a deserted, immune-cold counterpart [60]. Intriguingly, while the immune-reactive TME CAFs promote tumour organoid growth and are associated with lower disease-free survival, the immune-deserted TME CAFs confer chemoprotection to the organoids and are associated with lower overall survival [60]. Additionally, tumour organoids for CRC [61][62], HCC [63], and prostate cancer [64] have all shown a similar trophic effect of CAFs and the conferral of therapeutic resistance, seemingly relying on paracrine signalling. It is noted that patient-derived CAFs rapidly differentiate towards myCAFs in vitro, and thus protocol optimisation is key in preserving the CAF phenotype in organoids [62]. Investigation into the recruitment and reprogramming of CAFs, and their paracrine effect on tumour cells and immune cells, will yield exciting insight into the immunoregulatory functions of CAFs and offer new therapeutic opportunities, which are discussed next.
3. Testing of Novel Precision Immuno-Oncology Strategies
The past decade has witnessed the therapeutic revolution ICI has brought to the lives of many cancer patients simply by unleashing the brakes on effector T cells. Now, the reality of tumour heterogeneity, the advance of the researchers' knowledge in the TME, and the breakthroughs of the researchers' ability to interrogate tumour biology have all called for the advent of personalised I/O. Organoids are, and will continue to be, a key driver of precision I/O with their superior accessibility, rapid throughout, and good robustness [65][66]. The researchers begin by summarising in Figure 1 what the researchers have discovered about different cell types from tumour organoids and whether therapies targeting each cell type have been tested on them, discussed in detail next.
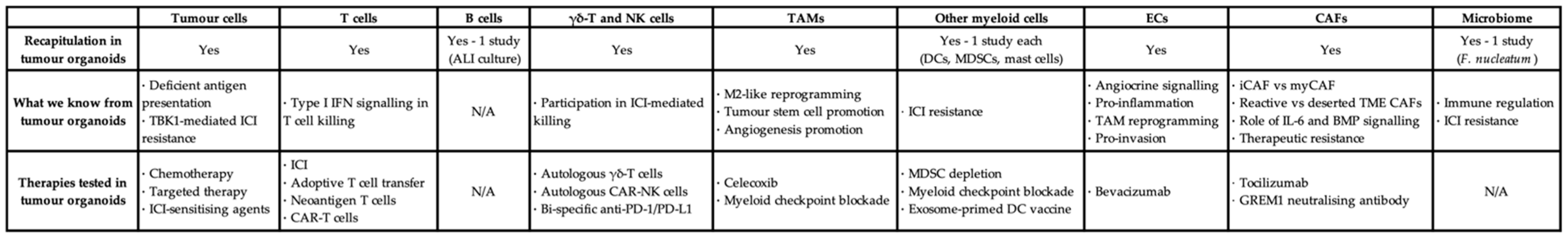
Figure 1. Tabulated summary of different cell types in tumour organoids, the knowledge added, and the therapeutics tested. TAM: tumour-associated macrophage; EC: endothelial cell; CAF: cancer-associated fibroblast; ALI: air–liquid interface; DC: dendritic cell; MDSC: myeloid-derived suppressor cell; ICI: immune checkpoint inhibitor; IFN: interferon; iCAF: inflammatory CAF; myCAF: myofibroblastic CAF; TME: tumour microenvironment; CAR: chimeric antigen receptor.
With combination immunotherapies proving superior survival outcomes across more and more cancers and even in the adjuvant setting [67], and with rising numbers of ongoing combination trials awaiting definitive results, combining ICIs and other therapeutic modalities will define a significant space in cancer immunotherapy strategies in the coming years [68]. New targets on the tumour cells have emerged that directly influence ICI-mediated cytotoxicity. PPARγ-agonists, such as pioglitazone, reprogramme the metabolism and inflammatory response of microsatellite-stable (MSS) CRC cells and increase their PD-L1 expression, sensitising these seemingly resistant organoids to ICI [69]. Targeting TBK1, a kinase with multifaceted functions in innate immunity and cell proliferation, also enhances ICI response, as seen in PDOTS of multiple cancers [70].
