Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kacper Nijakowski | -- | 3435 | 2023-04-22 01:08:49 | | | |
| 2 | Rita Xu | Meta information modification | 3435 | 2023-04-23 04:33:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nijakowski, K.; Ortarzewska, M.; Jankowski, J.; Lehmann, A.; Surdacka, A. Cellular Metabolism in Dentine-Pulp Complex. Encyclopedia. Available online: https://encyclopedia.pub/entry/43337 (accessed on 09 March 2026).
Nijakowski K, Ortarzewska M, Jankowski J, Lehmann A, Surdacka A. Cellular Metabolism in Dentine-Pulp Complex. Encyclopedia. Available at: https://encyclopedia.pub/entry/43337. Accessed March 09, 2026.
Nijakowski, Kacper, Martyna Ortarzewska, Jakub Jankowski, Anna Lehmann, Anna Surdacka. "Cellular Metabolism in Dentine-Pulp Complex" Encyclopedia, https://encyclopedia.pub/entry/43337 (accessed March 09, 2026).
Nijakowski, K., Ortarzewska, M., Jankowski, J., Lehmann, A., & Surdacka, A. (2023, April 22). Cellular Metabolism in Dentine-Pulp Complex. In Encyclopedia. https://encyclopedia.pub/entry/43337
Nijakowski, Kacper, et al. "Cellular Metabolism in Dentine-Pulp Complex." Encyclopedia. Web. 22 April, 2023.
Copy Citation
The cellular metabolic processes ensure the physiological integrity of the dentine-pulp complex. Odontoblasts and odontoblast-like cells are responsible for the defence mechanisms in the form of tertiary dentine formation. In turn, the main defence reaction of the pulp is the development of inflammation, during which the metabolic and signalling pathways of the cells are significantly altered.
cellular signalling
dental pulp
odontoblast
dental procedures
1. The Physiological Integrity of the Dentine-Pulp Complex
Extensive, detailed knowledge about the biology, physiology and structure of dentine-pulp complex is necessary in clinical dentistry, which mainly aims to preserve pulp vitality. It also helps clinicians select materials, methods, and techniques in restorative dentistry to provide appropriate treatment. Dental pulp consists of many constituents, such as cells, nerves, blood and lymph vessels, fibres and interstitial fluid. All the components participate in the response to dental procedures. Interstitial fluid, which is mostly similar to plasma, maintains the environment essential to cellular functions [1][2].
1.1. Dental Pulp Cells
Many metabolic processes are taking place in the pulp continually. Under physiological conditions, this activity reaches a lower rate; nevertheless, the intensity of metabolic processes increases after irritation with external factors, such as bacteria. One of the first in vivo studies about pulp metabolism was conducted in 1987 by Okiji et al. [3]. Scientists identified the main products of healthy rat dental pulp, such as 6-keto-prostaglandin F1 alpha (6-keto-PGF1α) and 12-hydroxyeicosatetraenoic acid (12-HETE) using high-performance liquid chromatography. A stable metabolite of prostaglandin I2 (PGI2), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2 alpha (PGF2α), and thromboxane B2 (TXB2) were also secreted, but at less than 30% of 6-keto-PGF1α. After that, inflammation was triggered by applying bacterial lipopolysaccharide. The results showed that the production of all these metabolites increased in inflamed rat pulp; however, the highest increase concerned PGE2, which was a 9.3-fold rise in comparison with normal pulp. The authors concluded that arachidonic acid metabolites, including lipoxygenase products, may be involved in the development of inflammation in the dental pulp. This experiment certainly initiated concern about pulpal metabolism issues.
As mentioned earlier, many cellular components, including multipotential mesenchymal stem cells, are found in the pulp, characterised by complicated biological features and promising therapeutic applications. Treatment success depends on the identification of stem cell markers to select the appropriate cell population. Both dental pulp stem cells and stem cells from human exfoliated deciduous teeth share a phenotypic profile of mesenchymal stem cells and express multiple standard markers, including but not limited to CD13, CD29, CD44, CD73, CD90, CD105, CD106, CD146, CD166, CD271, Stro-1, and Stro-3, while negative for CD3, CD8, CD11b (or CD14), CD15, CD19 (or CD79α), CD33, CD34, CD45, CD71, CD117 and HLA-DR. Furthermore, without any stimulation toward differentiation, osteogenic markers, such as osteonectin, osteocalcin, osteopontin, bone morphogenetic protein 2 or 4, runt-related transcription factor 2, and type I collagen, chondrogenic markers, such as type II collagen, adipogenic markers, such as leptin, adipophilin, and peroxisome proliferator-activated receptor gamma, myogenic markers, such as desmin, myogenin, myosin IIa, and alpha-smooth muscle actin are demonstrated. Moreover, octamer-binding transcription factor 4, reduced expression protein 1, Sox2, NANOG, forkhead box D3, and lin-28 homolog A, which are transcription factors responsible for pluripotency in early embryos and embryonic stem cells, were found as well. Notwithstanding, more in vivo studies are necessary because most of these markers are known only from in vitro experiments [4].
Other cells pivotal for pulp biology are fibroblasts. They are underrated, despite playing a critical role in immunoregulatory mechanisms, control of inflammation, and dentine-pulp regeneration [5]. In vitro study conducted by Chmilewsky et al. [6] demonstrated human pulp fibroblasts as the first non-immune cells capable of synthesising all complement proteins involved in initiating dentine-pulp regeneration. Pulp fibroblast metabolism is not as intense without bacterial stimulation. However, the presence of Gram-positive bacteria in carious areas causes complement activation. Moreover, cultured human pulp fibroblasts stimulated with lipoteichoic acid (LTA) express all complement components. This study showed membrane attack complex (C5b-9) formation and C5a active fragment production in the absence of plasma proteins. Furthermore, the experiment demonstrated that the activation of complement proteins produced by fibroblasts and the following release of C5a specifically induced pulp progenitor cell recruitment. To conclude, fibroblast cells participate in tissue regeneration by recruiting pulp progenitors via complement activation, which finds fibroblasts as a potential target in therapeutic strategy connected with dentine-pulp regeneration.
1.2. Odontoblasts—Primary and Secondary Dentine Formation
Odontoblasts are part of the dentine-pulp complex, which is considered a single functional unit responsible for dentine production, nourishment, and sensory function [7]. Cellular odontoblast metabolism is essential to the physiological and pathological function of the endodontium [8]. One of the components of the dentine-pulp complex is dentine, which is a product of odontoblast metabolism. This metabolism may be interpreted as releasing several building components required for synthesising various types of dentine and secreted substances that induce an immune response in the pulp (chemokines, cytokines).
