Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guilin Wang | -- | 1816 | 2023-04-21 12:35:29 | | | |
| 2 | Jessie Wu | + 14 word(s) | 1830 | 2023-04-23 04:04:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, G.; Zhang, J.; Wang, Y.; Tan, Y.; Li, Z.; Zhang, B.; Liu, Z. Decomposition Characteristics of Iron Ore during Smelting Reduction. Encyclopedia. Available online: https://encyclopedia.pub/entry/43320 (accessed on 08 February 2026).
Wang G, Zhang J, Wang Y, Tan Y, Li Z, Zhang B, et al. Decomposition Characteristics of Iron Ore during Smelting Reduction. Encyclopedia. Available at: https://encyclopedia.pub/entry/43320. Accessed February 08, 2026.
Wang, Guilin, Jianliang Zhang, Yaozu Wang, Yubo Tan, Zhen Li, Bo Zhang, Zhengjian Liu. "Decomposition Characteristics of Iron Ore during Smelting Reduction" Encyclopedia, https://encyclopedia.pub/entry/43320 (accessed February 08, 2026).
Wang, G., Zhang, J., Wang, Y., Tan, Y., Li, Z., Zhang, B., & Liu, Z. (2023, April 21). Decomposition Characteristics of Iron Ore during Smelting Reduction. In Encyclopedia. https://encyclopedia.pub/entry/43320
Wang, Guilin, et al. "Decomposition Characteristics of Iron Ore during Smelting Reduction." Encyclopedia. Web. 21 April, 2023.
Copy Citation
Against the background of low global carbonization, blast furnace ironmaking technology with coking puts huge amounts of pressure on the global steel industry to save energy and reduce emissions due to its high pollution levels and high energy consumption. Bath smelting reduction technology is globally favored and studied by metallurgists as a non-blast furnace ironmaking technology that directly reduces iron ore into liquid metal without using coke as the raw material. The smelting reduction reaction of iron ore, which is the core reaction of the process, is greatly significant to its productivity and energy saving.
iron
thermal decomposition
non-blast furnace ironmaking
energy
1. Research Progress of Experimental Methods
Iron ores used in the bath smelting reduction technology are generally hematite or hematite–limonite ore, minerals that contain Fe2O3, FeO(OH), SiO2 and Al2O3. The iron ore undergoes thermal decomposition reactions in a high-temperature environment due to heat transfer during smelting reduction.
For the study of the thermal decomposition behavior of iron ore during smelting reduction, previous experimental methods included a thermogravimetric analysis (TGA) device, a heated horizontal furnace, and a high-temperature drop tube furnace, as shown in Figure 1 [1]. This method is used to investigate the thermal decomposition temperature and properties of iron ores via the thermal decomposition temperature and mass weight loss of the sample at different heating rates. The TGA device (as shown in Figure 1a) is often used in combination with differential scanning calorimetry (DSC) equipment to test the endothermic peak at different heating rates. It requires a smaller sample mass, and it is more difficult to test the chemical composition of the sample with it after a reaction than other experimental methods are.
