Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anupam Mukherjee | -- | 1657 | 2023-04-24 10:49:12 | | | |
| 2 | Sirius Huang | Meta information modification | 1657 | 2023-04-25 03:40:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, I.; Harshithkumar, R.; More, A.; Mukherjee, A. Human Papilloma Virus and Associated Malignancies. Encyclopedia. Available online: https://encyclopedia.pub/entry/43378 (accessed on 07 February 2026).
Khan I, Harshithkumar R, More A, Mukherjee A. Human Papilloma Virus and Associated Malignancies. Encyclopedia. Available at: https://encyclopedia.pub/entry/43378. Accessed February 07, 2026.
Khan, Ishrat, R Harshithkumar, Ashwini More, Anupam Mukherjee. "Human Papilloma Virus and Associated Malignancies" Encyclopedia, https://encyclopedia.pub/entry/43378 (accessed February 07, 2026).
Khan, I., Harshithkumar, R., More, A., & Mukherjee, A. (2023, April 24). Human Papilloma Virus and Associated Malignancies. In Encyclopedia. https://encyclopedia.pub/entry/43378
Khan, Ishrat, et al. "Human Papilloma Virus and Associated Malignancies." Encyclopedia. Web. 24 April, 2023.
Copy Citation
HPV, or Human Papilloma Virus, has been the primary causative agent of genital warts and cervical cancer worldwide. It is a sexually transmitted infection mainly affecting women of reproductive age group, also infecting men and high-risk group individuals globally, resulting in high mortality.
epidemiology
disease burden
human papilloma virus
cervical cancer
oropharyngeal cancer
vaginal cancer
colorectal cancers
HPV associated malignancies
1. Introduction
Human Papilloma Virus (HPV) is one of the most common causes of sexually transmitted infections worldwide. It is the main etiological agent responsible for multiple types of cancers, primarily cervical cancer in women. HPV is a small, non-enveloped, double-stranded DNA virus infecting the skin and mucosal surfaces. It is classified into two categories: low-risk HPVs (Lr-HPVs) and high-risk HPVs (Hr-HPVs). Lr-HPV is responsible for the manifestation of anogenital and cutaneous warts, while Hr-HPV is majorly responsible for oropharyngeal (oral, tonsil and throat areas) cancers and anogenital cancers, including cervical, anal, vulvar, vaginal and penile cancers [1][2][3][4][5]. In most individuals, the HPV infection clears by itself without ever developing clinical manifestations. Thus, very few of them progress to invasive cancers depending upon the type of HPV variants. The disease is often characterized by subcutaneous and anogenital warts, which may or may not develop into malignancies. The causal association between HPV and cancer has been established for many squamous cell type carcinomas, with growing evidence to link it to cervical (99%), anal (88% of cases), penile (50%), vaginal (70%), vulvar (43%), colorectal (35%) and oropharyngeal (12–53%) cancer (Figure 1) [1].
