Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rajeshwar Sinha | -- | 2322 | 2023-04-21 12:16:22 | | | |
| 2 | Camila Xu | Meta information modification | 2322 | 2023-04-23 09:42:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Application of Microalgae in Cosmetics. Encyclopedia. Available online: https://encyclopedia.pub/entry/43319 (accessed on 04 March 2026).
Gupta A, Singh AP, Singh VK, Singh PR, Jaiswal J, Kumari N, et al. Application of Microalgae in Cosmetics. Encyclopedia. Available at: https://encyclopedia.pub/entry/43319. Accessed March 04, 2026.
Gupta, Amit, Ashish P. Singh, Varsha K. Singh, Prashant R. Singh, Jyoti Jaiswal, Neha Kumari, Vijay Upadhye, Suresh C. Singh, Rajeshwar P. Sinha. "Application of Microalgae in Cosmetics" Encyclopedia, https://encyclopedia.pub/entry/43319 (accessed March 04, 2026).
Gupta, A., Singh, A.P., Singh, V.K., Singh, P.R., Jaiswal, J., Kumari, N., Upadhye, V., Singh, S.C., & Sinha, R.P. (2023, April 21). Application of Microalgae in Cosmetics. In Encyclopedia. https://encyclopedia.pub/entry/43319
Gupta, Amit, et al. "Application of Microalgae in Cosmetics." Encyclopedia. Web. 21 April, 2023.
Copy Citation
Microalgae produce a number of secondary metabolites with anti-inflammatory, anti-blemish and antimicrobial activities. Certain microalgal extracts such as Arthrospira platensis, Chlorella vulgaris, and Dunaliella salina can be used for repairing skin aging, healing and preventing wrinkle formation. The microalgae or its components’ activity is the basis for the creation of several commercially available cosmetics and cosmeceuticals.
natural sunscreen
cosmetic
Microalgae
1. Introduction
Microalgae produce a number of secondary metabolites with anti-inflammatory, anti-blemish and antimicrobial activities [1]. Certain microalgal extracts such as Arthrospira platensis, Chlorella vulgaris, and Dunaliella salina can be used for repairing skin aging, healing and preventing wrinkle formation [2][3][4][5]. The microalgae or its components’ activity is the basis for the creation of several commercially available