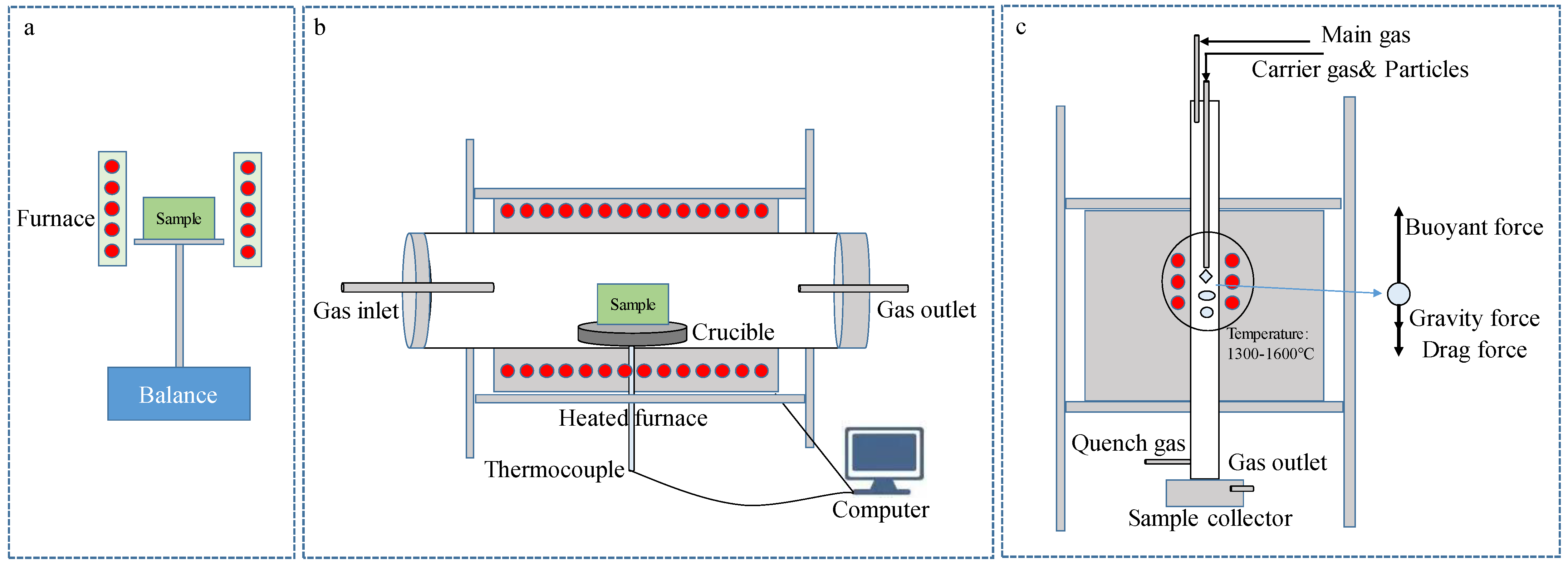
Figure 1. Experimental methods for the thermal decomposition of iron ore. (a) TGA; (b) heated horizontal furnace; (c) high-temperature drop tube furnace.
The heated horizontal furnace (as shown in Figure 1b) allows for experiments to be performed on a large number of samples, and samples at room temperature could be pushed directly into the constant temperature area for the thermal decomposition of samples at a constant temperature. Its advantage is that the chemical composition of a sample can be detected at different reaction times, and the atmosphere of experiment can be changed.
Figure 1c shows the high-temperature drop tube furnace that produces the thermal decomposition characteristics of iron ore during the falling process; therefore, it is closer to the actual environment of the HIsarna process. The method allows for the thermal decomposition characteristics of iron ore to be studied at different residence times, particle sizes, and temperatures, and in different atmospheres.
2. The Thermal Decomposition Reaction Mechanism and Influencing Factors of Iron Ore
The thermal decomposition of iron ore involves the decomposition of hematite and hydrated minerals (such as goethite, kaolinite, and gibbsite). Goethite, as a mineral produced through the weathering of hematite, contains crystalline hydrate. Its thermal decomposition reaction is the process of dehydration into hematite. Wolska’s study showed that goethite is converted into primary hematite, then into hydrogen hematite, and lastly into hematite in the pyrolytic process [2]. Goethite is converted directly into hematite during thermal decomposition without the formation of any intermediate phases [3][4]. The thermal decomposition reaction of goethite is shown in Equation (1):
The thermal decomposition temperature of hematite is higher than that of goethite. The step-by-step reaction process of the Fe2O3 decomposition is shown in Equations (2)–(4) [5]:
The results of partial oxygen pressure for the decomposition of iron oxides (Fe2O3, Fe3O4 and FeO) at different temperatures were calculated with FactSage, as shown in Figure 2. Partial oxygen pressure during the decomposition of Fe2O3 at 500 and 1000 °C was 9.4505 × 10−20 and 1.7968 × 10−6 atm, respectively. The partial oxygen pressure of hematite’s thermal decomposition at 1400 and 1500 °C was 0.1175 and 0.8919 atm, respectively. The partial oxygen decomposition pressure of Fe3O4 500 and at 1500 °C was 5.2033 × 10−30 and 5.1294 × 10−7 atm, respectively. The partial oxygen decomposition pressure of FeO at 1500 °C was 1.3700 × 10−9 atm. Hematite is more difficult to decompose into magnetite at low temperatures, and easier to decompose at high temperatures to produce magnetite. The decomposition reaction of Fe3O4 and FeO at normal pressure levels does not occur below 1500 °C.