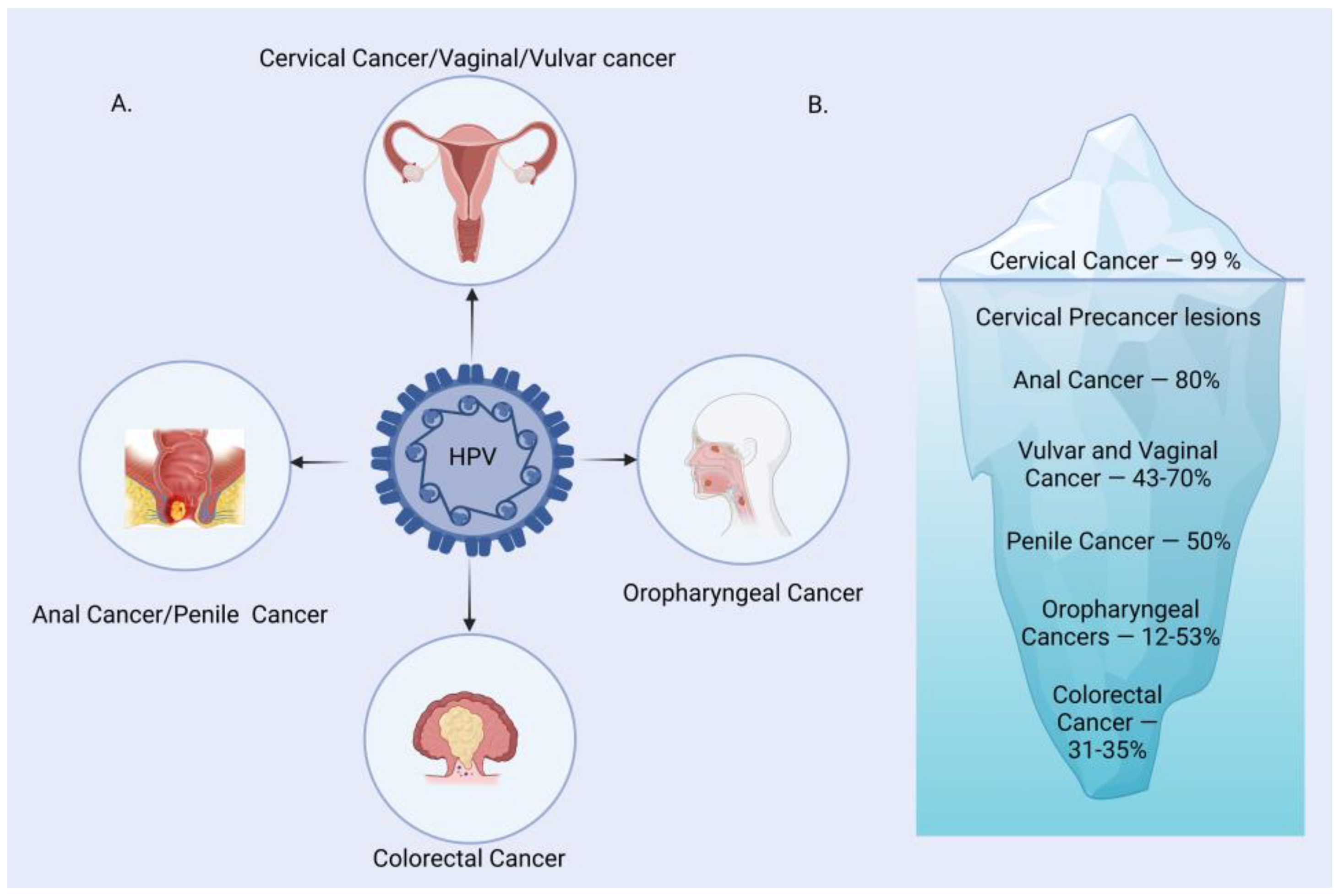
Figure 1. HPV-associated malignancies and their prevalence. (A) A major group of malignancies attributed to HPV. (B) Burden of HPV-related malignancies.
Globally, Cervical cancer is the primary cause of cancer-related mortality among women of reproductive age. Vaccination against HPV is the safest and the most reliable means of primary prevention of cervical carcinoma. Currently, there are three major vaccines available worldwide for HPV; Cervarix, Gardasil and Gardasil 9. But due to the high cost, these vaccines are not accessible to women in developing countries. The age-standardized incidence ratio for cervical cancer in the world is 14 per 100,000 women, while in India, it is 22, which is significantly higher in comparison [6][7]. More than 80 countries have introduced HPV vaccination in their national immunization programs, of which 33 are low- and middle-income countries (LMICs) [8]. Cost-effectiveness studies on HPV vaccination have shown that spending on HPV vaccinations is more cost-effective than treating cervical cancer [9][10]. Apart from cervical cancer, HPV has emerged to be one of the main causative reasons for HPV-induced other malignancies like oropharyngeal, anogenital, colorectal and breast cancers. Current guidelines are mainly focused on cervical cancer screening, but not much progress has been made toward screening for HPV-induced other cancers [11][12]. HPV-associated malignancies have different characteristics compared to other prevalent cancers in terms of variations in clinical, pathological, molecular and epidemiological profiles. As a result, their response towards therapies is markedly different and shows a better response in terms of survival rate [13][14][15]. The incidence and prevalence of HPV-associated malignancies vary remarkably in pathology and disease manifestation depending upon the HPV genotype, vaccine coverage, awareness and geographical and regional conditions [16]. Despite the improvement in vaccination coverage and other intervention studies, the high rate of morbidity related to HPV and HPV-induced cancers still persists. The worldwide burdens of HPV-associated malignancies keep increasing, and the major challenges causing the increase in the burden are associated with (A) incomplete immunization coverage due to lacunae in the established programs or programs not being followed or established successfully and (B) the time of vaccination, which usually is given late and/or not accepted among the populace. Additionally, the variability in the Hr-HPV subtypes and the resulting antigenic protection limit of current vaccines add to the already high burden of infections. These vaccines provide coverage against 