Combining ICIs with TME-targeting therapies may also lead to improved clinical response [71], and some of these strategies have been tested in tumour organoids. As discussed earlier, celecoxib in tumour organoids can abrogate the TAM-tumour stem cell interaction [26]. Celecoxib or other NSAIDs (e.g., aspirin in NCT00565708) may be attractive in targeting TAMs, yet their overall clinical effect remains uncertain. Depletion of MDSCs in gastric cancer PDOs increases anti-PD-1/PD-L1-induced killing [34], suggesting the immunosuppressive role of myeloid checkpoints besides PD-L1, such as arginase-1 [72] and VISTA [73][74]. Clinical trials are underway to test anti-VISTA monotherapy or combination with anti-PD-1 in advanced solid tumours (e.g., NCT05082610) [75]. Surprisingly, histamine, via H1 receptors on TAMs, confers ICI resistance by polarising TAMs towards M2 with increased VISTA expression [76]. Organoid testing for H1-antihistamines, anti-VISTA, and other myeloid checkpoint inhibitors may offer a rapid prediction, in-depth molecular insight, and longitudinal monitoring. In addition, ICI combined with bevacizumab has already shown benefits in phase 3 trials [67][77], which warrant organoid studies of EC-targeting therapies [54]. Furthermore, small molecule inhibitors or blocking antibodies targeting FGFR or TGF-β receptors may interfere with CAF activation and action [57], as seen in organoids [78]. However, their clinical benefit remains uncertain [79], suggesting the robustness of CAFs and the difficulty in targeting them. Intercepting the IL-6 axis may also abrogate tumour–CAF interaction [80], yet the effect of drugs (e.g., tocilizumab) both on the TME and systemically is concerning. Crucially, culture conditions, such as growth factor supply and hypoxia, may directly influence the readouts of organoid testing, as discussed in earlier sections.
Adoptive cell transfer of tumour infiltrating lymphocytes (TILs) or tumour-reactive T cells, especially neoantigen-specific T cells [81][82], has emerged as a promising strategy of precision I/O [83]. Lymphodepletion with fludarabine/cyclophosphamide or similar non-ablative conditioning regimens creates a favourable environment for the engraftment, survival, and optimal in vivo expansion of the infused therapeutic T cell product [83][84]. ICIs may be synergistic or additive to adoptive cell therapy [85]. Organoids can boost T cell therapy translation by rapidly enriching tumour-reactive T cells [5][6], unravelling the regulation [86][87] and heterogeneity [88] of MHC-I neoantigen presentation and, crucially, providing a platform for neoantigen validation [85] and autologous killing assays [8][89].
Chimeric antigen receptor (CAR)-T cells have shown unequivocal clinical efficacy against B cell malignancies and multiple myeloma. Testing against solid tumours has shown some encouraging clinical efficacy, although significant challenges from the immunosuppressive TME still exist [90]. The delicate design of the CAR-T cell construct and its activation mechanisms leads to their potent killing capacity and sustained presence and anti-tumour activity [91][92][93]. Organoid-killing assays have shown success in CAR-EGFRvIII T cells for glioblastoma [94], CAR-HBsAg for HBV-HCC [95], as well as CAR-CD70 for renal cell carcinoma [96]. Further investigation on organoid-CAR-T co-culture may offer solutions to CAR-T cell exhaustion in vivo and discover novel targets tackling tumour heterogeneity. For instance, the efficacy of CAR-T cells might be limited by the physical barrier of a fibrotic tumour stroma [97]. Organoid modelling of tissue fibrosis has shown the deposition of collagen and other extracellular matrix proteins [98][99]. By modifying the mechanical and biochemical properties of the matrix or hydrogels in organoid culture, the researchers may unravel the spatiotemporal dynamics of CAR-T infiltration into and their killing of tumouroids.
