Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roxana Nartea | -- | 1877 | 2023-04-21 16:03:45 | | | |
| 2 | Rita Xu | Meta information modification | 1877 | 2023-04-23 04:13:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nartea, R.; Mitoiu, B.I.; Ghiorghiu, I. Magnesium Supplements and Statin Medication. Encyclopedia. Available online: https://encyclopedia.pub/entry/43327 (accessed on 07 February 2026).
Nartea R, Mitoiu BI, Ghiorghiu I. Magnesium Supplements and Statin Medication. Encyclopedia. Available at: https://encyclopedia.pub/entry/43327. Accessed February 07, 2026.
Nartea, Roxana, Brindusa Ilinca Mitoiu, Ioana Ghiorghiu. "Magnesium Supplements and Statin Medication" Encyclopedia, https://encyclopedia.pub/entry/43327 (accessed February 07, 2026).
Nartea, R., Mitoiu, B.I., & Ghiorghiu, I. (2023, April 21). Magnesium Supplements and Statin Medication. In Encyclopedia. https://encyclopedia.pub/entry/43327
Nartea, Roxana, et al. "Magnesium Supplements and Statin Medication." Encyclopedia. Web. 21 April, 2023.
Copy Citation
Many investigations have discovered a connection between statins and magnesium supplements. On one hand, increasing research suggests that chronic hypomagnesemia may be an important factor in the etiology of some metabolic illnesses, including obesity and overweight, insulin resistance and type 2 diabetes mellitus, hypertension, alterations in lipid metabolism, and low-grade inflammation. Chronic metabolic problems seem to be prevented by a high Mg intake combined with diet and/or supplements.
magnesium supplements
metabolic syndrome
lipoproteins
statins
MetS
Dyslipidemia
1. Introduction
Dyslipidemia, an important factor in defining metabolic syndrome (MetS), represents a worldwide challenge, due to the increased risk of atherosclerosis and cardiovascular diseases (CVD) [1]. Dyslipidemia is characterized by elevated levels of total cholesterol and/or triglycerides, with decreased high-density lipoprotein (HDL) levels. Elevated levels of LDL (low-density lipoprotein) and VLDL (very low-density lipoprotein-density) can be also observed [1][2][3].
The progression to atherosclerosis depends on the vascular endothelial metabolism (Kruppel-like factor 2 expression) and LDL serum level (above 20–40 mg/dL) [4][5][6].
Statins are the main treatment for LDL cholesterol reduction because they demonstrably lower cardiovascular morbidity and mortality (according to International Guidelines for the Management of Dyslipidemia 2022), in cases of high-risk conditions, such as clinical atherosclerosis, abdominal aortic aneurysm, diabetes mellitus, chronic kidney disease (age ≥50 years), and patients with LDL-C ≥ 5.0 mmol/L. However, many studies highlight a series of adverse reactions and/or rather modest results in reducing the level of LDL cholesterol and indirectly reducing dyslipidemia, after statin administration [7][8].
There are several factors involved in abnormal lipid profiles such as genetic background, a Western-style diet, alcohol abuse, being overweight or obese, insulin resistance, or some chronic conditions, such as nephrotic syndrome [8][2][3][9][10]. Recent studies suggest that serum magnesium levels can be associated with lipid abnormalities. As epidemiological research is refined by the description of the biological and pathophysiological mechanisms, the goal of this research is to summarize the current understanding of the pathological relationships between dyslipidemia, statin use, and serum magnesium levels.
It is not just theoretically interesting to learn about the connections between dyslipidemia and serum magnesium levels. Magnesium, through its action on lecithin–cholesterol acyl transferase (LCAT), can improve the metabolism of lipoproteins, and implicitly dyslipidemia [11][12][13][14]. If correctly controlled, interventions like diet, exercise, and magnesium supplementation could be very beneficial and lessen the effect of dyslipidemia on CVD [14].
2. Magnesium and Dyslipidemia
2.1. Magnesium Deficiency
Magnesium, the most common intracellular divalent cation, participates in around 300 enzymatic processes, including the metabolism of lipoprotein lipase (LPL), HMG-CoA reductase, and lecithin-cholesterol acyl transferase (LCAT), as well as the attenuation of Na-K ATPase and the breakdown of glycogen [15].