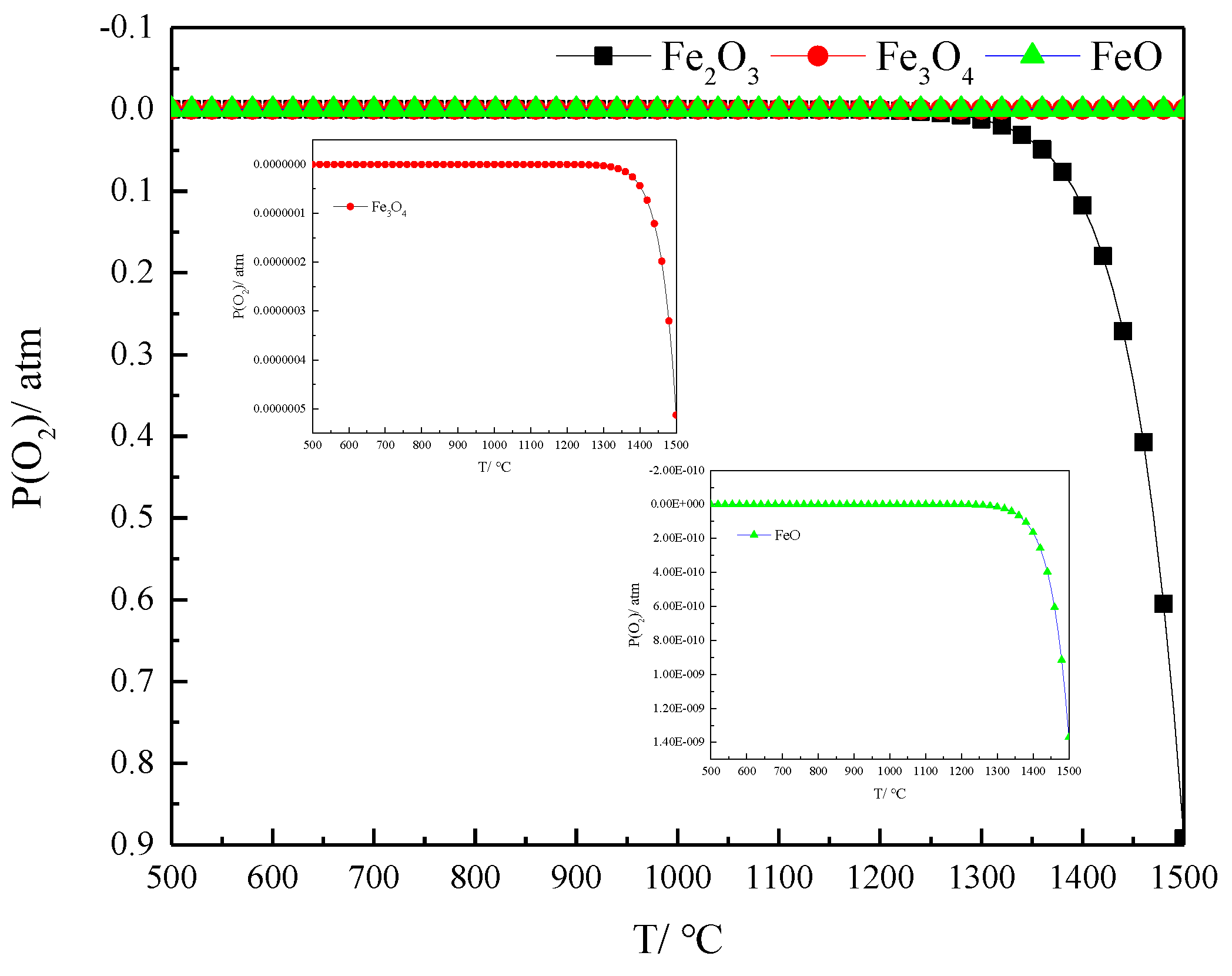
Figure 2. Partial oxygen pressure results of iron oxide decomposition at different temperatures.
The decomposition products of hematite at 1400 and 1500 °C are solid magnetite, and liquid magnetite and FeO at 1600 °C [1]. Optical-microscopy observations based on the thermal decomposition process of hematite also indicated that the core unreacted mineral is hematite, and the edge decomposition product is magnetite [5]. The results of the study correspond to the theoretical calculations in Figure 2.