Finally, NK and γδ-T cell therapies, both autologous and allogenic, have received greater attention now due to their potent anti-tumour capabilities. NK cells typically react to stress ligands via interactions including NKG2A–HLA-E and NKG2D–MIC-A/B [100], while γδ-T cells recognise phosphoantigens, such as exogenous zoledronic acid (ZOL), to exert cytotoxicity [101]. Strikingly, a recent study with ovarian cancer organoids shows that bi-specific PD-1/PD-L1 antibody activates NK cells in addition to CTLs [102], while another study with CRC organoids demonstrates the anti-tumour effect of ICIs from γδ-T cells (rather than αβ-T) when there is MHC-I defect on tumour cells [103], underlining their importance in I/O effectiveness. Organoid testing has also shown tumoricidal activity of γδ-T cells [104] and (CAR-)NK cells [105] for melanoma and mesothelioma, respectively. Importantly, while 3D killing assays have been performed and published extensively for NK and γδ-T cells, most “tumours” are only spheroid aggregates of cancer cell lines, which do not recapitulate the TME. Killing assays on organoids, on the other hand, will unveil distinct mechanisms that facilitate or inhibit cell therapies, many of which arise from interactions with the TME rather than with tumour cells alone. For instance, the researchers' group is studying the distinct phenotypes between NKG2A(+) and NKG2A(-) γδ-T cells, where tumour organoid co-culture may offer a better understanding.
References
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723.
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018, 174, 1586–1598.e1512.
- Cattaneo, C.M.; Dijkstra, K.K.; Fanchi, L.F.; Kelderman, S.; Kaing, S.; van Rooij, N.; van den Brink, S.; Schumacher, T.N.; Voest, E.E. Tumor organoid-T-cell coculture systems. Nat. Protoc. 2020, 15, 15–39.
- Dekkers, J.F.; Alieva, M.; Cleven, A.; Keramati, F.; Wezenaar, A.K.L.; van Vliet, E.J.; Puschhof, J.; Brazda, P.; Johanna, I.; Meringa, A.D.; et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 2022, 41, 60–69.
- Ding, S.; Hsu, C.; Wang, Z.; Natesh, N.R.; Millen, R.; Negrete, M.; Giroux, N.; Rivera, G.O.; Dohlman, A.; Bose, S.; et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 2022, 29, 905–917.
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D.; et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018, 8, 196–215.
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid modeling of the tumor immune microenvironment. Cell 2018, 175, 1972–1988.
- Jabbari, N.; Kenerson, H.L.; Lausted, C.; Yan, X.; Meng, C.; Sullivan, K.M.; Baloni, P.; Bergey, D.; Pillarisetty, V.G.; Hood, L.E.; et al. Modulation of immune checkpoints by chemotherapy in human colorectal liver metastases. Cell Rep. Med. 2020, 1, 100160.
- Voabil, P.; de Bruijn, M.; Roelofsen, L.M.; Hendriks, S.H.; Brokamp, S.; van den Braber, M.; Broeks, A.; Sanders, J.; Herzig, P.; Zippelius, A.; et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat. Med. 2021, 27, 1250–1261.
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018, 8, 216–233.
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’Agostino, R., Jr.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid platform in preclinical investigation of personalized immunotherapy efficacy in appendiceal cancer: Feasibility study. Clin. Cancer Res. 2021, 27, 5141–5150.
- Gao, Y.; Bi, D.; Xie, R.; Li, M.; Guo, J.; Liu, H.; Guo, X.; Fang, J.; Ding, T.; Zhu, H.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 398.
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe. 2015, 17, 763–774.
- McCracken, K.W.; Cata, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404.
- Xu, H.; Van der Jeught, K.; Zhou, Z.; Zhang, L.; Yu, T.; Sun, Y.; Li, Y.; Wan, C.; So, K.M.; Liu, D.; et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J. Clin. Investig. 2021, 131.
- Zhou, Z.; Van der Jeught, K.; Fang, Y.; Yu, T.; Li, Y.; Ao, Z.; Liu, S.; Zhang, L.; Yang, Y.; Eyvani, H.; et al. An organoid-based screen for epigenetic inhibitors that stimulate antigen presentation and potentiate T-cell-mediated cytotoxicity. Nat. Biomed. Eng. 2021, 5, 1320–1335.
