
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emad Ahmed | -- | 2351 | 2023-04-21 16:08:33 | | | |
| 2 | Lindsay Dong | Meta information modification | 2351 | 2023-04-23 09:50:25 | | |
Video Upload Options
Osteoarthritis (OA) and rheumatoid arthritis (RA) are two common disorders that disrupt the quality of life of millions of people. Sex-determining region Y-related (SRY) high-mobility group (HMG) box C, SOXC, is a superfamily of transcription factors that have been recently shown to be involved in various physiological and pathological processes. These include embryonic development, cell differentiation, fate determination, and autoimmune diseases, as well as carcinogenesis and tumor progression. The SOXC superfamily includes SOX4, SOX11, and SOX12, all have a similar DNA-binding domain, i.e., HMG.
1. Introduction
2. SOXC Transcription Factors under In Vivo Inflammatory Conditions Associated with Arthritis
3. SOXC Transcription Factors as Potential Diagnostic Biomarkers of Arthritis
3.1. SOX4 as a Potential Diagnostic Biomarker of Osteoarthritis
3.2. SOX4 as a Potential Diagnostic Biomarker of Rheumatoid Arthritis
3.3. SOX11 Is Dysregulated during Osteoarthritis
4. Signaling Mechanisms Involved in SOXC TFs Promoting Arthritis
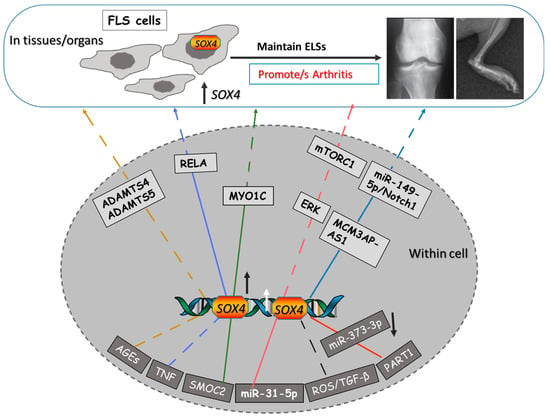
Mechanistically, SOX4 promoting arthritis has been documented to occur through regulating several signaling pathways. (1) As a critical mediator of the TNF-induced transformation of FLS, SOX4 interacts with RELA (NF-κB signaling molecule) to regulate the expression of TNF downstream genes and thus maintains the transformation of FLS and inflammatory pathology in arthritis [10][26]. (2) SOX4 is regulated by the ROS/TGF-β signal to enhance OA pathogenesis and FLS senescence [5]. (3) SOX4 is involved in osteoarthritis onset by increasing the levels of two major aggrecanase-degrading articular cartilage enzymes, Adamts4 and Adamts5, through binding to their gene promoters [8]. The degradation of the cartilage extracellular matrix (ECM) is one of the main features associated with arthritis. The degradation of aggrecans in the ECM of aggrecan was found to regulated by these two enzymes. Thus, SOX4 appears to regulate the level of these enzymes and then promote arthritis. (4) SOX4 is upregulated in synovial CD4+ T cells and contributes to the production of CXCL13 and the formation of ELSs at the inflammatory sites in RA patients [17]. (5) SOX4 activated the long noncoding MCM3AP-AS1, aggravated OA progression via targeting the miR-149-5p/Notch1 axis and then modulated autophagy and ECM degradation [7].
5. SOX4 as a Therapeutic Target of Arthritis
6. Upstream Molecules That Can Target SOX4 to Treat Arthritis
Through direct and indirect binding to SOX4 TF, several upstream factors and signaling molecules have been recently reported to target SOX4 during arthritis, such as ROS)/TGF, TNF, SMOC2, AGEs, Lnc PART1, and the miRNA, miR-31 (Figure 1).
7. Transcriptional Activity of SOX4 in Arthritis
8. SOX4 and Its Implications in Osteoporosis
9. SOX4 Involvement in Other Autoimmune Disorders
References
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—Two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615.
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121.
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144.
- Zhu, Y.-J.; Jiang, D.-M. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8175–8185.
- Ye, X.; Yin, C.; Huang, X.; Huang, Y.; Ding, L.; Jin, M.; Wang, Z.; Wei, J.; Li, X. ROS/TGF-β signal mediated accumulation of SOX4 in OA-FLS promotes cell senescence. Exp. Gerontol. 2021, 156, 111616.