Just 1% of the body’s magnesium is found in circulation (0.3% in plasma/serum), while the majority of the body’s magnesium is found in the bony tissue (60%) and intracellular (40%) compartments [16]. Magnesium is distributed differently inside each individual compartment. For instance, bone has two deposits of magnesium: one in the bone network and the other present on the bone surface. Magnesium within cells is found in the cell membrane, intracellular components, and the nucleus in some amounts. The content of magnesium in red blood cells (RBC) is three times higher in blood than it is in plasma [16]. There are currently two recognized types of magnesium shortage: acute hypomagnesemia and chronic magnesium deficit. Extreme cramps, nystagmus, refractory hypokalemia, refractory hypocalcemia, eclampsia during pregnancy, and cardiac arrhythmias are only a few of the clinical signs and symptoms of extracellular acute hypomagnesemia [17]. In some instances, they respond quickly to intravenous magnesium. Although a chronic magnesium shortage is frequently coupled with normal magnesium levels in serum (0.75–0.95 mmol/L), which is mistakenly thought to eliminate the magnesium deficiency, it represents decreased levels of magnesium inside the cells and bone [16][17]. The overall amount of magnesium absorbed and eliminated through the kidneys, as well as factors such as food intake or supplementation and serum albumin level, can all affect the body’s magnesium levels [18]. Magnesium insufficiency is also made worse by other concomitant conditions such as inflammatory bowel disease, unmanaged diabetes, or renal dysfunction [19][20]. The causes that produce magnesium deficiency are presented in Table 1.
Table 1. Causes of magnesium deficiency.
| Causes of Magnesium Deficiency | |
|---|---|
| Insufficient intake A diet low in Magnesium [18][21] Slimming cures [18][22] |
Intensification of losses At the gastrointestinal level: vomiting; purgatives/laxatives; trailing diarrhea [19][23][24] By the renal route: nephropathies; diuretics; chronic alcoholism; diabetes [20][25][26][27][28][29][30][31] |
| Decreased intestinal absorption Conditions after intestinal resection [19][32] Diarrhea [19][23][24] Malabsorption [19][23][24][33] Chron’s disease [19][24][33] Ulcerative colitis [19][33] Coeliac disease [19][23] |
Stressing Factors Pregnancy [18][34][35][36][37] Lactation [18][34][35][36][37] The growing period [18][38] Sport performance [18][39][40][41] Old age [19][23][25][37][42][43] Convalescence [18][44][45] |
| Endocrine disorders Hyperthyroidism [20][46][47] Aldosteronism [20][46][47] Hyperparathyroidism [20][46][47][48] Poorly controlled diabetes [20][25][49] |
Interference of absorption Increased calcium intake [18][33][48] Hyper protein diet [18][24] Lipid foods [18][48][50][51] Excessive alcohol consumption [18][37][52] |
However, numerous epidemiological, experimental, and clinical studies (not dependent on the type, design, measured indexes, size, and statistical approach of these studies) have demonstrated that chronic magnesium deficiency is connected with higher risk and prevalence of the pathologies listed in Figure 1.
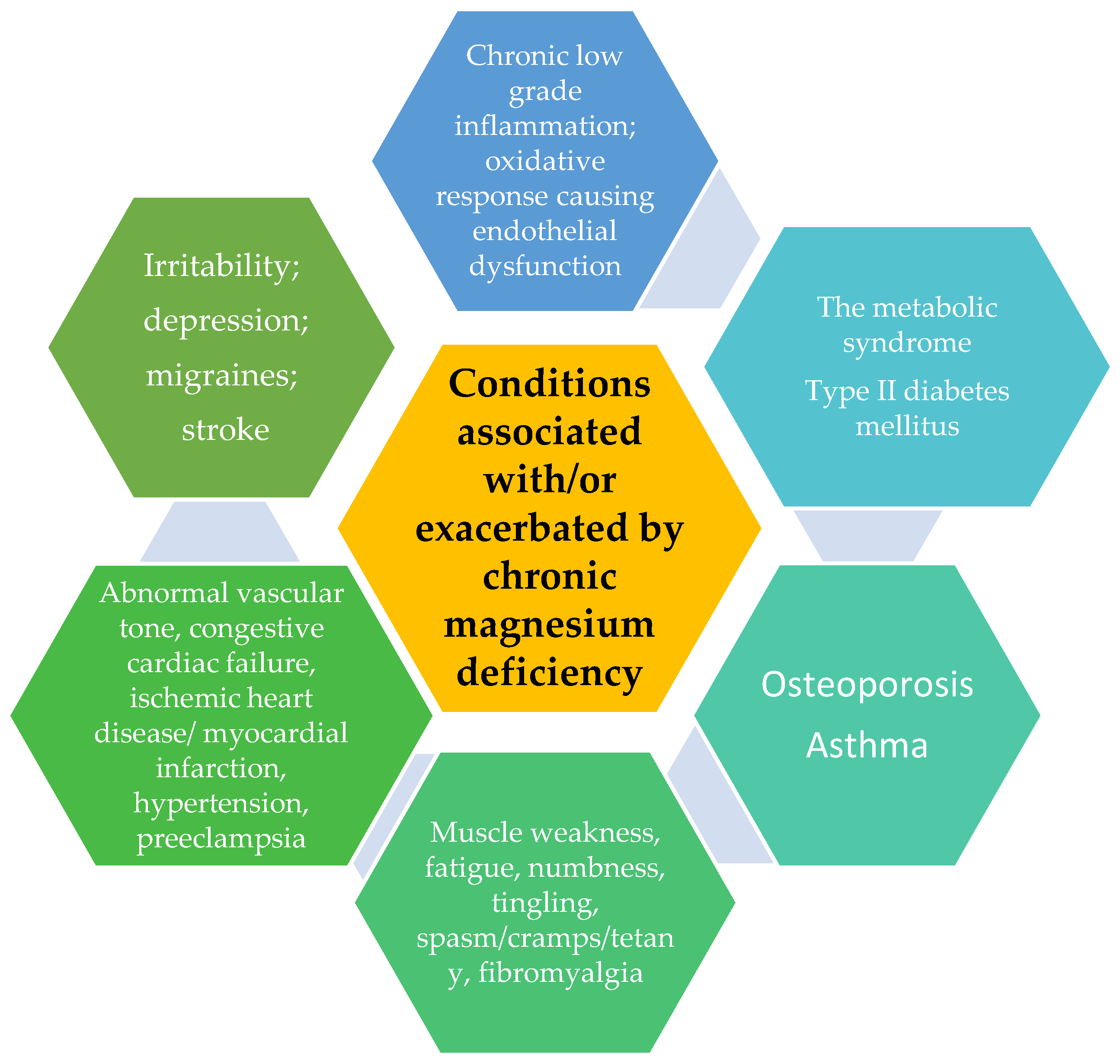
Figure 1. Pathologies connected to/or increased by chronic magnesium deficiency.
Also, it was discovered that a magnesium deficit could predict negative outcomes, and when supplementation or treatment was started, there was a decreased chance of pathology [17].
2.2. Magnesium and MetS