Odontoblasts are post-mitotic, mesenchymal cells derived from the neural crest [9]. These cells are situated on the tooth pulp’s periphery [10] and are the first cells commonly affected by bacteria from tooth caries [11]. Because of their position, odontoblasts serve as a conduit for delivering nutrients, oxygen and inflammatory cells to the odontoblasts. They also maintain a tight relationship with the living section of the tooth, notably with nerve endings and blood capillaries [12].
Couve et al. [13] found that the localisation and composition of organelles in odontoblasts depend on the cell lifecycle stage, as evidenced by the pre-odontoblast, secretory, mature, and old odontoblast phenotypes. Odontoblasts have a large, oval, eccentrically located nucleus. Except for lysosomes, autophagic vacuoles, Golgi complex, smooth endoplasmic reticulum (SER) and the rough endoplasmic reticulum (RER) in mature odontoblasts are located in mitochondria. These organelles are distributed around the odontoblast to provide energy for the movement of secretory molecules inside and towards the apical pole of the cell process. Additionally, proteins are created in the RER and delivered to the Golgi complex for packaging and distribution. These compounds may serve as substrates for dentinogenesis [8].
Odontoblasts are composed of a large number of junctional complexes between cells, creating a selective barrier that may regulate the interaction between dentine and pulp and vice versa under normal and pathological conditions [7]. Secretory odontoblasts have intercellular junctions and connect with fibroblasts at the subodontoblast region in the dental pulp. This enables the transfer of ions and molecules and communication between cells, as well as the active transport of secretory molecules to the odontoblast processes and their release during the secretory phase by the odontoblasts [14].
Under physiological conditions, cellular metabolism comprises the formation of primary and secondary dentine. During primary dentinogenesis (prior to tooth eruption), the secretory stage of the odontoblasts produces and regulates the mineralisation of the predentine matrix to form primary dentine. At their apical pole, cells release substances that are part of the extracellular matrix (ECM) [15], such as collagen type I and non-collagenous proteins [12], including SIBLINGs—phosphorylated matrix proteins, non-phosphorylated matrix proteins and proteoglycans, which participate in the mineralisation of the dentine matrix [7][16]. The odontoblasts move pulpally as additional matrix is deposited, leaving behind one or more cytoplasmic processes that are encircled by predentine matrix, which later mineralises to increase the dentine width [17]. The biomarkers for active dentine synthesis, dentine matrix protein-1 (DMP-1) and human dentine sialophosphoprotein (DSPP) show that the odontoblast is at the secretory stage [18].
By delivering calcium ions and inorganic phosphate to the mineralising front, odontoblasts actively contribute to dentine mineralisation. Via voltage-gated Ca2+ channels (L-type Ca2+ channels) at their basal part and Ca-ATPase and Ca-Na exchangers at their apical pole, respectively, odontoblasts may take in and release calcium ions. In contrast, phosphate transport mechanisms need further research [12].
When teeth erupt and crown development is complete, the odontoblast transitions from the secretory to the mature stage. At this stage, odontoblasts significantly decrease their dentine formation activity in order to produce secondary dentine. Physiological secondary dentine formation will lead to a slow reduction in the pulp chamber size as the matrix is deposited circumpulpally [19]. Secondary dentine is formed at a significantly slower rate of 0.4 μm/day, whereas primary dentine is deposited at 4–20 μm/day [12][20][21]. This secondary dentine is similar to the primary dentine in terms of chemical composition and structural organisation. After primary dentinogenesis, the cell essentially enters a resting state, and the restricted secondary dentine development over many years indicates a basic level of activity of the odontoblast in this resting state [12].
The stage of the lifecycle has a considerable impact on the odontoblast metabolism, which in turn has a significant effect on the functioning of the endodontium since odontoblasts secrete metabolites necessary for the synthesis of primary and secondary dentine.
1.3. Odontoblasts and Odontoblast-Like Cells—Tertiary Dentine Formation
Damage to the tooth tissue leads to the deposition of the tertiary dentine below the injury site [22]. This type of dentine differs from primary and secondary dentine in its composition as well as in the rate of deposition. There are two subtypes of tertiary dentine: reactionary and reparative [21][22]. This classification distinguishes subtypes mainly due to the conditions in which the dentine was formed and the severity of the damaging factor [23]—Figure 1.