- Xiong, X.; Liu, L.; Xu, F.; Wu, X.; Yin, Z.; Dong, Y.; Qian, P. Feprazone Ameliorates TNF-α-Induced Loss of Aggrecan via Inhibition of the SOX-4/ADAMTS-5 Signaling Pathway. ACS Omega 2021, 6, 7638–7645.
- Xu, F.; Hu, Q.-F.; Li, J.; Shi, C.-J.; Luo, J.-W.; Tian, W.-C.; Pan, L.-W. SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine 2022, 152, 155805.
- Takahata, Y.; Nakamura, E.; Hata, K.; Wakabayashi, M.; Murakami, T.; Wakamori, K.; Yoshikawa, H.; Matsuda, A.; Fukui, N.; Nishimura, R. Sox4 is involved in osteoarthritic cartilage deterioration through induction of ADAMTS4 and ADAMTS5. FASEB J. 2019, 33, 619–630.
- Xu, F.; Lv, Y.-M.; Wang, H.-B.; Song, Y.-C. miR-31-5p/SOX4 Axis Affects Autophagy and Apoptosis of Chondrocytes by Regulating Extracellular Regulated Protein Kinase/Mechanical Target of Rapamycin Kinase Signalling. Pathobiology 2022, 89, 63–73.
- Jones, K.; Ramirez-Perez, S.; Niu, S.; Gangishetti, U.; Drissi, H.; Bhattaram, P. SOX4 and RELA Function as Transcriptional Partners to Regulate the Expression of TNF- Responsive Genes in Fibroblast-Like Synoviocytes. Front. Immunol. 2022, 13, 789349.
- A Ahmed, E.; Ibrahim, H.-I.M.; Khalil, H.E. Pinocembrin Reduces Arthritic Symptoms in Mouse Model via Targeting Sox4 Signaling Molecules. J. Med. Food 2021, 24, 282–291.
- Xu, S.; Yu, J.; Wang, Z.; Ni, C.; Xia, L.; Tang, T. SOX11 promotes osteoarthritis through induction of TNF-α. Pathol. Res. Pr. 2019, 215, 152442.
- Kan, A.; Ikeda, T.; Fukai, A.; Nakagawa, T.; Nakamura, K.; Chung, U.-I.; Kawaguchi, H.; Tabin, C.J. SOX11 contributes to the regulation of GDF5 in joint maintenance. BMC Dev. Biol. 2013, 13, 4.
- Wang, X.; Fan, J.; Ding, X.; Sun, Y.; Cui, Z.; Liu, W. Tanshinone I Inhibits IL-1β-Induced Apoptosis, Inflammation And Extracellular Matrix Degradation In Chondrocytes CHON-001 Cells And Attenuates Murine Osteoarthritis. Drug Des. Dev. Ther. 2019, ume 13, 3559–3568.
- Pan, W.; Wang, H.; Ruan, J.; Zheng, W.; Chen, F.; Kong, J.; Wang, Y. lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis. Open Life Sci. 2021, 16, 511–522.
- Yi, P.; Xu, X.; Yao, J.; Qiu, B. Analysis of mRNA Expression and DNA Methylation Datasets According to the Genomic Distribution of CpG Sites in Osteoarthritis. Front. Genet. 2021, 12, 618803.
- Yoshitomi, H.; Kobayashi, S.; Miyagawa-Hayashino, A.; Okahata, A.; Doi, K.; Nishitani, K.; Murata, K.; Ito, H.; Tsuruyama, T.; Haga, H.; et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat. Commun. 2018, 9, 3762.
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462.
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892.
- Corsiero, E.; Nerviani, A.; Bombardieri, M.; Pitzalis, C. Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Front. Immunol. 2016, 7, 430.
- Nerviani, A.; Pitzalis, C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J. Leukoc. Biol. 2018, 104, 333–341.
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333.
- Lefebvre, V.; Bhattaram, P. SOXC Genes and the Control of Skeletogenesis. Curr. Osteoporos. Rep. 2016, 14, 32–38.
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-Hora, M.; Kodama, T.; Tanaka, S.; A Bluestone, J.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68.
- Di Liu, D.; Li, R.; Xu, S.; Shi, M.; Kuang, Y.; Wang, J.; Shen, C.; Qiu, Q.; Liang, L.; Xiao, Y.; et al. SMOC2 promotes aggressive behavior of fibroblast-like synoviocytes in rheumatoid arthritis through transcriptional and post-transcriptional regulating MYO1C. Cell Death Dis. 2022, 13, 1035.