- Scognamiglio, G.; De Chiara, A.; Parafioriti, A.; Armiraglio, E.; Fazioli, F.; Gallo, M.; Aversa, L.; Camerlingo, R.; Cacciatore, F.; Colella, G.; et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br. J. Cancer 2019, 121, 979–982.
- Lu, L.G.; Zhou, Z.L.; Wang, X.Y.; Liu, B.Y.; Lu, J.Y.; Liu, S.; Zhang, G.B.; Zhan, M.X.; Chen, Y. PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut 2022, 71, 2551–2560.
- Underhill, D.M.; Gordon, S.; Imhof, B.A.; Nunez, G.; Bousso, P. Elie Metchnikoff (1845–1916): Celebrating 100 years of cellular immunology and beyond. Nat. Rev. Immunol. 2016, 16, 651–656.
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686.
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416.
- Song, M.; Yeku, O.O.; Rafiq, S.; Purdon, T.; Dong, X.; Zhu, L.; Zhang, T.; Wang, H.; Yu, Z.; Mai, J.; et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat. Commun. 2020, 11, 6298.
- He, X.; Smith, S.E.; Chen, S.; Li, H.; Wu, D.; Meneses-Giles, P.I.; Wang, Y.; Hembree, M.; Yi, K.; Zhao, X.; et al. Tumor-initiating stem cell shapes its microenvironment into an immunosuppressive barrier and pro-tumorigenic niche. Cell Rep. 2021, 36, 109674.
- Haque, M.R.; Wessel, C.R.; Leary, D.D.; Wang, C.; Bhushan, A.; Bishehsari, F. Patient-derived pancreatic cancer-on-a-chip recapitulates the tumor microenvironment. Microsyst. Nanoeng. 2022, 8, 36.
- Fang, H.; Huang, Y.; Luo, Y.; Tang, J.; Yu, M.; Zhang, Y.; Zhong, M. SIRT1 induces the accumulation of TAMs at colorectal cancer tumor sites via the CXCR4/CXCL12 axis. Cell Immunol. 2022, 371, 104458.
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456.
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507.
- Lim, J.T.C.; Kwang, L.G.; Ho, N.C.W.; Toh, C.C.M.; Too, N.S.H.; Hooi, L.; Benoukraf, T.; Chow, P.K.; Dan, Y.Y.; Chow, E.K.; et al. Hepatocellular carcinoma organoid co-cultures mimic angiocrine crosstalk to generate inflammatory tumor microenvironment. Biomaterials 2022, 284, 121527.
- Cui, X.; Morales, R.T.; Qian, W.; Wang, H.; Gagner, J.P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M.; et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 2018, 161, 164–178.
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark Res. 2021, 9, 1.
- Koh, V.; Chakrabarti, J.; Torvund, M.; Steele, N.; Hawkins, J.A.; Ito, Y.; Wang, J.; Helmrath, M.A.; Merchant, J.L.; Ahmed, S.A.; et al. Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 2021, 518, 59–71.
- Holokai, L.; Chakrabarti, J.; Lundy, J.; Croagh, D.; Adhikary, P.; Richards, S.S.; Woodson, C.; Steele, N.; Kuester, R.; Scott, A.; et al. Murine- and human-derived autologous organoid/immune cell co-cultures as pre-clinical models of pancreatic ductal adenocarcinoma. Cancers 2020, 12, 3816.
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45.
- Cao, K.; Zhang, G.; Zhang, X.; Yang, M.; Wang, Y.; He, M.; Lu, J.; Liu, H. Stromal infiltrating mast cells identify immunoevasive subtype high-grade serous ovarian cancer with poor prognosis and inferior immunotherapeutic response. Oncoimmunology 2021, 10, 1969075.
- Frede, A.; Czarnewski, P.; Monasterio, G.; Tripathi, K.P.; Bejarano, D.A.; Ramirez Flores, R.O.; Sorini, C.; Larsson, L.; Luo, X.; Geerlings, L.; et al. B cell expansion hinders the stroma-epithelium regenerative cross talk during mucosal healing. Immunity 2022, 55, 2336–2351.