Factors affecting the thermal decomposition of iron ore include the system atmosphere, particle size, and residence time at high temperatures. The results of the relevant studies are as follows.
- (1)
-
The influence of atmosphere
Monica et al. [6] investigated the thermal decomposition of hematite (α-phase, 99% metal basis) with TGA, with the first and second decomposition peaks at 358 and 600 °C, respectively. Mohammad et al. treated hematite up to 1450 °C in an inert (N2) and air atmosphere [7], and showed that the decomposition reaction of hematite occurred at 1180 °C in the inert atmosphere and was basically completed at 1270 °C. However, the decomposition of hematite started at 1360 °C in the air atmosphere. Qu et al. [1] found that the decomposition temperature of hematite was 1264 °C at a heating rate of 10 °C/min. Darken et al. [8] also reported that hematite’s decomposition temperature was 1264 °C in an inert atmosphere, 1457 °C in an oxygen atmosphere, and 1392 °C in an air atmosphere. Hematite decomposes at a temperature of 1385 °C in an air atmosphere [9]. The decomposition temperature of hematite is lower and lasts longer in an inert atmosphere than it does in an air atmosphere. The results of the partial oxygen pressure for the decomposition reactions calculated with FactSage show that the partial oxygen pressure of the system was lower in the inert atmosphere, and its decomposition temperature was thereby lower. However, even for the same atmosphere, previous studies on the decomposition temperature of hematite vary, with large differences in the decomposition temperature.
In addition, the differences in the decomposition behavior of hematite in CO2 and inert (N2) atmospheres were compared by previous authors [1]. The oxygen loss percentage (R) is the ratio of the weight loss of oxygen of a sample, Δmoxygen, to the total oxygen mass of the iron oxide in sample, mtot-oxygen, as shown in Equation (5).
The R of hematite in CO2 atmosphere was 3–4% higher than that in the N2 atmosphere, as shown in Figure 3. This is mainly related to the structure of the gas molecules, as CO2 has a higher thermal radiation capacity than that of N2, as the particles heat up faster through radiation in a CO2 atmosphere than they do in an N2 or Ar atmosphere [1].
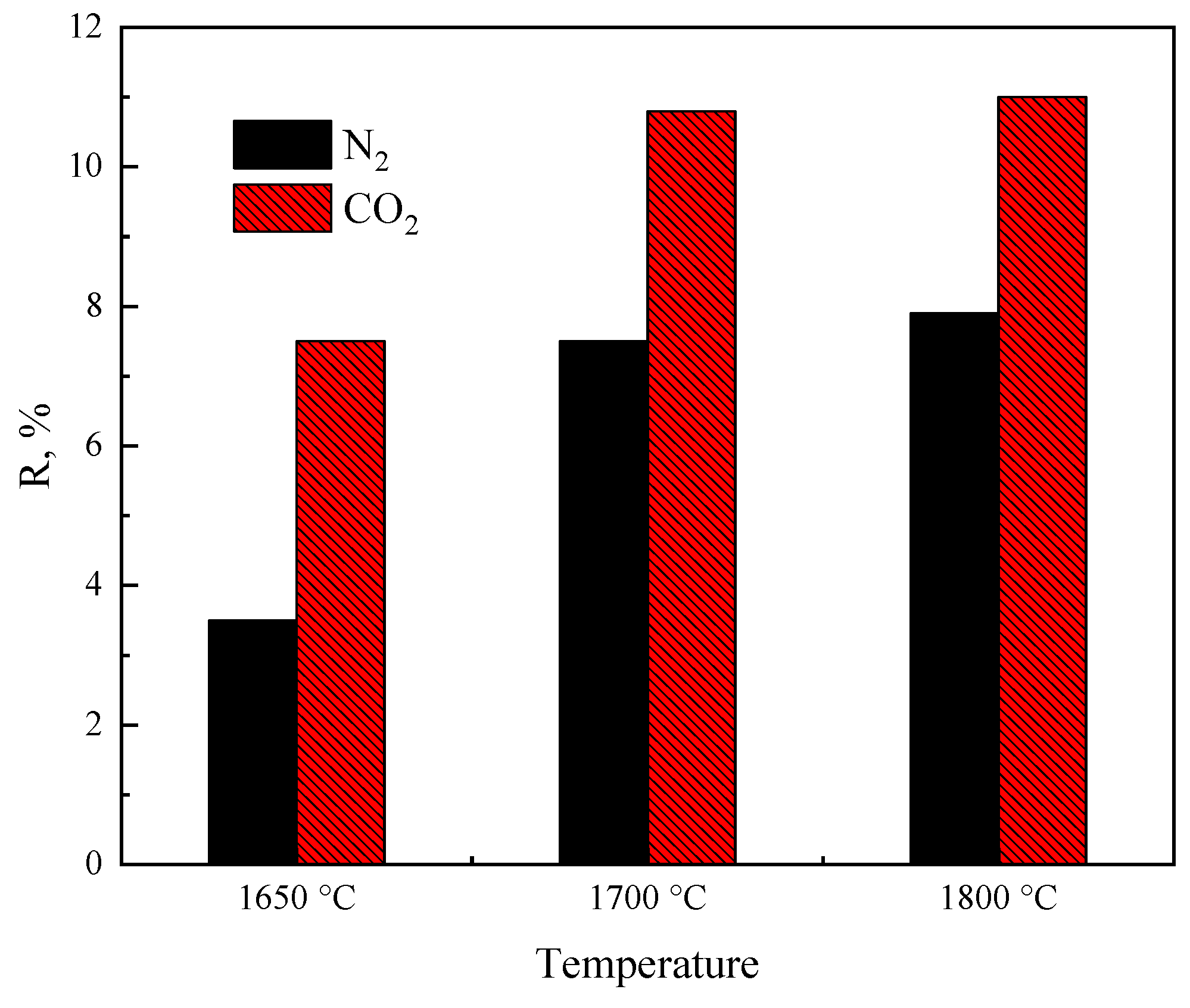
Figure 3. Thermal decomposition of hematite in different atmospheres [1].
- (2)
-
Particle size