7 Hr-HPV, offering 89.6% protection. The high prevalence of other viral and bacterial diseases (epidemiologic cluster) like HIV, EBV and Bacterial Vaginosis also increases the burden associated with HPV infection and HPV-associated diseases in developing countries. Also, the worldwide vaccine uptake is limited, with only a few countries reaching vaccination coverage of 90% of the adolescent female population, which is the 2030 goal of the WHO Cervical Cancer Elimination Strategy [17]. These estimates and analysis make it imperative to study the global burden of HPV and associated malignancies to combat this rising public health concern.
2. Burden of HPV Infection
It is crucial to have a clear epidemiological knowledge of the distribution of cervical human papillomavirus infection in the general population. This knowledge is important to devise vaccine strategies and assess the case burden of HPV over a period of time. Genital HPV infection is one of the most common sexually transmitted infections worldwide. On the basis of cross-sectional observations and meta-analysis done in 2007, it was found that approximately 10% of women worldwide with normal cytological findings through traditional methods carry a detectable cervical HPV infection, although a broad range of estimates (6.1–35.5%) has been documented, depending on the HPV testing technology, study size, the age groups and geographical region studied [18][19].
A second meta-analysis done in 2010 among 100 thousand women from 59 countries found the prevalence of HPV infection among women with normal cytological findings to be 11.7% (adjusted) [20]. The data estimated on the basis of age showed, in all the regions, a peak in HPV infection at younger ages (25 years), declining to a plateau in middle age [20]. Although this data varied on the basis of geographical locations, it gives a clear idea that during the young adult age, the possibility of acquiring HPV infection is much higher compared to other age groups. Though the vaccine-targeted types 16 and 18 are the common variant worldwide, with HPV-16 being the most common one, the other HPV types like 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 are also frequently detected in the general female population worldwide, making for 70% of HPV infections in normal cytological findings [20]. In a study conducted in 2017 in India, the overall prevalence of HPV infection was 60.33%. Out of which, the prevalence of HPV infection was 93.80% (197/210) in invasive cervical cancer (ICC) cases, 54.32% (88/162) in inflammatory smears and 19.11% (13/ 68) in normal cervical cytology. The most prevalent genotype was found to be HPV-16 (87.28%), followed by HPV-18 (24.56%) and HPV-51 (3.46%) [21].