According to the World Health Organization (WHO), coronary heart disease (CHD) accounts for more than 7 million deaths each year and is the leading cause of mortality regardless the gender [53]. It is commonly recognized that type 2 diabetes (T2DM) is a significant risk factor for coronary heart disease (CHD). Patients with diabetes have twice the risk of developing coronary heart disease than people without the disease [20][54]. Several studies have demonstrated a link connecting CHD and metabolic syndrome (MetS), type 2 diabetes, and increased levels of oxidative and inflammatory stress biomarkers [55][56]. A complex biological process called oxidative stress is defined by an excessive creation of reactive oxygen species (ROS), which function as destabilizers of the body’s REDOX balance and cause oxidative dysfunction. Oxidative stress impairs every process and even affects the equilibrium of nucleic acids. ROS interacts with proteins and phospholipids, which causes structural alterations in tissues and organs. Those who are predisposed to heart problems typically develop a constellation of cardiovascular risk factors (CVRFs). Oxidative stress is frequently linked to cardiovascular diseases (CVDs) such as coronary artery disease (CAD), cardiomyopathy, or heart failure (HF), which may occur in people with hypertension (HTN), diabetes mellitus (DM), obesity, and other disorders [57]. According to some studies, patients with CHD and T2DM had significantly lower levels of magnesium [12][38][58][59][60]. There is also strong evidence linking magnesium deficiency to metabolic syndrome [23][55][61][62]. The elements of MetS, such as hyperglycemia, hypertension, hypertriglyceridemia, and insulin resistance, have been linked to hypomagnesemia [12]. The components of MetS, such as insulin sensitivity, fasting blood glucose (FBG), triglyceride (TG) levels, high-density lipoprotein cholesterol (HDL-C), and high blood pressure (BP), is said to respond favorably to magnesium supplementation [58]. The aforementioned metabolic illnesses all share the common pathophysiological feature of chronic low-grade inflammation, and magnesium deficiency can both directly and indirectly cause inflammation by altering the gut flora [24]. In addition, a lack of magnesium worsens immunological function by priming phagocytes, promoting granulocyte oxidative burst, activating endothelial cells, and raising cytokine production levels [12]. Magnesium has anti-inflammatory, glucose, and insulin metabolism-improving, endothelium-dependent vasodilation-improving, and lipid profile-normalizing properties [63]. C-reactive protein (CRP), a measure of systemic inflammation and a predictor of future cardiovascular events in individuals with MetS, and serum magnesium levels are inversely correlated [20][64]. At the same time, changes in the metabolism of several micronutrients were observed in obese patients, such as decreased magnesium concentrations in the serum, plasma, and erythrocytes [21][65][66]. Magnesium supplementation has been shown to have positive effects on patients with metabolic disorders in terms of their metabolic profile [25]. For example, Asemi et al. demonstrated that magnesium supplementation in pregnant women with gestational diabetes (GDM), in the form of magnesium oxide at a dose of 250 mg per day, significantly improved glycemic control, lipoproteins, and the biomarkers of oxidative stress and inflammation [25]. Higher magnesium intakes were associated with lower fasting insulin levels in healthy women without diabetes [67]. The relationship between total dietary magnesium intake and insulin responses to an oral glucose tolerance test was inverse [23][39].