Monica et al. [6] found that the decomposition temperature of fine-grained hematite (99% metal basis) was lower after ball milling, and that the grain size had an important influence on the thermal stability of hematite. Similarly, in TGA–DSC experiments, the initial decomposition temperature was 1383 °C for 125–250 μm hematite and 1264 °C for 45–53 μm hematite on the basis of a 10 °C/min heating rate, showing that the decomposition temperature increased for the larger iron-ore fines due to the slow heating rate [1]. However, TGA thermal decomposition experiments with different hematite particle sizes of −110 μm were also conducted, and the results showed that the particle size of hematite had no significant effect on the thermal decomposition temperature [5].
However, in the thermal decomposition experiments based on a high-temperature drop tube furnace, the variation in iron-ore particle size had no effect on the weight loss of its thermal decomposition when both were −250 μm, mainly because the decomposition of hematite into magnetite had been completed before it fell to the bottom [1]. Although the particle size causes changes in the decomposition temperature, it is not a major factor in the HIsarna bath smelting reduction process, and decomposition was completed with the falling process in CCF.
- (3)
-
Residence time/Heating rate
For the decomposition of goethite, an increase in residence time leads to an increase in its weight loss, suggesting that the heating rate is the limiting step in its thermal decomposition. Xing et al. [5] showed that the start and end temperatures of the thermal decomposition reaction in the TGA experiments increased with the heating rate. There is hysteresis in the reaction at faster heating rates. The residence time of iron ore with N2 in the vertical furnace had a significant effect on the thermal decomposition, while the residence time in CO2 atmosphere had no effect on the thermal decomposition, indicating that the heating rate of the particles is the limiting step of thermal iron-ore decomposition in an N2 atmosphere.
3. Study of the Kinetics of Iron Ore Thermal Decomposition
The kinetic behavior of thermal iron-ore decomposition reactions has been extensively studied by previous authors. The main investigations regarded the rate-controlled model of thermal decomposition reactions and the activation energy of the reactions. The activation energy characterizes the ease of the thermal iron-ore decomposition reaction and is the energy absorbed by the reactant molecules to convert them from a stable state into activated molecules. The activation energy of hematite decomposition is higher than that of hematite–limonite ores.
Beuria [10] investigated the kinetics of the thermal decomposition of hematite–limonite ore. The rate-limiting model of thermal decomposition reaction is dominated by random nucleation and chemical reaction control. At higher temperatures, the random nucleation model dominates the thermal decomposition process.