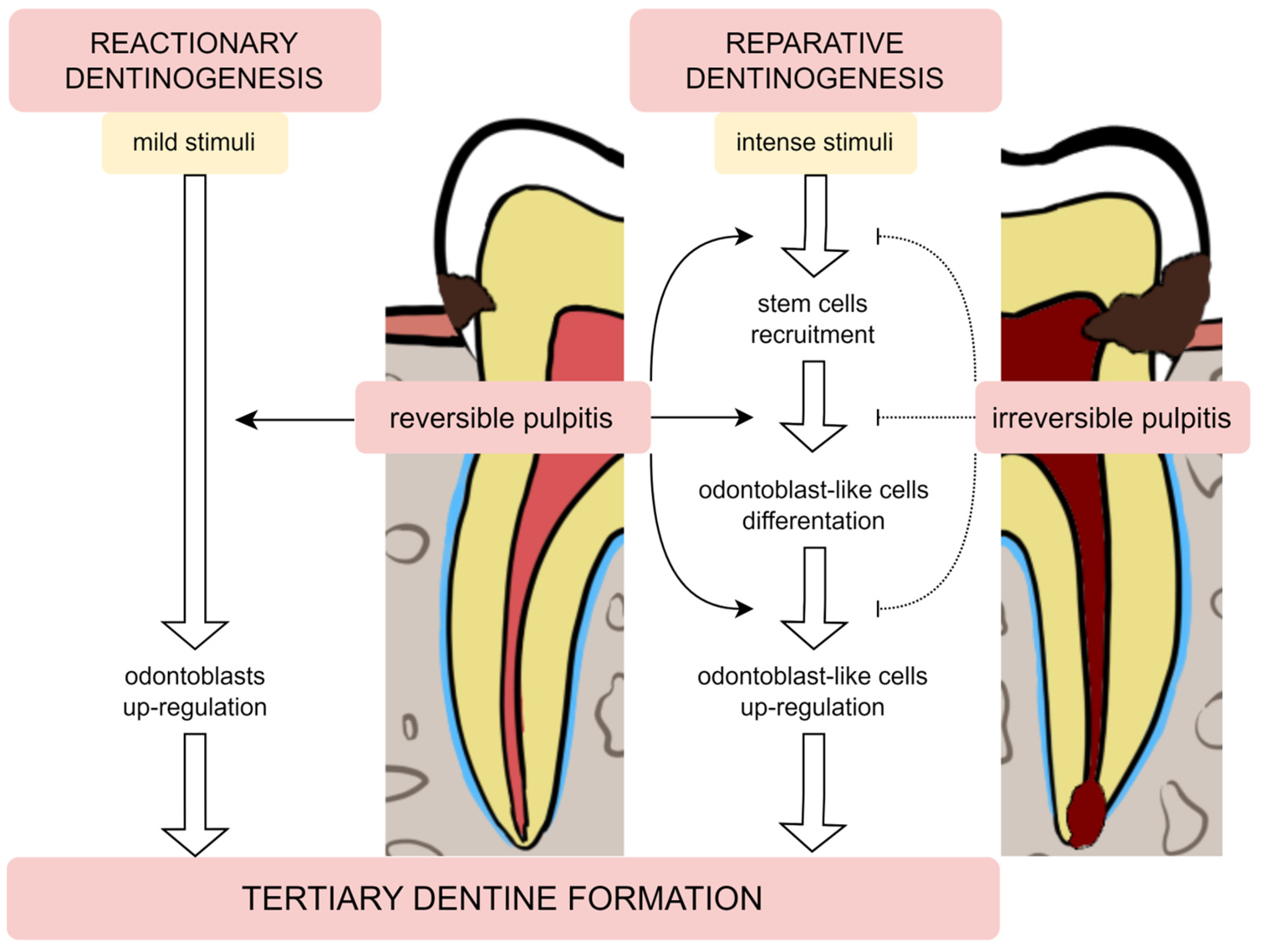
Figure 1. The tertiary dentine formation—reactionary and reparative dentinogenesis during reversible and irreversible pulpitis.
Odontoblasts may endure damage produced by mild stimulation, such as slowly increasing caries or attrition, as well as some restorative methods or proinflammatory mediators [10][24]. Odontoblasts create reactionary dentine in response to mild stimulation by upregulating their baseline secretory activity. Moreover, the release of transforming growth factor β1 (TGF-β1) during dentine demineralisation is a significant activator of odontoblast differentiation and dentine matrix secretion [22][25]. This factor is responsible for the increased odontoblast secretory activity [26].
In contrast, when the noxious stimulus is stronger (e.g., quickly expanding carious lesion), it can lead to the odontoblasts’ death. To replace these cells, the differentiation of stem cell populations into odontoblast-like cells is initiated. These newly differentiated cells secrete reparative dentine, which has an amorphous structure, an atubular shape and imprisoned cells [26]. The formation of this dentine is a more complicated process than the production of reactive dentine, as it requires the recruitment of pulp cells, their differentiation and induction to secrete the matrix of this dentine [27][28].
When the bacteria infect the dentine, it demineralises (as mentioned earlier) and releases dentine matrix components into the dentine-pulp complex [8]. Released growth and angiogenic factors may affect the metabolism of the dentine-pulp complex by promoting angiogenesis or proliferation and differentiation of progenitor cells in the pulp [25][26][29][30]. In addition, some of the substances (DSP) also stimulate the migration and proinflammatory activation of immune system cells [31][32]. However, previous studies mentioned that released dentine matrix components, cytokines and growth factors (TNF-α and TGF-β1) have detrimental effects on pulpal tissue and can cause cellular death [24][32][33][34].
It can be assumed that odontoblasts induce pulp cell metabolism indirectly through the secretion of cytokines and chemokines. Nuclear factor kappa B (NF-κB) is an intracellular signalling pathway activated when harmful bacterial substances, such as lipoteichoic acids (LTA), bind to TLR receptors on dental pulp cells like odontoblasts or fibroblasts. The pulp can then generate a cascade of molecules as a result of the binding of cytokines and chemokines to their specific receptors, which will further activate the pulp’s response to infection. The differentiation processes, however, may be inhibited and obstructed due to the activation of the NF-κB proinflammatory signalling cascade [35][36].
2. The Changes in Metabolic and Signalling Pathways during the Pulp Inflammation
The complications of dental caries are the main cause of triggering inflammatory responses in the dental pulp by the penetration of oral bacteria via the enamel and dentine layers [21]. In response to the release of the metabolically active bacterial components, the dental pulp cells express pattern-recognition receptors, in particular, Toll-like (TLR) and NOD-like receptors (NLR), which can activate nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MPK) signalling [26].
Also, bacterial invasion is closely related to the enhanced release of a wide range of mediators, especially interleukins and matrix metalloproteinases, from the pulp cells [37]. Surprisingly, the dental pulp may be susceptible to SARS-CoV2 infection, and then the patients present worse outcomes of pulp inflammation [38]. In general, pulp inflammation is strictly associated with lipopolysaccharide (LPS) induction. The bacterial LPS mainly affects NF-κB stimulation via TLR receptor activation but also can regulate reactive oxygen species (ROS) production and DNA methylation [39].
Additionally, severe chronic periodontal disease may negatively influence both cementum and dental pulp functions. In the inflamed pulp, the upregulated expression of IL-17 and IL-1β and autophagy markers LC3B and P62 were observed [40]. In addition, reduced oxygen saturation was found in the pulp of teeth with more advanced periodontitis [41]. The dental pulp cells manifest the dynamic response to hypoxia through the modulation of their metabolism by vascular endothelial growth factor (VEGF) expression (regulated by hypoxia-inducible factor-1α) [42]. Similarly, exposure to tumour necrosis factor-α (TNF-α) promotes apoptosis with upregulated VEGF expression and enhanced NF-κB signalling [43].
In a recent study, Yan et al. [44] analysed the cytokine signalling pathways in dental pulp. The activity of TRAIL, NO, IL3, CXCL12 and IL1A was high in the majority of cells in the dental pulp. The dental pulp stem cells demonstrated the elevated activity of NO, TRAIL, CXCL12, BMP4 and BMP6, whereas pulp cells presented the high activity of CXCL12, BMP4, BMP6, BMP2 and IFN1.