Recent evidence from some meta-analysis studies demonstrated the efficacy of oral magnesium supplementation in significantly decreasing various inflammatory markers, especially CRP, and increasing nitric oxide (NO) [68]. The reduction of fasting blood glucose was also observed, as well as a 3–4 mm Hg reduction in systolic blood pressure (SBP) and 2–3 mmHg in diastolic blood pressure (DBP), with a beneficial impact on the lipid profile [15][22]. The experimental data show that magnesium deficiency worsens atherosclerosis while magnesium supplementation slows atherogenesis [11][69][70]. In addition, randomized controlled trials have shown that magnesium supplementation improves endothelial function while lowering blood pressure [26][27][36][42], arterial stiffness [71], fasting hyperglycemia [27][72], insulin resistance [73], and postoperative arrhythmias [70][74].
2.3. Magnesium–Statin-like Effect
Magnesium is crucial for regulating the activity of several key enzymes involved in lipid metabolism, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) [46][75], which controls cholesterol biosynthesis; lecithin–cholesterol acyltransferase (LCAT), which lowers plasma concentrations of LDL-c and VLDL-c [15][21]; desaturase (DS) [12][15]; and lipoprotein lipase (LPL) [47][76]. LPL, DS, and LCAT activity are suppressed by hypomagnesemia, and Mg supplementation can positively affect their expression [50][75]. On the other hand, Mg deficiency is associated with increased activity of HMG-CoA reductase [43]. The ratio of saturated to unsaturated fatty acids increases and the levels of TG, LDL, HDL, and VLDL decrease when the activity of this enzyme is impaired [77]. Modulation of the gene expression of LDLR (LDL receptor) and other transcription factors, such as sterol regulatory element-binding proteins SREBP-1a and SREBP-2, may also contribute to the hypercholesterolemic effect of an inadequate intake of Mg, although the increase in LDL concentration is mediated by increased LDLR and SREBP expression [78]. According to a study in type 1 diabetes mellitus (T1DM) patients, the Mg levels and blood levels of oxidized low-density lipoprotein (ox-LDL) are linked [12]. Magnesium also seems to affect the expression of genes that regulate a number of processes, including adipogenesis, lipolysis, and inflammation, such as peroxisome proliferator-activated receptor gamma (PPARγ) [21][79]. Also, research has shown that a dietary magnesium deficiency causes a higher activity of the enzyme’s serine palmitoyl CoA transferase 1 and 2, which, in turn, triggers the processes of atherogenesis, angiogenesis, and immunoreaction [12][21][80].
A 12-week randomized, double-blind, placebo-controlled clinical trial comparing the effects of magnesium oxide (250 mg/day) versus placebo on anthropometric indices, blood pressure, blood glucose, insulin, C-reactive protein, uric acid, and a lipid profile was performed on individuals with prediabetes (n = 86). Compared to the placebo group, subjects who took magnesium supplements had significantly increased levels of HDL cholesterol at the end of the study. However, magnesium supplementation at the dose and time mentioned above did not alter other cardiometabolic parameters [81].
Participants with moderate coronary artery disease (CAD) lowered their serum levels of LDL-C, SGOT, SGPT, and ox-LDL by taking 300 mg/day of MgSO4 for six months in a double-blind, randomized clinical investigation [66].
In addition, a recent meta-analysis of studies that included people with hypertriglyceridemia revealed an important decrease in triglyceride levels after magnesium supplementation, indicating a potential beneficial effect of magnesium supplementation on dyslipidemic diseases [15].