- Purwada, A.; Singh, A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc. 2017, 12, 168–182.
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Silvestri, V.L.; Henriet, E.; Linville, R.M.; Wong, A.D.; Searson, P.C.; Ewald, A.J. A tissue-engineered 3D microvessel model reveals the dynamics of mosaic vessel formation in breast cancer. Cancer Res. 2020, 80, 4288–4301.
- Mazio, C.; Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Recapitulating spatiotemporal tumor heterogeneity in vitro through engineered breast cancer microtissues. Acta Biomater. 2018, 73, 236–249.
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520.
- Kambayashi, T.; Laufer, T.M. Atypical MHC class II-expressing antigen-presenting cells: Can anything replace a dendritic cell? Nat. Rev. Immunol. 2014, 14, 719–730.
- Sakai, M.; Troutman, T.D.; Seidman, J.S.; Ouyang, Z.; Spann, N.J.; Abe, Y.; Ego, K.M.; Bruni, C.M.; Deng, Z.; Schlachetzki, J.C.M.; et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity 2019, 51, 655–670.e658.
- Sharma, A.; Seow, J.J.W.; Dutertre, C.A.; Pai, R.; Bleriot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell 2020, 183, 377–394.e321.
- Winkler, M.; Staniczek, T.; Kurschner, S.W.; Schmid, C.D.; Schonhaber, H.; Cordero, J.; Kessler, L.; Mathes, A.; Sticht, C.; Nessling, M.; et al. Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling. J. Hepatol. 2021, 74, 380–393.
- Choi, J.I.; Jang, S.I.; Hong, J.; Kim, C.H.; Kwon, S.S.; Park, J.S.; Lim, J.B. Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Lett. 2021, 498, 42–53.
- Lai Benjamin, F.L.; Lu Rick, X.; Hu, Y.; Davenport, H.L.; Dou, W.; Wang, E.Y.; Radulovich, N.; Tsao, M.S.; Sun, Y.; Radisic, M. Recapitulating pancreatic tumor microenvironment through synergistic use of patient organoids and organ-on-a-chip vasculature. Adv. Funct. Mater. 2020, 30, 2000545.
- Hoarau-Vechot, J.; Blot-Dupin, M.; Pauly, L.; Touboul, C.; Rafii, S.; Rafii, A.; Pasquier, J. Akt-activated endothelium increases cancer cell proliferation and resistance to treatment in ovarian cancer cell organoids. Int. J. Mol. Sci. 2022, 23, 14173.
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Investig. 2016, 126, 4157–4173.
- Zhang, S.; Wan, Z.; Kamm, R.D. Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature. Lab. Chip. 2021, 21, 473–488.
- Shigeta, K.; Datta, M.; Hato, T.; Kitahara, S.; Chen, I.X.; Matsui, A.; Kikuchi, H.; Mamessier, E.; Aoki, S.; Ramjiawan, R.R.; et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 2020, 71, 1247–1261.
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566.
- Maishi, N.; Annan, D.A.; Kikuchi, H.; Hida, Y.; Hida, K. Tumor endothelial heterogeneity in cancer progression. Cancers 2019, 11, 1511.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596.
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019, 9, 282–301.
- Grunwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M.; et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021, 184, 5577–5592.e5518.
- Luo, X.; Fong, E.L.S.; Zhu, C.; Lin, Q.X.X.; Xiong, M.; Li, A.; Li, T.; Benoukraf, T.; Yu, H.; Liu, S. Hydrogel-based colorectal cancer organoid co-culture models. Acta. Biomater. 2021, 132, 461–472.
- Atanasova, V.S.; de Jesus Cardona, C.; Hejret, V.; Tiefenbacher, A.; Mair, T.; Tran, L.; Pfneissl, J.; Draganić, K.; Binder, C.; Kabiljo, J.; et al. Mimicking tumor cell heterogeneity of colorectal cancer in a patient-derived organoid-fibroblast model. Cell. Mol. Gastroenterol. Hepatol. 2023.