For the study of goethite’s kinetic model’s thermal decomposition, previous studies showed that the thermal decomposition process of goethite is controlled by the random nucleation with nucleation growth models [11][12][13]. The activation energies for the decomposition reactions of goethite were also extensively reported. The activation energy for decomposition was around 80 kJ·mol−1 for most researchers, with some activation energy
For the kinetic study of the thermal decomposition reaction, previous studies had consistent results regarding its thermal decomposition kinetic model, but they vary considerably for the activation energy of decomposition reactions.
The activation energy of the thermal decomposition of hematite has also been extensively studied with the isoconversion method. The activation energy of hematite in air and inert atmospheres was 382 and 324 kJ/mol through an isoconversion methodogy, respectively. The activation energy calculated with the DSC method was significantly higher than the values achieved with TG [7]. However, two stages of the thermal decomposition of hematite in an inert atmosphere were reported with activation energies of 636 and 325 kJ/mol [9][14]. Xing’s results indicated that activation energy for the thermal decomposition of hematite was 1256 kJ/mol with a pre-exponential factor of 1.94 × 1041 s−1 with the TGA method [5].
References
- Qu, Y.; Yang, Y.; Zou, Z.; Zeilstra, C.; Meijer, K.; Boom, R. Thermal Decomposition Behaviour of Fine Iron Ore Particles. ISIJ Int. 2014, 54, 2196–2205.
- Wolska, E. Relations between the Existence of Hydroxyl Ions in the Anionic Sublattice of Hematite and Its Infrared and X-Ray Characteristics. Solid. State Ion. 1988, 28–30, 1349–1351.
- Watari, F.; Delavignette, P.; Amelinckx, S. Electron Microscopic Study of Dehydration Transformations. II. The Formation of “Superstructures” on the Dehydration of Goethite and Diaspore. J. Solid. State Chem. 1979, 29, 417–427.
- Goss, C.J. The Kinetics and Reaction Mechanism of the Goethite to Hematite Transformation. Mineral. Mag. 1987, 51, 437–451.
- Xing, L.; Qu, Y.; Wang, C.; Shao, L.; Zou, Z.; Song, W. Kinetic Study on Thermal Decomposition Behavior of Hematite Ore Fines at High Temperature. Metall. Mater. Trans. B. 2020, 51, 395–406.
- Sorescu, M.; Xu, T. Particle Size Effects on the Thermal Behavior of Hematite. J. Therm. Anal. Calorim. 2012, 107, 463–469.
- Salmani, M.; Alamdari, E.K.; Firoozi, S. Isoconversional Analysis of Thermal Dissociation Kinetics of Hematite in Air and Inert Atmospheres. J. Therm. Anal. Calorim. 2017, 128, 1385–1390.
- Darken, L.S.; Gurry, R.W. The System Iron—Oxygen. II. Equilibrium and Thermodynamics of Liquid Oxide and Other Phases. J. Am. Chem. Soc. 1946, 68, 798–816.
- Ferreira, S.; Siguin, D.; Garcia, F. Thermal Analysis of Sintering of Magnetite Pellets. Ironmak. Steelmak 1994, 21, 119–123.
- Beuria, P.C.; Biswal, S.K.; Mishra, B.K.; Roy, G.G. Kinetics Study on Removal of LOI by Thermal Decomposition of Hydrated Minerals Associated in Hematite Ore. J. Therm. Anal. Calorim. 2016, 126, 1231–1241.
- Przepiera, K.; Przepiera, A. Kinetics of Thermal Transformations of Precipitated Magnetite and Goethite. J. Therm. Anal. Calorim. 2004, 65, 497–503.
- Diamandescu, L.; Mihãilã-Tãrãbãşanu, D.; Calogero, S. Mössbauer Study of the Solid Phase Transformation α-FeOOH → Fe2O3. Mater. Chem. Phys. 1997, 48, 170–173.
- Grygar, T.; Ruan, I.H.D.; Gilkes, R.J. Re-Examination of the Kinetics of the Thermal Dehydroxylation of Goethite. J. Therm. Anal. Calorim. 1999, 55, 301–309.
- Chen, Z.; Zeilstra, C.; Van Der Stel, J.; Sietsma, J.; Yang, Y. Thermal Decomposition Reaction Kinetics of Hematite Ore. ISIJ Int. 2020, 60, 65–72.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