Clinical research using oral Mg supplementation provides the majority of the evidence for relationships between Mg and the blood lipid profile [28][29][46][76][77][82][83][84][85][86]. The authors of various meta-analyses describing this link stressed the inconsistency of the findings of this research [87][88]. The significant variety of groups examined, the numerous approaches to measuring blood serum Mg concentration, and the absence of stratification of the subgroups of patients compared have all contributed to the ambiguity of the results produced in this sector [12].
References
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029.
- Otelea, M.R.; Nartea, R.; Popescu, F.G.; Covaleov, A.; Mitoiu, B.I.; Nica, A.S. The Pathological Links between Adiposity and the Carpal Tunnel Syndrome. Curr. Issues Mol. Biol. 2022, 44, 2646–2663.
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240.
- Mir, R.; Elfaki, I.; Javid, J.; Barnawi, J.; Altayar, M.A.; Albalawi, S.O.; Jalal, M.M.; Tayeb, F.J.; Yousif, A.; Ullah, M.F.; et al. Genetic Determinants of Cardiovascular Disease: The Endothelial Nitric Oxide Synthase 3 (eNOS3), Krüppel-Like Factor-14 (KLF-14), Methylenetetrahydrofolate Reductase (MTHFR), MiRNAs27a and Their Association with the Predisposition and Susceptibility to Coronary Artery Disease. Life 2022, 12, 1905.
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56.
- Elfaki, I.; Mir, R.; Abu-Duhier, F.; Alotaibi, M.; Alalawy, A.; Barnawi, J.; Babakr, A.; Mir, M.; Mirghani, H. Clinical Implications of MiR128, Angiotensin I Converting Enzyme and Vascular Endothelial Growth Factor Gene Abnormalities and Their Association with T2D. Curr. Issues Mol. Biol. 2021, 43, 1859–1875.
- Padam, P.; Barton, L.; Wilson, S.; David, A.; Walji, S.; de Lorenzo, F.; Ray, K.K.; Jones, B.; Cegla, J. Lipid lowering with inclisiran: A real-world single-center experience. Open Heart 2022, 9, e002184.
- Aygun, S.; Tokgozoglu, L. Comparison of Current International Guidelines for the Management of Dyslipidemia. J. Clin. Med. 2022, 11, 7249.
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444.
- Li, S.; Chen, W.; Srinivasan, S.R.; Bond, M.G.; Tang, R.; Urbina, E.M.; Berenson, G.S. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 2003, 290, 2271–2276.
- Morais, J.B.S.; Severo, J.S.; Santos, L.R.D.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26.
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714.
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; da Costa, T.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114.
- Jacobs, D.R.; Woo, J.G.; Sinaiko, A.R.; Daniels, S.R.; Ikonen, J.; Juonala, M.; Kartiosuo, N.; Lehtimäki, T.; Magnussen, C.G.; Viikari, J.S.A.; et al. Childhood cardiovascular risk factors and adult cardiovascular events. N. Engl. J. Med. 2022, 386, 1877–1888.
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Pharmacol. 2017, 73, 525–536.
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993.
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic Magnesium Deficiency and Human Disease; Time for Reappraisal? QJM Int. J. Med. 2018, 111, 759–763.
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694.
- Ebrahimi Mousavi, S.; Ghoreishy, S.M.; Hemmati, A.; Mohammadi, H. Association between Magnesium Concentrations and Prediabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 24388.
- Alkazemi, D.; Alsouri, N.; Zafar, T.; Kubow, S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients 2022, 14, 5257.
- Dos Santos, L.R.; Melo, S.R.D.S.; Severo, J.S.; Morais, J.B.S.; da Silva, L.D.; de Paiva Sousa, M.; de Sousa, T.G.V.; Henriques, G.S.; do Nascimento Marreiro, D. Cardiovascular Diseases in Obesity: What Is the Role of Magnesium? Biol. Trace Elem. Res. 2021, 199, 4020–4027.
- Rodríguez-Morán, M.; Simental-Mendía, L.E.; Gamboa-Gómez, C.I.; Guerrero-Romero, F. Oral Magnesium Supplementation and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv. Chronic Kidney Dis. 2018, 25, 261–266.
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463.
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271.
- Hamedifard, Z.; Farrokhian, A.; Reiner, Ž.; Bahmani, F.; Asemi, Z.; Ghotbi, M.; Taghizadeh, M. The Effects of Combined Magnesium and Zinc Supplementation on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Lipids Health Dis. 2020, 19, 112.
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral Magnesium Supplementation Improves Endothelial Function and Attenuates Subclinical Atherosclerosis in Thiazide-Treated Hypertensive Women. J. Hypertens. 2017, 35, 89–97.