- Liu, J.; Li, P.; Wang, L.; Li, M.; Ge, Z.; Noordam, L.; Lieshout, R.; Verstegen, M.M.A.; Ma, B.; Su, J.; et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 407–431.
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor microenvironment-derived NRG1 promotes antiandrogen resistance in prostate cancer. Cancer Cell 2020, 38, 279–296.
- Ooft, S.N.; Weeber, F.; Schipper, L.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van de Haar, J.; Prevoo, W.; van Werkhoven, E.; Snaebjornsson, P.; et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open 2021, 6, 100103.
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of immunotherapy combination strategies in cancer. Cancer Discov. 2021, 11, 1368–1397.
- Gutting, T.; Hauber, V.; Pahl, J.; Klapproth, K.; Wu, W.; Dobrota, I.; Herweck, F.; Reichling, J.; Helm, L.; Schroeder, T.; et al. PPARgamma induces PD-L1 expression in MSS+ colorectal cancer cells. Oncoimmunology 2021, 10, 1906500.
- Sun, Y.; Revach, O.Y.; Anderson, S.; Kessler, E.A.; Wolfe, C.H.; Jenney, A.; Mills, C.E.; Robitschek, E.J.; Davis, T.G.R.; Kim, S.; et al. Targeting TBK1 to overcome resistance to cancer immunotherapy. Nature 2023, 615, 158–167.
- Kirchhammer, N.; Trefny, M.P.; Auf der Maur, P.; Laubli, H.; Zippelius, A. Combination cancer immunotherapies: Emerging treatment strategies adapted to the tumor microenvironment. Sci. Transl. Med. 2022, 14, eabo3605.
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020, 11, 938.
- Xu, W.; Dong, J.; Zheng, Y.; Zhou, J.; Yuan, Y.; Ta, H.M.; Miller, H.E.; Olson, M.; Rajasekaran, K.; Ernstoff, M.S.; et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol. Res. 2019, 7, 1497–1510.
- Zong, L.; Mo, S.; Sun, Z.; Lu, Z.; Yu, S.; Chen, J.; Xiang, Y. Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod. Pathol. 2022, 35, 266–273.
- Thakkar, D.; Paliwal, S.; Dharmadhikari, B.; Guan, S.; Liu, L.; Kar, S.; Tulsian, N.K.; Gruber, J.J.; DiMascio, L.; Paszkiewicz, K.H.; et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J. Immunother. Cancer 2022, 10, e003382.
- Li, H.; Xiao, Y.; Li, Q.; Yao, J.; Yuan, X.; Zhang, Y.; Yin, X.; Saito, Y.; Fan, H.; Li, P.; et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 2022, 40, 36–52.e39.
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.H.; He, A.R.; Ryoo, B.Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611.
- Kobayashi, H.; Gieniec, K.A.; Wright, J.A.; Wang, T.; Asai, N.; Mizutani, Y.; Lida, T.; Ando, R.; Suzuki, N.; Lannagan, T.R.M.; et al. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology 2021, 160, 1224–1239.e1230.
- Melisi, D.; Oh, D.Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and activity of the TGFbeta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 2021, 9, e002068.
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018, 78, 4957–4970.
- Lowery, F.J.; Krishna, S.; Yossef, R.; Parikh, N.B.; Chatani, P.D.; Zacharakis, N.; Parkhurst, M.R.; Levin, N.; Sindiri, S.; Sachs, A.; et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 2022, 375, 877–884.
- Tran, E.; Robbins, P.F.; Rosenberg, S.A. ‘Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017, 18, 255–262.
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68.
- Tran, E.; Robbins, P.F.; Lu, Y.C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 2016, 375, 2255–2262.
- Wang, W.; Yuan, T.; Ma, L.; Zhu, Y.; Bao, J.; Zhao, X.; Zhao, Y.; Zong, Y.; Zhang, Y.; Yang, S.; et al. Hepatobiliary tumor organoids reveal HLA class I neoantigen landscape and antitumoral activity of neoantigen peptide enhanced with immune checkpoint inhibitors. Adv. Sci. 2022, 9, e2105810.