3. HPV and Associated Malignancies
HPV-associated malignancies commonly manifest in the form of cervical, vulvar, anal, colorectal and oropharyngeal cancer. Nearly all cases of cervical cancer can be attributed to HPV infection. Although most HPV infections clear up on their own and most pre-cancerous lesions resolve spontaneously, there is a risk for all women that HPV infection may become chronic and pre-cancerous lesions progress to invasive cervical cancer. HPV being the oncogenic virus, modulates most of the hallmarks of cancer (Figure 2) [22]. HPV oncoproteins play a major role in the progression of malignancies. The hallmarks of cancer comprise eight biological capabilities along with two enabling factors acquired during neoplastic progression. HPV oncoproteins E1 and E2 play a significant role in the initiation and regulation of HPV infection. The E4 protein is majorly involved in viral release, transmission and post-translational modifications [23]. E5, E6 and E7 are major oncoproteins involved in cellular proliferation, invasion and metastasis, cell cycle arrest, angiogenesis, resisting cell death, tumor-promoting inflammation, genomic instability, evading growth suppressors, deregulation of cellular energetics, avoiding immune destruction and enabling replicative immortality [22][23]. The brief role of HPV oncoproteins responsible for their role in acquiring biological capabilities of cancer hallmarks is shown in Figure 2 [23][24][25][26][27]. The prevalence, screening, diagnosis and treatment for all types of malignancies differ in the type of organ affected. Hr-HPVs express E6 and E7, two major oncoproteins, which are responsible for the inhibition of p53 and pRB proteins in human keratinocytes and cellular immortalization (Figure 2). p53 and pRB proteins are responsible for the regulation of many key signaling pathways and gene expression [28]. HPV-associated malignancies differ from their non-HPV counterparts. The unique features of most HPV-positive cancers are summarized earlier [29]. The differences are majorly related to increased mutation activity associated with Apolipoprotein B mRNA Editing Catalytic Polypeptide-like (APOBEC) family of proteins, disrupted DNA repair pathways and altered tumor microenvironment, although it has been interesting to note that HPV-positive malignancies have better prognostic outcomes compared to their negative counterparts (Table 1) [29]. The pathogenesis of HPV-independent carcinoma is basically attributed to specific mutations in the genome. In the case of HPV-negative cervical carcinoma, HPV-negative tumors are characterized by distinct molecular profiles and lower proliferative abilities, p53 immunostaining, decreased expression of p16, p27 and p14 and alterations in PTEN, p53, KRAS, CTNNB1, ARID1A and ARID5B along with lower expression of inflammatory associated genes which explain their poor response