- Verma, H.; Garg, R. Effect of Magnesium Supplementation on Type 2 Diabetes Associated Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633.
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low Serum Magnesium Levels and Metabolic Syndrome. Acta Diabetol. 2002, 39, 209–213.
- Rasheed, H.; Elahi, S.; Ajaz, H. Serum Magnesium and Atherogenic Lipid Fractions in Type II Diabetic Patients of Lahore, Pakistan. Biol. Trace Elem. Res. 2012, 148, 165–169.
- Toprak, O.; Sarı, Y.; Koç, A.; Sarı, E.; Kırık, A. The Impact of Hypomagnesemia on Erectile Dysfunction in Elderly, Non-Diabetic, Stage 3 and 4 Chronic Kidney Disease Patients: A Prospective Cross-Sectional Study. Clin. Interv. Aging 2017, 12, 437–444.
- Dey, R.; Rajappa, M.; Parameswaran, S.; Revathy, G. Hypomagnesemia and Atherogenic Dyslipidemia in Chronic Kidney Disease: Surrogate Markers for Increased Cardiovascular Risk. Clin. Exp. Nephrol. 2015, 19, 1054–1061.
- Bain, L.K.; Myint, P.K.; Jennings, A.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Welch, A.A. The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int. J. Cardiol. 2015, 196, 108–114.
- Yuan, Z.; Liu, C.; Tian, Y.; Zhang, X.; Ye, H.; Jin, L.; Ruan, L.; Sun, Z.; Zhu, Y. Higher Levels of Magnesium and Lower Levels of Calcium in Whole Blood Are Positively Correlated with the Metabolic Syndrome in a Chinese Population: A Case-Control Study. Ann. Nutr. Metab. 2016, 69, 125–134.
- Raitakari, O.; Kartiosuo, N.; Pahkala, K.; Hutri-Kähönen, N.; Bazzano, L.A.; Chen, W.; Urbina, E.M.; Jacobs, D.R.; Sinaiko, A.; Steinberger, J.; et al. Lipoprotein(a) in Youth and Prediction of Major Cardiovascular Outcomes in Adulthood. Circulation 2023, 147, 23–31.
- Wilson, D.P.; Koschinsky, M.L.; Moriarty, P.M. Expert position statements: Comparison of recommendations for the care of adults and youth with elevated lipoprotein(a). Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 159–173.
- Rosanoff, A.; Costello, R.B.; Johnson, G.H. Effectively Prescribing Oral Magnesium Therapy for Hypertension: A Categorized Systematic Review of 49 Clinical Trials. Nutrients 2021, 13, 195.
- Jin, H.; Nicodemus-Johnson, J. Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients 2018, 10, 1760.
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R.; Savage, P.J. Magnesium Intake and Incidence of Metabolic Syndrome Among Young Adults. Circulation 2006, 113, 1675–1682.
- Humphries, S.; Kushner, H.; Falkner, B. Low Dietary Magnesium Is Associated with Insulin Resistance in a Sample of Young, Nondiabetic Black Americans. Am. J. Hypertens. 1999, 12, 747–756.
- Itoh, K.; Kawasaka, T.; Nakamura, M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br. J. Nutr. 1997, 78, 737–750.
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013.
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2021, 13, 139.
- Rashvand, S.; Mobasseri, M.; Tarighat-Esfanjani, A. Effects of Choline and Magnesium Concurrent Supplementation on Coagulation and Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Pilot Clinical Trial. Biol. Trace Elem. Res. 2020, 194, 328–335.
- Karie, S.; Launay-Vacher, V.; Deray, G.; Isnard-Bagnis, C. Statins in patients with kidney failure: Efficacy, tolerance, and prescription guidelines in patients with chronic kidney disease and renal transplant. Presse Med. 2006, 35 Pt 1, 219–229.
- Zhao, L.; Li, S.; Gao, Y. Efficacy of statins on renal function in patients with chronic kidney disease: A systematic review and meta-analysis. Ren Fail. 2021, 43, 718–728.
- Rotter, I.; Kosik-Bogacka, D.; Dolegowska, B.; Safranow, K.; Karakiewicz, B.; Laszczynska, M. Relationship between Serum Magnesium Concentration and Metabolic and Hormonal Disorders in Middle-Aged and Older Men. Magnes. Res. 2015, 28, 99–107.