- Westcott, P.M.K.; Sacks, N.J.; Schenkel, J.M.; Ely, Z.A.; Smith, O.; Hauck, H.; Jaeger, A.M.; Zhang, D.; Backlund, C.M.; Beytagh, M.C.; et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat. Cancer 2021, 2, 1071–1085.
- Newey, A.; Griffiths, B.; Michaux, J.; Pak, H.S.; Stevenson, B.J.; Woolston, A.; Semiannikova, M.; Spain, G.; Barber, L.J.; Matthews, N.; et al. Immunopeptidomics of colorectal cancer organoids reveals a sparse HLA class I neoantigen landscape and no increase in neoantigens with interferon or MEK-inhibitor treatment. J. Immunother. Cancer 2019, 7, 309.
- Demmers, L.C.; Kretzschmar, K.; Van Hoeck, A.; Bar-Epraim, Y.E.; van den Toorn, H.W.P.; Koomen, M.; van Son, G.; van Gorp, J.; Pronk, A.; Smakman, N.; et al. Single-cell derived tumor organoids display diversity in HLA class I peptide presentation. Nat. Commun. 2020, 11, 5338.
- Liu, T.; Tan, J.; Wu, M.; Fan, W.; Wei, J.; Zhu, B.; Guo, J.; Wang, S.; Zhou, P.; Zhang, H.; et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut 2021, 70, 1965–1977.
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365.
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464.
- Imai, C.; Mihara, K.; Andreansky, M.; Nicholson, I.C.; Pui, C.H.; Geiger, T.L.; Campana, D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004, 18, 676–684.
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270.
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 2020, 180, 188–204.
- Zou, F.; Tan, J.; Liu, T.; Liu, B.; Tang, Y.; Zhang, H.; Li, J. The CD39(+) HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8(+) T cells exhibit potent anti-HCC activity. Mol. Ther. 2021, 29, 1794–1807.
- Li, Z.; Xu, H.; Yu, L.; Wang, J.; Meng, Q.; Mei, H.; Cai, Z.; Chen, W.; Huang, W. Patient-derived renal cell carcinoma organoids for personalized cancer therapy. Clin. Transl. Med. 2022, 12, e970.
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69.
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019, 30, 374–384.e376.
- Leite, S.B.; Roosens, T.; El Taghdouini, A.; Mannaerts, I.; Smout, A.J.; Najimi, M.; Sokal, E.; Noor, F.; Chesne, C.; van Grunsven, L.A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 2016, 78, 1–10.
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218.
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. Gammadelta T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404.
- Wan, C.; Keany, M.P.; Dong, H.; Al-Alem, L.F.; Pandya, U.M.; Lazo, S.; Boehnke, K.; Lynch, K.N.; Xu, R.; Zarrella, D.T.; et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021, 81, 158–173.
- de Vries, N.L.; van de Haar, J.; Veninga, V.; Chalabi, M.; Ijsselsteijn, M.E.; van der Ploeg, M.; van den Bulk, J.; Ruano, D.; van den Berg, J.G.; Haanen, J.B.; et al. Gammadelta T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature 2023, 613, 743–750.
- Ou, L.; Wang, H.; Liu, Q.; Zhang, J.; Lu, H.; Luo, L.; Shi, C.; Lin, S.; Dong, L.; Guo, Y.; et al. Dichotomous and stable gamma delta T-cell number and function in healthy individuals. J. Immunother. Cancer 2021, 9, e002274.
- Knelson, E.H.; Ivanova, E.V.; Tarannum, M.; Campisi, M.; Lizotte, P.H.; Booker, M.A.; Ozgenc, I.; Noureddine, M.; Meisenheimer, B.; Chen, M.; et al. Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol. Res. 2022, 10, 947–961.
More
Information
Subjects:
Oncology; Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
663
Revisions:
2 times
(View History)
Update Date:
25 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