- Bhattaram, P.; Muschler, G.; Wixler, V.; Lefebvre, V. Inflammatory Cytokines Stabilize SOXC Transcription Factors to Mediate the Transformation of Fibroblast-Like Synoviocytes in Arthritic Disease. Arthritis Rheumatol. 2018, 70, 371–382.
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Ohkawa, M.; Ruengsinpinya, L. Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 2020, 21, 1340.
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793.
- Kato, K.; Bhattaram, P.; Penzo-Méndez, A.; Gadi, A.; Lefebvre, V. SOXC Transcription Factors Induce Cartilage Growth Plate Formation in Mouse Embryos by Promoting Noncanonical WNT Signaling. J. Bone Miner Res. 2015, 30, 1560–1571.
- Ngondo, R.P.; Carbon, P. Transcription factor abundance controlled by an auto-regulatory mechanism involving a transcription start site switch. Nucleic Acids Res. 2014, 42, 2171–2184.
- Li, G.; Gu, Z.; He, Y.; Wang, C.; Duan, J. The effect of SOX4 gene 3′UTR polymorphisms on osteoporosis. J. Orthop. Surg. Res. 2021, 16, 321.
- Duncan, E.L.; Danoy, P.; Kemp, J.P.; Leo, P.J.; McCloskey, E.; Nicholson, G.C.; Eastell, R.; Prince, R.L.; Eisman, J.A.; Jones, G.; et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 2011, 7, e1001372.
- Nissen-Meyer, L.S.H.; Jemtland, R.; Gautvik, V.T.; Pedersen, M.E.; Paro, R.; Fortunati, D.; Pierroz, D.D.; Stadelmann, V.A.; Reppe, S.; Reinholt, F.P.; et al. Osteopenia, decreased bone formation and impaired osteoblast development in Sox4 heterozygous mice. J. Cell Sci. 2007, 120, 2785–2795.
- Lefebvre, V. Roles and regulation of SOX transcription factors in skeletogenesis. Vertebr. Skelet. Dev. Curr. Top. Dev. Biol. 2019, 133, 171–193.
- Kodrič, K.; Čamernik, K.; Černe, D.; Komadina, R.; Marc, J. P4 medicine and osteoporosis: A systematic review. Wien. Klin. Wochenschr. 2016, 128 (Suppl. 7), 480–491.
- Kuwahara, M.; Yamashita, M.; Shinoda, K.; Tofukuji, S.; Onodera, A.; Shinnakasu, R.; Motohashi, S.; Hosokawa, H.; Tumes, D.; Iwamura, C.; et al. The Transcription Factor Sox4 Is a Downstream Target of Signaling by the Cytokine TGF-beta and Suppresses T(H)2 Differentiation. Nat. Immunol. 2012, 13, 778–786.
- Gerner, M.C.; Ziegler, L.S.; Schmidt, R.L.J.; Krenn, M.; Zimprich, F.; Uyanik-Ünal, K.; Konstantopoulou, V.; Derdak, S.; Del Favero, G.; Schwarzinger, I.; et al. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J. 2020, 34, 8367–8384.
- Moncrieffe, H.; Nistala, K.; Hunter, P.; Kamhieh, Y.; Wedderburn, L. CD39: A regulatory role in childhood arthritis. Pediatr. Rheumatol. 2008, 6 (Suppl. 1), P10.
- Hinrichs, A.C.; Blokland, S.L.M.; Lopes, A.P.; Wichers, C.G.K.; Kruize, A.A.; Pandit, A.; Radstake, T.R.D.J.; van Roon, J.A.G. Transcriptome Analysis of CCR9+ T Helper Cells From Primary Sjögren’s Syndrome Patients Identifies CCL5 as a Novel Effector Molecule. Front. Immunol. 2021, 12, 702733.
- Jin, L.; Kawai, T.; Cha, S.; Yu, Q. Interleukin-7 Enhances the Th1 Response to Promote the Development of Sjögren’s Syndrome-like Autoimmune Exocrinopathy in Mice. Arthritis Rheum. 2013, 65, 2132–2142.
- Potzner, M.R.; Griffel, C.; Lütjen-Drecoll, E.; Bösl, M.R.; Wegner, M.; Sock, E. Prolonged Sox4 Expression in Oligodendrocytes Interferes with Normal Myelination in the Central Nervous System. Mol. Cell. Biol. 2007, 27, 5316–5326.
- Panach, L.; Serna, E.; Tarín, J.J.; Cano, A.; García-Pérez, M.Á. A translational approach from an animal model identifies CD80 as a candidate gene for the study of bone phenotypes in postmenopausal women. Osteoporos. Int. 2017, 28, 2445–2455.