- Suliburska, J.; Cofta, S.; Gajewska, E.; Kalmus, G.; Sobieska, M.; Samborski, W.; Krejpcio, Z.; Drzymala-Czyz, S.; Bogdanski, P. The Evaluation of Selected Serum Mineral Concentrations and Their Association with Insulin Resistance in Obese Adolescents. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2396–2400.
- Dai, B.; Li, X.; Xu, J.; Zhu, Y.; Huang, L.; Tong, W.; Yao, H.; Chow, D.H.; Qin, L. Synergistic effects of magnesium ions and simvastatin on attenuation of high-fat diet-induced bone loss. Bioact Mater. 2021, 6, 2511–2522.
- Cambray, S.; Ibarz, M.; Bermudez-Lopez, M.; Marti-Antonio, M.; Bozic, M.; Fernandez, E.; Valdivielso, J.M. Magnesium Levels Modify the Effect of Lipid Parameters on Carotid Intima Media Thickness. Nutrients 2020, 12, 2631.
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of Oral Selenium and Magnesium Co-Supplementation on Lipid Metabolism, Antioxidative Status, Histopathological Lesions, and Related Gene Expression in Rats Fed a High-Fat Diet. Lipids Health Dis. 2018, 17, 165.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010.
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project. Nutrients 2020, 13, 125.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186.
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium Intake, C-Reactive Protein, and the Prevalence of Metabolic Syndrome in Middle-Aged and Older U.S. Women. Diabetes Care 2005, 28, 1438–1444.
- Barbagallo, M. Role of Magnesium in Insulin Action, Diabetes and Cardio-Metabolic Syndrome X. Mol. Aspects Med. 2003, 24, 39–52.
- Akyüz, O.; Gücün, M.; Demirci, R.; Celik, M. Relationship Between Serum Magnesium Level and Insulin Resistance in Turkey Non-Obese Adult Population. Biol. Trace Elem. Res. 2022, 200, 3070–3077.
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238.
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodríguez-Morán, M. Magnesium in Metabolic Syndrome: A Review Based on Randomized, Double-Blind Clinical Trials. Magnes. Res. 2016, 29, 146–153.
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care 2004, 27, 134–140.
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. Dietary Magnesium Intake in Relation to Plasma Insulin Levels and Risk of Type 2 Diabetes in Women. Diabetes Care 2004, 27, 59–65.
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical Magnesium Deficiency: A Principal Driver of Cardiovascular Disease and a Public Health Crisis. Open Heart 2018, 5, e000668.
- Barbagallo, M.; Dominguez, L.J. Magnesium Metabolism in Type 2 Diabetes Mellitus, Metabolic Syndrome and Insulin Resistance. Highlight Issue Cell. Regul. Magnes. 2007, 458, 40–47.
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44.
- Ridker, P.; Buring, J.; Cook, N.; Rifai, N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events. An 8-year Follow-Up of 14,719 Initially Healthy American Women. Circulation 2003, 12, 391–397.
- Cruz, K.J.C.; de Oliveira, A.R.S.; Pinto, D.P.; Morais, J.B.S.; da Silva Lima, F.; Colli, C.; Torres-Leal, F.L.; do Nascimento Marreiro, D. Influence of Magnesium on Insulin Resistance in Obese Women. Biol. Trace Elem. Res. 2014, 160, 305–310.
- Farshidi, H.; Sobhani, A.R.; Eslami, M.; Azarkish, F.; Eftekhar, E.; Keshavarz, M.; Soltani, N. Magnesium Sulfate Administration in Moderate Coronary Artery Disease Patients Improves Atherosclerotic Risk Factors: A Double-Blind Clinical Trial Study. J. Cardiovasc. Pharmacol. 2020, 76, 321–328.
- Fung, T.T.; Manson, J.E.; Solomon, C.G.; Liu, S.; Willett, W.C.; Hu, F.B. The Association between Magnesium Intake and Fasting Insulin Concentration in Healthy Middle-Aged Women. J. Am. Coll. Nutr. 2003, 22, 533–538.
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679.
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium Deficiency and Oxidative Stress: An Update. BioMedicine 2016, 6, 20.
- Larsson, S.C.; Burgess, S.; Michaëlsson, K. Serum Magnesium Levels and Risk of Coronary Artery Disease: Mendelian Randomisation Study. BMC Med. 2018, 16, 68.
- Joris, P.; Plat, J.; Bakker, S.; Mensink, R. Long-Term Magnesium Supplementation Improves Arterial Stiffness in Overweight and Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Intervention Study. Atherosclerosis 2016, 252, e90.