rate to checkpoint inhibitor-based immunotherapy such as PD1/PD-L1 inhibitors [30]. In HPV-independent head and neck cancers (HNSCC), p53 mutations and p16 deactivation play an important role in transformation [31]. The main mechanism of p16 deactivation in these carcinomas is attributed to homozygous deletions, mutations and promoter hypermethylation leading to loss of CDKN2A [32]. However, p16 upregulation in HPV-negative head and neck cancers makes them difficult to differentiate them from HPV-positive cancers associated with p16 up or downregulation. Comprehensive molecular profiles of HPV-independent vulvar squamous cell carcinoma also reported alterations in TP53, TERTp, CDKN2A, CCND1 and EGFR [33].
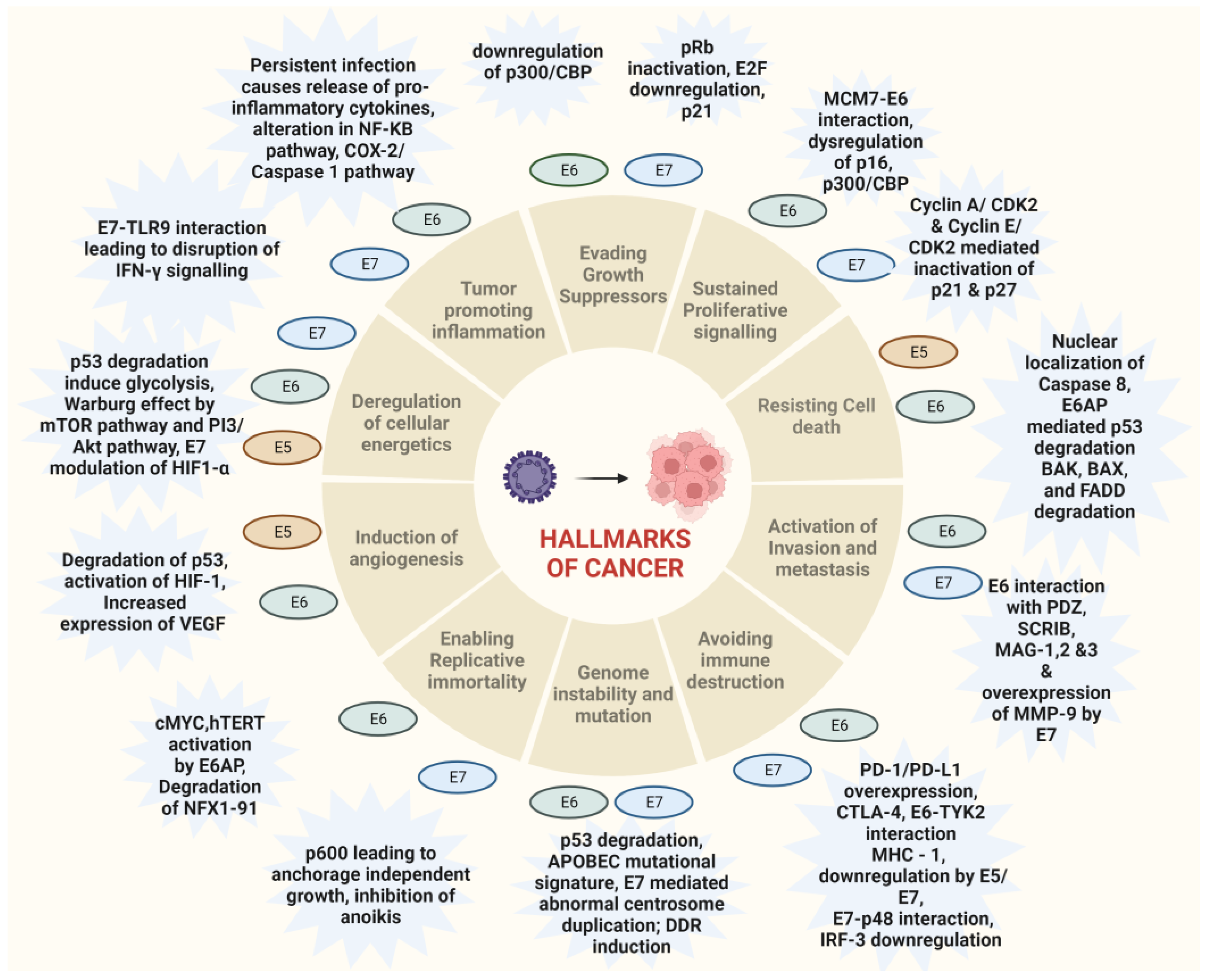
Figure 2. Hallmarks of cancer associated with HPV-driven oncogenic process. The major oncoproteins E6 and E7 and their interactions with specific proteins result in the modulation of major pathways associated with carcinogenesis and drive the hallmarks of cancer.
| Significant Feature of HPV -Associated Malignancy |
Mechanism Involved | Type of Cancer | |
|---|---|---|---|
| HPV Positive Cancers | Enhanced Cell cycle progression | E6 and E7 mediated degradation of p53 and pRb | All |
| Increased radiosensitivity | Regulation of wt-p53, accumulation of double-stranded DNA breaks | HNSCC, Cervical cancer, Anal cancer | |
| Impaired DNA repair | Dysregulation of homologous recombination by the overexpression of p16, Dysregulation of non-homologous end joining by pRB | HNSCC | |
| Upregulated immune checkpoints proteins like PD-L1 and CTLA-4 | E7 mediated PD-L1 expression, Hypermethylation of DNA repair genes | HNSCC, Cervical Cancer | |
| Altered tumor immune microenvironment | Abundance of CD8+ cells, Increased levels of IL-6, IL-8 and CXCL1 | HNSCC, Cervical Cancer | |
| Accumulation of mutations | Higher APOBEC mutations, Disrupted DNA repair mechanisms | HNSCC, Cervical Cancer | |
| CpG methylation in both repetitive and non-repetitive regions | Higher Line -1 Methylation and higher genomic instability | HNSCC |
References
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615.
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global Burden of Human Papillomavirus and Related Diseases. Vaccine 2012, 30, F12–F23.
- Buchanan, T.R.; Graybill, W.S.; Pierce, J.Y. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum. Vaccines Immunother. 2016, 12, 1352–1356.
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2014, 23, 206–224.
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- NCDIR-NCRP ICMR. National Cancer Registry Programme: Three-Year Report of Population-Based Cancer Registries 2012–2014; NCDIR-NCRP (ICMR): Bengaluru, India, 2016.
- Sankaranarayanan, R.; Bhatla, N.; Basu, P. Current global status & impact of human papillomavirus vaccination: Implications for India. Indian J. Med. Res. 2016, 144, 169–180.