Furthermore, Worsley et al. [45] demonstrated that chronic pulpitis promoted the persistent activation of phosphorylated extracellular signal-regulated kinase (pERK) and p38 (pp38) bilaterally in the trigeminal nuclei, and their expression could be further elevated in case of inflammation exacerbation. Yang et al. [46] found that the glutamate signalling mediated by vesicular glutamate transporter-2 could be enhanced in the pulpal axons during the inflammation, leading to hyperalgesia. The pain from the inflamed pulp can refer to other oral sites, e.g., tongue via IL-1RI and TRPV1 signalling in the trigeminal ganglion [47].
Moreover, the expressed miRNAs can show both positive and negative effects during pulp inflammation processes. In inflamed dental pulp, miRNAs can be upregulated or downregulated in inflamed pulpal tissues. They can regulate pulp cell differentiation, cellular metabolism and signalling, migration and apoptosis [48]. Also, DNA methylation can modulate the inflammatory response of human dental pulp cells by regulating miRNA expression [49]. Similarly, the different directions of the lncRNAs expression in pulpitis [50].
3. The Impact of Selected Dental Procedures on Cellular Metabolism in the Dental Pulp
3.1. The Orthodontic Treatment
Orthodontic tooth movements induce vasodilatation and remodelling at the cellular level in the dental pulp [51][52]. Yu et al. [53] suggested that the elevated proinflammatory IL-17A secretion could promote pulp remodelling and alterations in the pulp microenvironment.
The orthodontic intrusion could cause metabolic changes in the dental pulp, reflected by the increased activity of aspartate aminotransferase (AST). Veberiene et al. [54] speculated that the orthodontic force application could promote hypoxia following circulatory disturbances in the pulp cells, thereby altering the mitochondrial oxidative phosphorylation system. The study by Perinetti et al. [55] observed that the elevated levels of AST activity in orthodontically treated teeth were comparable with teeth with reversible pulpitis [56].
However, the controlled mechanical forces during orthodontic treatment led to reversible temporary metabolic changes in pulp tissue. These findings were confirmed by the previous studies assessing the AST activity elevations after 7 days of the orthodontic intrusion, which gradually reduced to the control levels [57][58]. Moreover, Chavarria-Bolanos et al. [59] found that the increased expression of substance P and calcitonin gene-related peptide, as well as the increasing expression trends of β-endorphins and methionine-enkephalin, were found in the dental pulp after the controlled orthodontic intrusion, even in asymptomatic teeth without pain.
3.2. The Conservative Dentistry Procedures
Via ROS signalling, the BisGMA monomers stimulate prostaglandin E2 (PE2) production by the pulp cells, enhancing their MEK/ERK signalling and leading to the higher production of alkaline phosphatase and IL-8 [60][61][62]. Elevated PGE2 levels accelerate neutrophil infiltration and vascular permeability, causing pulpal inflammation common after the application of composite resin restorations [63][64]. The study by Chang et al. [65] found that the pulpal expression of CES2 could protect against the cytotoxicity of the resin monomers and their metabolites (e.g., methacrylic acid).
Also, the other resin monomers, such as UDMA and TEGDMA, can alter the mitochondrial metabolism of fibroblasts, inducing inflammatory processes in the pulp tissue [66][67][68]. In contrast, the polyacrylic acid released from glass-ionomer cement contains long interconnected and intertwined polymer chains, preventing migration via the dentinal tubules and harmful reactions in the pulp. However, several studies reported the cytotoxicity of HEMA [69][70][71]. Davidovic et al. [72] analysed the impact of various liners on dental pulp in experimental animals. Only in individual cases were the increased vasodilatation and hyperaemia observed, which was explained by the fact of performing the cavity preparation during the restorative procedure.
Based on the systematic review conducted by Rathinam et al. [73], tricalcium silicate cement (e.g., mineral trioxide aggregate or Biodentine) promotes the odontogenic capacity of dental pulp cells via the activation of the extracellular signal-regulated kinase ½, nuclear factor E2 related factor 2, p38, c-Jun N-terminal kinase mitogen-activated protein kinase, p42/p44 mitogen-activated protein kinase, nuclear factor kappa B, and fibroblast growth factor receptor pathways. Silicium ions influence increased metabolism, collagen synthesis, bone mineralisation, and connective tissue cross-linking [74]. Both released calcium and phosphate ions can activate the MAPK signalling pathway, inducing odontoblastic differentiation [75][76].
In a recent study, Mendes Soares et al. [77] evaluated if resin infiltration system components could interfere with pulp metabolism. During infiltrating enamel white spot-like lesions, the hydrochloric acid molecules diffuse via enamel and dentine to the pulp cells, reaching toxic concentrations. It led to a significant reduction in total protein production and alkaline phosphatase activity, as well as decreased cell viability and mineralised nodule formation. These impaired metabolic processes influence on defence abilities of the dentine-pulp complex, involving enhanced mineralisation of the dentine matrix. In response to the etchant application, the mineralisation-related genes for alkaline phosphatase, dentine protein 1 and dentine sialophosphoprotein were downregulated, although they are crucial for the proper defensive dentine formation. Additionally, the significantly upregulated expression of genes for IL-1β and TNF-α was found. Interestingly, the biological effects of the only etchant application demonstrated significantly higher toxicity in comparison with the combined resin application after etching. This may be related to the interaction between the residual resin monomers and unreacted dissociated HCl molecules. In in vitro conditions, the resin infiltration negatively affected the metabolic activity of pulp cells and interfered with dentine-pulp homeostasis. Therefore, although the resin infiltration is considered a minimally invasive conservative dentistry procedure, special care should be taken with lesions reaching the external third of the dentine due to the possibility of diffusion of components into the pulp.
3.3. The Dental Bleaching
During bleaching procedures, H2O2 and its by-products can diffuse to the pulp via the layers of enamel and dentine, triggering the release of inflammatory mediators, increasing vascular permeability, decreasing cellular metabolism, and even leading to pulp necrosis [78][79]. The study by Chen et al. [80] reported that H2O2 from bleaching gel could induce the pain response via upregulation of transient receptor potential ankyrin 1 (TRPA1) expression in dental pulp stem cells, activating the inflammatory genes TNF-α and IL-6 and the hyperalgesia gene PANX1. The secreted neurotransmitter ATP develops hyperalgesia. In parallel, increased intracellular ROS and calcium ions levels were found.