- Song, Y.; He, K.; Levitan, E.B.; Manson, J.E.; Liu, S. Effects of Oral Magnesium Supplementation on Glycaemic Control in Type 2 Diabetes: A Meta-Analysis of Randomized Double-Blind Controlled Trials. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 1050–1056.
- Simental-Mendía, L.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effects of Magnesium Supplementation on Insulin Sensitivity and Glucose Control. Pharmacol. Res. 2016, 111, 272–282.
- Lee, H.; Ghimire, S.; Kim, E. Magnesium Supplementation Reduces Postoperative Arrhythmias After Cardiopulmonary Bypass in Pediatrics: A Metaanalysis of Randomized Controlled Trials. Pediatr. Cardiol. 2013, 34, 1396–1403.
- Rosanoff, A.; Seelig, M. Comparison of Mechanism and Functional Effects of Magnesium and Statin Pharmaceuticals. J. Am. Coll. Nutr. 2004, 23, 501S–505S.
- Oliveira, A.R.; Cruz, K.; Severo, J.; Morais, J.; Freitas, T.; Araújo, R.; Marreiro, D. Hypomagnesemia and Its Relation with Chronic Low-Grade Inflammation in Obesity. Rev. Assoc. Médica Bras. 2017, 63, 156–163.
- Randell, E.W.; Mathews, M.; Gadag, V.; Zhang, H.; Sun, G. Relationship between Serum Magnesium Values, Lipids and Anthropometric Risk Factors. Atherosclerosis 2008, 196, 413–419.
- Jamilian, M.; Samimi, M.; Faraneh, A.E.; Aghadavod, E.; Shahrzad, H.D.; Chamani, M.; Mafi, A.; Asemi, Z. Magnesium Supplementation Affects Gene Expression Related to Insulin and Lipid in Patients with Gestational Diabetes. Magnes. Res. 2017, 30, 71–79.
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.; Downes, M.; Evans, R. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566.
- Altura, B.; Shah, N.; Li, Z.; Jiang, X.-C.; Perez-Albela, J.; Altura, B.T. Magnesium Deficiency Upregulates Serine Palmitoyl Transferase (SPT 1 and SPT 2) in Cardiovascular Tissues: Relationship to Serum Ionized Mg and Cytochrome c. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H932–H938.
- Salehidoost, R.; Taghipour Boroujeni, G.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of Oral Magnesium Supplement on Cardiometabolic Markers in People with Prediabetes: A Double Blind Randomized Controlled Clinical Trial. Sci. Rep. 2022, 12, 18209.
- Ham, J.Y.; Shon, Y.H. Natural Magnesium-Enriched Deep-Sea Water Improves Insulin Resistance and the Lipid Profile of Prediabetic Adults: A Randomized, Double-Blinded Crossover Trial. Nutrients 2020, 12, 515.
- Guerrero-Romero, F.; Rodríguez-Morán, M. Hypomagnesemia Is Linked to Low Serum HDL-Cholesterol Irrespective of Serum Glucose Values. J. Diabetes Complicat. 2000, 14, 272–276.
- Shahbah, D.; Naga, A.; Hassan, T.; Zakaria, M.; Beshir, M.; El-Morshedy, S.E.; Abdalhady, M.; Kamel, E.; Rahman, D.; Kamel, L.; et al. Status of Serum Magnesium in Egyptian Children with Type 1 Diabetes and Its Correlation to Glycemic Control and Lipid Profile. Medicine 2016, 95, e5166.
- Mishra, S.; Padmanaban, P.; Deepti, G.; Sarkar, G.; Sumathi, S.; Toora, B. Serum Magnesium and Dyslipidemia in Type-2 Diabetes Mellitus. Biomed. Res. 2011, 23, 295–300.
- Mahalle, N.; Kulkarni, M.; Naik, S. Is Hypomagnesaemia a Coronary Risk Factor among Indians with Coronary Artery Disease? J. Cardiovasc. Dis. Res. 2012, 3, 280–286.
- Asbaghi, O.; Moradi, S.; Nezamoleslami, S.; Moosavian, S.P.; ali Hojjati Kermani, M.; Lazaridi, A.V.; Miraghajani, M. The Effects of Magnesium Supplementation on Lipid Profile Among Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Trace Elem. Res. 2021, 199, 861–873.
- Yu, L.; Zhang, J.; Wang, L.; Li, S.; Zhang, Q.; Xiao, P.; Wang, K.; Zhuang, M.; Jiang, Y. Association between Serum Magnesium and Blood Lipids: Influence of Type 2 Diabetes and Central Obesity. Br. J. Nutr. 2018, 120, 250–258.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.4K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