- Jit, M.; Brisson, M.; Portnoy, A.; Hutubessy, R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. Lancet Glob. Health 2014, 2, e406–e414.
- Mehrotra, R.; Hariprasad, R.; Rajaraman, P.; Mahajan, V.; Grover, R.; Kaur, P.; Swaminathan, S. Stemming the Wave of Cervical Cancer: Human Papillomavirus Vaccine Introduction in India. J. Glob. Oncol. 2018, 4, JGO.17.00030.
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2017, 67, 100–121.
- American Cancer Society American Cancer Society Guidelines for the Early Detection of Cancer 2022. Available online: https://www.cancer.org/healthy/find-cancer-early/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html (accessed on 11 February 2023).
- Sharma, S.J.; Wagner, S.; Reder, H.S.F.; Kroll, T.; Wuerdemann, N.; Klussmann, J.P.; Wittekindt, C. The 8th edition AJCC/UICC TNM staging for p16-positive oropharyngeal carcinoma: Is there space for improvement? Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 3087–3091.
- Westra, W.H. Detection of human papillomavirus (HPV) in clinical samples: Evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014, 50, 771–779.
- Lombard, I.; Vincent-Salomon, A.; Validire, P.; Zafrani, B.; de la Rochefordière, A.; Clough, K.; Favre, M.; Pouillart, P.; Sastre-Garau, X. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 2613–2619.
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028.
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399.
- de Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459.
- Clifford, G.M.; Gallus, S.; Herrero, R.; Muñoz, N.; Snijders, P.J.F.; Vaccarella, S.; Anh, P.T.H.; Ferreccio, C.; Hieu, N.T.; Matos, E.; et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet 2005, 366, 991–998.
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799.
- Senapati, R.; Nayak, B.; Kar, S.K.; Dwibedi, B. HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect. Dis. 2017, 17, 30.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675.
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327.
- Martínez-Ramírez, I.; Carrillo-García, A.; Contreras-Paredes, A.; Ortiz-Sánchez, E.; Cruz-Gregorio, A.; Lizano, M. Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses. Int. J. Mol. Sci. 2018, 19, 1839.
- Cuninghame, S.; Jackson, R.; Zehbe, I. Hypoxia-inducible factor 1 and its role in viral carcinogenesis. Virology 2014, 456–457, 370–383.
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511.
- Vieira, G.V.; Somera Dos Santos, F.; Lepique, A.P.; da Fonseca, C.K.; Innocentini, L.M.A.R.; Braz-Silva, P.H.; Quintana, S.M.; Sales, K.U. Proteases and HPV-Induced Carcinogenesis. Cancers 2022, 14, 3038.
- Otter, S.; Whitaker, S.; Chatterjee, J.; Stewart, A. The Human Papillomavirus as a Common Pathogen in Oropharyngeal, Anal and Cervical Cancers. Clin. Oncol. 2019, 31, 81–90.
- Fernandes, A.; Viveros-Carreño, D.; Hoegl, J.; Ávila, M.; Pareja, R. Human papillomavirus-independent cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7.
- Dufour, X.; Beby-Defaux, A.; Agius, G.; Lacau St Guily, J. HPV and head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 26–31.
- Sartor, M.A.; Dolinoy, D.C.; Jones, T.R.; Colacino, J.A.; Prince, M.E.P.; Carey, T.E.; Rozek, L.S. Genome-wide methylation and expression differences in HPV(+) and HPV(-) squamous cell carcinoma cell lines are consistent with divergent mechanisms of carcinogenesis. Epigenetics 2011, 6, 777–787.
- Williams, E.A.; Werth, A.J.; Sharaf, R.; Montesion, M.; Sokol, E.S.; Pavlick, D.C.; McLaughlin-Drubin, M.; Erlich, R.; Toma, H.; Williams, K.J.; et al. Vulvar Squamous Cell Carcinoma: Comprehensive Genomic Profiling of HPV+ Versus HPV- Forms Reveals Distinct Sets of Potentially Actionable Molecular Targets. JCO Precis. Oncol. 2020, 4, PO.19.00406.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
25 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