Da Silva et al. [81] determined that a violet LED affected only the most superficial dental tissues and only accelerated the maturation of dentine collagen fibers without any inflammation and fibrosis processes in the rat pulp tissue after bleaching. Similarly, Barboza et al. [82] observed no impact of the violet LED on dentine collagen biostability. The 17.5% hydrogen peroxidase-based gel used in this study did not influence the pulp collagen fibre maturations compared to the previous studies using 35% concentrations [83][84].
The systematic review conducted by Benetti et al. [85] assessed the bleaching effects on the inflammatory response, as well as the cytotoxicity and cell metabolism of the pulp tissue. The included in vitro studies demonstrated that light could influence pulp cell metabolism. The one using halogen light to activate the bleaching gel indicated negative effects [86], in contrast to three other studies [87][88][89]. During the laser therapy, the light was able to compensate for the cytotoxic effects in one study [90] and was incapable of positively modulating the cell metabolism in two others [91][92]. Low-level laser therapy (LLLT) can stimulate cellular metabolism, collagen synthesis, and angiogenesis [93][94], thereby minimising the oxidative damage of the pulp cells caused by the bleaching agents. According to the beneficial effects of LLLT on pulp cell metabolism, Lima et al. [92] found increased alkaline phosphatase activity when using a lower LLLT intensity (4 J/cm2).
Interestingly, Ferreira et al. [95] observed that oxidative stress generates increased levels of IL-6 and TNF-α in the rat pulp tissue, regardless of diabetes mellitus, as well as of IL-17 only in the early periods in normoglycemic ones. After dental bleaching, the normoglycemic group demonstrated an inflammatory response limited to more tissue and cellular disorganisation near the pulp horns.
References
- Mjör, I.A.; Sveen, O.B.; Heyeraas, K.J. Pulp-Dentin Biology in Restorative Dentistry. Part 1: Normal Structure and Physiology. Quintessence Int. 2001, 32, 427–446.
- Van Hassel, H. Reprint of: Physiology of the Human Dental Pulp. J. Endod. 2021, 47, 690–695.
- Okiji, T.; Morita, I.; Kobayashi, C.; Sunada, I.; Murota, S. Arachidonic-acid metabolism in normal and experimentally-inflamed rat dental pulp. Arch. Oral Biol. 1987, 32, 723–727.
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. STEM CELLS Transl. Med. 2020, 9, 445–464.
- Álvarez-Vásquez, J.L.; Castañeda-Alvarado, C.P. Dental Pulp Fibroblast: A Star Cell. J. Endod. 2022, 48, 1005–1019.
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. Pulp Fibroblasts Synthesize Functional Complement Proteins Involved in Initiating Dentin–Pulp Regeneration. Am. J. Pathol. 2014, 184, 1991–2000.
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153.
- Rajan, S.; Ljunggren, A.; Manton, D.J.; Björkner, A.E.; McCullough, M. Post-Mitotic Odontoblasts in Health, Disease, and Regeneration. Arch. Oral Biol. 2020, 109, 104591.
- Thesleff, I.; Nieminen, P. Tooth morphogenesis and cell differentiation. Curr. Opin. Cell Biol. 1996, 8, 844–850.
- Arana-Chavez, V.E.; Massa, L.F. Odontoblasts: The cells forming and maintaining dentine. Int. J. Biochem. Cell Biol. 2004, 36, 1367–1373.
- Zhang, L.; Chen, Z. Autophagy in the dentin-pulp complex against inflammation. Oral Dis. 2018, 24, 11–13.
- Bleicher, F. Odontoblast physiology. Exp. Cell Res. 2014, 325, 65–71.
- Couve, E.; Osorio, R.; Schmachtenberg, O. The Amazing Odontoblast: Activity, Autophagy, and Aging. J. Dent. Res. 2013, 92, 765–772.
- Sasaki, T.; Garant, P.R. Structure and Organization of Odontoblasts. Anat. Rec. 1996, 245, 235–249.
- Kawasaki, K.; Weiss, K. SCPP Gene Evolution and the Dental Mineralization Continuum. J. Dent. Res. 2008, 87, 520–531.
- Balic, A.; Mina, M. Identification of secretory odontoblasts using DMP1-GFP transgenic mice. Bone 2011, 48, 927–937.
- Embery, G.; Hall, R.; Waddington, R.; Septier, D.; Goldberg, M. Proteoglycans in Dentinogenesis. Crit. Rev. Oral Biol. Med. 2001, 12, 331–349.
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin structure composition and mineralization. Front. Biosci. 2011, 3, 711–735.
- Tziafas, D.; Smith, A.J.; Lesot, H. Designing New Treatment Strategies in Vital Pulp Therapy. J. Dent. 2000, 28, 77–92.
- Couve, E. Ultrastructural changes during the life cycle of human odontoblasts. Arch. Oral Biol. 1986, 31, 643–651.
- Farges, J.-C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251.
- Smith, A.J.; Cassidy, N.; Perry, H.; Bègue-Kirn, C.; Ruch, J.V.; Lesot, H. Reactionary dentinogenesis. Int. J. Dev. Biol. 1995, 39, 273–280.
- Murray, P.; About, I.; Lumley, P.; Franquin, J.-C.; Windsor, L.; Smith, A. Odontoblast morphology and dental repair. J. Dent. 2003, 31, 75–82.
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation–regeneration interplay in the dentine–pulp complex. J. Dent. 2010, 38, 687–697.
- Simon, S.R.J.; Berdal, A.; Cooper, P.R.; Lumley, P.J.; Tomson, P.L.; Smith, A.J. Dentin-Pulp Complex Regeneration: From Lab to Clinic. Adv. Dent. Res. 2011, 23, 340–345.
- Galler, K.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480.
- Smith, A.J.; Lesot, H. Induction and Regulation of Crown Dentinogenesis: Embryonic Events as a Template for Dental Tissue Repair? Crit. Rev. Oral Biol. Med. 2001, 12, 425–437.
- Sloan, A.; Smith, A. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral Dis. 2007, 13, 151–157.
- Roberts-Clark, D.; Smith, A. Angiogenic growth factors in human dentine matrix. Arch. Oral Biol. 2000, 45, 1013–1016.
- Cassidy, N.; Fahey, M.; Prime, S.S.; Smith, A.J. Comparative analysis of transforming growth factor-β isoforms 1–3 in human and rabbit dentine matrices. Arch. Oral Biol. 1997, 42, 219–223.
- Silva, T.A.; Lara, V.S.; Silva, J.S.; Garlet, G.P.; Butler, W.T.; Cunha, F.Q. Dentin Sialoprotein and Phosphoprotein Induce Neutrophil Recruitment: A Mechanism Dependent on IL-1β, TNF-α, and CXC Chemokines. Calcif. Tissue Int. 2004, 74, 532–541.
- Silva, T.; Lara, V.; Silva, J.S.; Oliveira, S.; Butler, W.; Cunha, F.Q. Macrophages and Mast Cells Control the Neutrophil Migration Induced by Dentin Proteins. J. Dent. Res. 2005, 84, 79–83.
- He, W.-X.; Niu, Z.-Y.; Zhao, S.-L.; Smith, A.J. Smad protein mediated transforming growth factor β1 induction of apoptosis in the MDPC-23 odontoblast-like cell line. Arch. Oral Biol. 2005, 50, 929–936.
- Lara, V.; Figueiredo, F.; da Silva, T.; Cunha, F. Dentin-induced in vivo Inflammatory Response and in vitro Activation of Murine Macrophages. J. Dent. Res. 2003, 82, 460–465.
- Chang, J.; Zhang, C.; Tani-Ishii, N.; Shi, S.; Wang, C.-Y. NF-κB Activation in Human Dental Pulp Stem Cells by TNF and LPS. J. Dent. Res. 2005, 84, 994–998.
- Pevsner-Fischer, M.; Morad, V.; Cohen-Sfady, M.; Rousso-Noori, L.; Zanin-Zhorov, A.; Cohen, S.; Cohen, I.R.; Zipori, D. Toll-like Receptors and Their Ligands Control Mesenchymal Stem Cell Functions. Blood 2007, 109, 1422–1432.
- Kritikou, K.; Greabu, M.; Imre, M.; Miricescu, D.; Totan, A.R.; Burcea, M.; Stanescu-Spinu, I.-I.; Spinu, T. ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules 2021, 26, 4129.
- Galicia, J.C.; Guzzi, P.H.; Giorgi, F.M.; Khan, A.A. Predicting the Response of the Dental Pulp to SARS-CoV2 Infection: A Transcriptome-Wide Effect Cross-Analysis. Genes Immun. 2020, 21, 360–363.
- Brodzikowska, A.; Ciechanowska, M.; Kopka, M.; Stachura, A.; Włodarski, P.K. Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis-A Systematic Review. Biomolecules 2022, 12, 138.
- Li, X.; Hu, L.; Ma, L.; Chang, S.; Wang, W.; Feng, Y.; Xu, Y.; Hu, J.; Zhang, C.; Wang, S. Severe periodontitis may influence cementum and dental pulp through inflammation, oxidative stress, and apoptosis. J. Periodontol. 2019, 90, 1297–1306.
- Giovanella, L.B.; Barletta, F.B.; Felippe, W.T.; Bruno, K.F.; de Alencar, A.H.G.; Estrela, C.; Giovanella, L.B.; Barletta, F.B.; Felippe, W.T.; Bruno, K.F.; et al. Assessment of Oxygen Saturation in Dental Pulp of Permanent Teeth with Periodontal Disease. J. Endod. 2014, 40, 1927–1931.
- Amemiya, K.; Kaneko, Y.; Muramatsu, T.; Shimono, M.; Inoue, T. Pulp cell responses during hypoxia and reoxygenation in vitro. Eur. J. Oral Sci. 2003, 111, 332–338.
- Boyle, M.; Chun, C.; Strojny, C.; Narayanan, R.; Bartholomew, A.; Sundivakkam, P.; Alapati, S. Chronic Inflammation and Angiogenic Signaling Axis Impairs Differentiation of Dental-Pulp Stem Cells. PLoS ONE 2014, 9, e113419.
- Yan, L.; Lihua, L.; Sha, Z.; Hongli, W.; Wu, Z.; Guijun, T.; Kai, Z.; Yahui, L. The Activity of Cytokines in Dental Pulp. J. Gene Med. 2022, 24, e3444.
- Worsley, M.A.; Allen, C.E.; Billinton, A.; King, A.E.; Boissonade, F.M. Chronic Tooth Pulp Inflammation Induces Persistent Expression of Phosphorylated ERK (PERK) and Phosphorylated P38 (Pp38) in Trigeminal Subnucleus Caudalis. Neuroscience 2014, 269, 318–330.
- Yang, E.S.; Jin, M.U.; Hong, J.H.; Kim, Y.S.; Choi, S.Y.; Kim, T.H.; Cho, Y.S.; Bae, Y.C. Expression of Vesicular Glutamate Transporters VGLUT1 and VGLUT2 in the Rat Dental Pulp and Trigeminal Ganglion Following Inflammation. PLoS ONE 2014, 9, e109723.
- Kanno, K.; Shimizu, K.; Shinoda, M.; Hayashi, M.; Takeichi, O.; Iwata, K. Role of Macrophage-Mediated Toll-like Receptor 4-Interleukin-1R Signaling in Ectopic Tongue Pain Associated with Tooth Pulp Inflammation. J. Neuroinflamm. 2020, 17, 312.
- Muñoz-Carrillo, J.L.; Vázquez-Alcaraz, S.J.; Vargas-Barbosa, J.M.; Ramos-Gracia, L.G.; Alvarez-Barreto, I.; Medina-Quiroz, A.; Díaz-Huerta, K.K. The Role of MicroRNAs in Pulp Inflammation. Cells 2021, 10, 2142.
- Mo, Z.; Li, Q.; Cai, L.; Zhan, M.; Xu, Q. The effect of DNA methylation on the miRNA expression pattern in lipopolysaccharide-induced inflammatory responses in human dental pulp cells. Mol. Immunol. 2019, 111, 11–18.
- Huang, X.; Chen, K. Differential Expression of Long Noncoding RNAs in Normal and Inflamed Human Dental Pulp. J. Endod. 2018, 44, 62–72.
- Niklas, A.; Proff, P.; Gosau, M.; Römer, P. The Role of Hypoxia in Orthodontic Tooth Movement. Int. J. Dent. 2013, 2013, 841840.
- Wong, V.S.; Freer, T.J.; Joseph, B.K.; Daley, T.J. Tooth Movement and Vascularity of the Dental Pulp: A Pilot Study. Aust. Orthod. J. 1999, 15, 246–250.
- Yu, W.; Zhang, Y.; Jiang, C.; He, W.; Yi, Y.; Wang, J. Orthodontic treatment mediates dental pulp microenvironment via IL17A. Arch. Oral Biol. 2016, 66, 22–29.
- Veberiene, R.; Smailiene, D.; Danielyte, J.; Toleikis, A.; Dagys, A.; Machiulskiene, V. Effects of Intrusive Force on Selected Determinants of Pulp Vitality. Angle Orthod. 2009, 79, 1114–1118.
- Perinetti, G.; Varvara, G.; Festa, F.; Esposito, P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 88–92.
- Spoto, G.; Fioroni, M.; Rubini, C.; Tripodi, D.; Perinetti, G.; Piattelli, A. Aspartate Aminotransferase Activity in Human Healthy and Inflamed Dental Pulps. J. Endod. 2001, 27, 394–395.
- Veberiene, R.; Smailiene, D.; Baseviciene, N.; Toleikis, A.; Machiulskiene, V. Change in dental pulp parameters in response to different modes of orthodontic force application. Angle Orthod. 2010, 80, 1018–1022.
- Rohaya, M.; Hisham, Z.S.; Khazlina, K. Preliminary Study of Aspartate Aminotransferase Activity in Gingival Crevicular Fluids During Orthodontic Tooth Movement. J. Appl. Sci. 2009, 9, 1393–1396.
- Chavarria-Bolaños, D.; Flores-Reyes, H.; Lombana-Sanchez, N.; Cerda-Cristerna, B.; Pozos-Guillen, A. Sensory Neuropeptides and Endogenous Opioids Expression in Human Dental Pulp with Asymptomatic Inflammation: In Vivo Study. Mediat. Inflamm. 2015, 2015, 879126.
- Chang, M.-C.; Chen, Y.-J.; Tai, T.-F.; Tai, M.-R.; Li, M.-Y.; Tsai, Y.-L.; Lan, W.-H.; Wang, Y.-L.; Jeng, J.-H. Cytokine-induced prostaglandin E2production and cyclooxygenase-2 expression in dental pulp cells: Downstream calcium signalling via activation of prostaglandin EP receptor. Int. Endod. J. 2006, 39, 819–826.
- Chang, M.C.; Chen, Y.J.; Lee, M.Y.; Lin, L.D.; Wang, T.M.; Chan, C.P.; Tsai, Y.L.; Wang, C.Y.; Lin, B.R.; Jeng, J.H. Prostaglandin F2α stimulates MEK-ERK signalling but decreases the expression of alkaline phosphatase in dental pulp cells. Int. Endod. J. 2010, 43, 461–468.
- Chang, M.-C.; Chang, H.-H.; Lee, M.-Y.; Lin, C.-C.; Yeh, H.-W.; Yang, T.-T.; Lin, P.-S.; Tseng, W.-Y.; Jeng, J.-H. Prostaglandin F2α-Induced Interleukin-8 Production in Human Dental Pulp Cells Is Associated With MEK/ERK Signaling. J. Endod. 2009, 35, 508–512.
- Maltos, K.L.; Menezes, G.B.; Caliari, M.V.; Rocha, O.A.; Santos, J.M.; Alves, D.L.; Duarte, I.D.; Francischi, J.N. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch. Oral Biol. 2004, 49, 443–450.
- Hebling, J.; Giro, E.; Costa, C. Human pulp response after an adhesive system application in deep cavities. J. Dent. 1999, 27, 557–564.
- Chang, M.-C.; Lin, L.-D.; Chuang, F.-H.; Chan, C.-P.; Wang, T.-M.; Lee, J.-J.; Jeng, P.-Y.; Tseng, W.-Y.; Lin, H.-J.; Jeng, J.-H. Carboxylesterase expression in human dental pulp cells: Role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012, 8, 1380–1387.
- Rakich, D.R.; Wataha, J.C.; Lefebvre, C.A.; Weller, R.N. Effect of dentin bonding agents on the secretion of inflammatory mediators from macrophages. J. Endod. 1999, 25, 114–117.
- Noda, M.; Wataha, J.C.; Lockwood, P.E.; Volkmann, K.R.; Kaga, M.; Sano, H. Sublethal, 2-week exposures of dental material components alter TNF-α secretion of THP-1 monocytes. Dent. Mater. 2003, 19, 101–105.
- Lehmann, A.; Nijakowski, K.; Drożdżyńska, A.; Przybylak, M.; Woś, P.; Surdacka, A. Influence of the Polymerization Modes on the Methacrylic Acid Release from Dental Light-Cured Materials—In Vitro Study. Materials 2022, 15, 8976.
- Hamid, A.; Okamoto, A.; Iwaku, M.; Hume, W.R. Component release from light-activated glass ionomer and compomer cements. J. Oral Rehabilitation 1998, 25, 94–99.
- Kawai, K.; Takaoka, T. Fluoride, Hydrogen Ion and HEMA Release from Light-Cured GIC Restoratives. Am. J. Dent. 2002, 15, 149–152.
- Lehmann, A.; Nijakowski, K.; Nowakowska, M.; Woś, P.; Misiaszek, M.; Surdacka, A. Influence of Selected Restorative Materials on the Environmental pH: In Vitro Comparative Study. Appl. Sci. 2021, 11, 11975.
- Davidovic, L.; Cuk, M.; Zivkovic-Sandic, M.; Grga, D.; Zivkovic, S. The influence of liners on the pulp inflammation. Srp. Arh. za Celok. Lek. 2015, 143, 261–266.
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Dental Pulp Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endod. 2015, 41, 1805–1817.
- Peng, W.; Liu, W.; Zhai, W.; Jiang, L.; Li, L.; Chang, J.; Zhu, Y. Effect of Tricalcium Silicate on the Proliferation and Odontogenic Differentiation of Human Dental Pulp Cells. J. Endod. 2011, 37, 1240–1246.
- Woo, S.-M.; Hwang, Y.-C.; Lim, H.-S.; Choi, N.-K.; Kim, S.-H.; Kim, W.-J.; Kim, S.-M.; Jung, J.-Y. Effect of Nifedipine on the Differentiation of Human Dental Pulp Cells Cultured with Mineral Trioxide Aggregate. J. Endod. 2013, 39, 801–805.
- Wang, Y.; Zhu, R.; Ni, Y.; Kokot, S. Competitive Interactions of Anti-Carcinogens with Serum Albumin: A Spectroscopic Study of Bendamustine and Dexamethasone with the Aid of Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 123, 241–248.
- Soares, I.P.M.; Anovazzi, G.; Anselmi, C.; Leite, M.L.; Scheffel, D.L.S.; Soares, D.G.; Costa, C.A.D.S.; Hebling, J. Response of pulp cells to resin infiltration of enamel white spot-like lesions. Dent. Mater. 2021, 37, e329–e340.
- Benetti, F.; Gomes-Filho, J.E.; Ferreira, L.L.; Ervolino, E.; Briso, A.L.F.; Sivieri-Araújo, G.; Dezan-Júnior, E.; Cintra, L.T.A. Hydrogen Peroxide Induces Cell Proliferation and Apoptosis in Pulp of Rats after Dental Bleaching In Vivo: Effects of the Dental Bleaching in Pulp. Arch. Oral Biol. 2017, 81, 103–109.
- Soares, D.G.; Basso, F.G.; Scheffel, D.S.; Hebling, J.; Costa, C.A.D.S. Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch. Oral Biol. 2015, 60, 1428–1436.
- Chen, C.; Huang, X.; Zhu, W.; Ding, C.; Huang, P.; Li, R. TRPA1 triggers hyperalgesia and inflammation after tooth bleaching. Sci. Rep. 2021, 11, 17418.
- Da Silva, L.M.A.V.; Cintra, L.T.A.; de Alcântara, S.; Machado, N.E.d.S.; Benetti, F.; Ervolino, E.; Briso, A.L.F. Influence of Violet LED Associated or Not with Peroxide Gel on Inflammation, Mineralization, and Collagen Fiber Maturation in Dentin and Pulp Tissue. Photodiagnosis Photodyn. Ther. 2022, 39, 102959.
- Barboza, A.C.S.; dos Santos, P.H.; Vale, L.R.D.; Gallinari, M.D.O.; Assmann, A.; Vidal, C.M.P.; Fagundes, T.C.; Briso, A.L.F. Dental bleaching with violet LED: Effects on dentin color change, resin-dentin bond strength, hybrid layer nanohardness and dentinal collagen biostability. Photodiagn. Photodyn. Ther. 2021, 33, 102141.
- Cintra, L.T.A.; Ferreira, L.L.; Benetti, F.; Gastélum, A.A.; Gomes-Filho, J.E.; Ervolino, E.; Briso, A.L.F. The Effect of Dental Bleaching on Pulpal Tissue Response in a Diabetic Animal Model. Int. Endod. J. 2017, 50, 790–798.
- Terayama, A.M.; Benetti, F.; Lopes, J.M.D.A.; Barbosa, J.G.; Silva, I.J.P.; Sivieri-Araújo, G.; Briso, A.L.F.; Cintra, L.T.A. Influence of low-level laser therapy on inflammation, collagen fiber maturation, and tertiary dentin deposition in the pulp of bleached teeth. Clin. Oral Investig. 2020, 24, 3911–3921.
- Benetti, F.; Lemos, C.A.A.; de Oliveira Gallinari, M.; Terayama, A.M.; Briso, A.L.F.; de Castilho Jacinto, R.; Sivieri-Araújo, G.; Cintra, L.T.A. Influence of Different Types of Light on the Response of the Pulp Tissue in Dental Bleaching: A Systematic Review. Clin. Oral Investig. 2018, 22, 1825–1837.
- Ribeiro, A.P.D.; Sacono, N.T.; Lessa, F.C.R.; Nogueira, I.; Coldebella, C.R.; Hebling, J.; Costa, C.A.D.S. Cytotoxic effect of a 35% hydrogen peroxide bleaching gel on odontoblast-like MDPC-23 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 458–464.
- Trindade, F.Z.; Ribeiro, A.P.D.; Sacono, N.T.; Oliveira, C.F.; Lessa, F.C.R.; Hebling, J.; Costa, C.A.S. Trans-enamel and trans-dentinal cytotoxic effects of a 35% H2O2 bleaching gel on cultured odontoblast cell lines after consecutive applications. Int. Endod. J. 2009, 42, 516–524.
- Gonçalves, R.; Costa, C.; Soares, D.; dos Santos, P.; Cintra, L.; Briso, A. Effect of Different Light Sources and Enamel Preconditioning on Color Change, H2O2 Penetration, and Cytotoxicity in Bleached Teeth. Oper. Dent. 2016, 41, 83–92.
- Coldebella, C.R.; Ribeiro, A.P.D.; Sacono, N.T.; Trindade, F.Z.; Hebling, J.; Costa, C.A.D.S. Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz. Dent. J. 2009, 20, 267–274.
- Dantas, C.M.G.; Vivan, C.L.; Ferreira, L.S.; De Freitas, P.M.; Marques, M.M. In vitro effect of low intensity laser on the cytotoxicity produced by substances released by bleaching gel. Braz. Oral Res. 2010, 24, 460–466.
- Lima, A.F.; Basso, F.G.; Ribeiro, A.P.D.; Bagnato, V.S.; Hebling, J.; Marchi, G.M.; de Souza Costa, C.A. Effects of Laser Irradiation on Pulp Cells Exposed to Bleaching Agents. Photochem. Photobiol. 2014, 90, 201–206.
- Lima, A.F.; Ribeiro, A.P.D.; Basso, F.G.; Bagnato, V.S.; Hebling, J.; Marchi, G.M.; de Souza Costa, C.A. Effect of Low-Level Laser Therapy on Odontoblast-like Cells Exposed to Bleaching Agent. Lasers Med. Sci. 2014, 29, 1533–1538.
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-Level Laser Therapy for Wound Healing: Mechanism and Efficacy. Dermatol. Surg. 2005, 31, 334–340.
- Park, I.-S.; Chung, P.-S.; Ahn, J.C. Adipose-derived stromal cell cluster with light therapy enhance angiogenesis and skin wound healing in mice. Biochem. Biophys. Res. Commun. 2015, 462, 171–177.
- Ferreira, L.L.; Gomes-Filho, J.E.; Benetti, F.; Carminatti, M.; Ervolino, E.; Briso, A.L.F.; Cintra, L.T.A. The Effect of Dental Bleaching on Pulpal Tissue Response in a Diabetic Animal Model: A Study of Immunoregulatory Cytokines. Int. Endod. J. 2018, 51, 347–356.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