cosmetics and cosmeceuticals. Anti-aging creams, refreshing/regenerating care items, emollients and anti-irritants in peelers, sunscreen cream, and hair care items are a few examples of commercialized microalgae sources in the skin care industry. These cosmetics and cosmeceuticals contain bioactive microalgae components or algal extract (Table 1 and Figure 1). The products could offer promising and innovative alternatives to existing cosmetics and drive the development of new functions for cosmetic products.
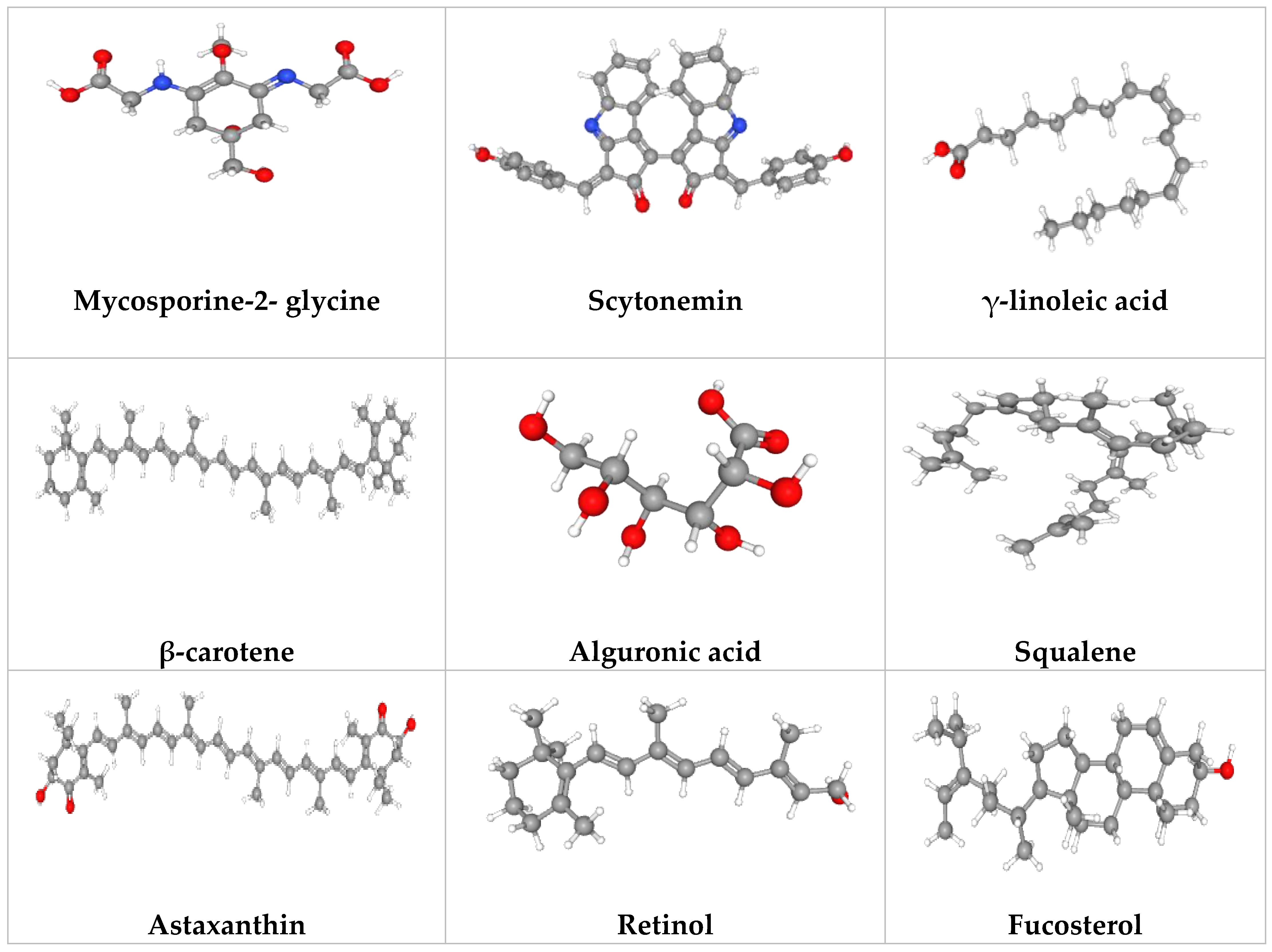
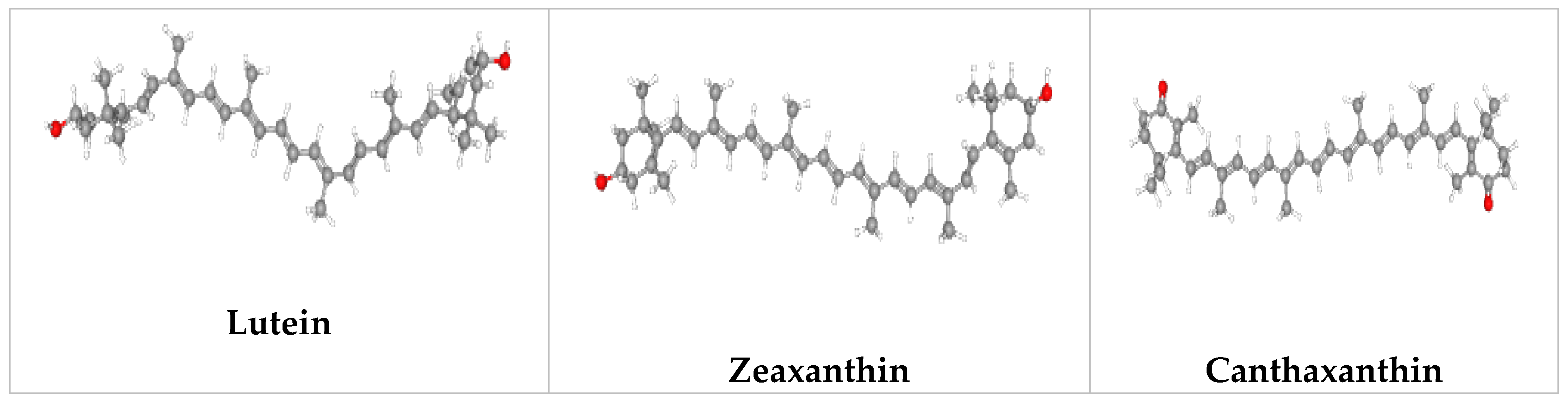
Figure 1. Potential microalgal compounds used in cosmetics such as sunscreen and anti-aging.
Cosmetic and cosmeceutical products must safeguard the natural dermal qualities and improve its appearance of health. They are typically applied when skin becomes dry due to a change in the filaggrin gene, which produces the skin’s natural moisturizing component. Additionally, by protecting against both external and internal influences, they could be used to lessen the signs and symptoms of aging [6]. In addition to offering these advantages, they should have anti-bacterial and anti-fungal activities against organisms such as Mucor ramaniannus and Candida albicans to protect the equilibrium of the skin flora. Moreover, extracellular matrix stability, acne management, cell regeneration, skin whitening, inflammation control, stimulation of angiogenesis, and oxidative stress management are all areas where microalgal antimicrobial peptides play distinctive roles. Cosmetics may contain chemicals that have unintended adverse effects, such as triggering hypersensitivity reactions, anaphylactic shock, or fatal poisoning [7]. To select compounds as safe cosmetics, some crucial studies such as genetic toxicity, phototoxicity, photo genotoxicity, toxicokinetics, and carcinogenicity should be conducted. Due to the growing popularity of cosmetics, there is a greater demand for natural and sustainable resources for the manufacture of cosmetics [5]. Cosmetics made from microalgae can replace current goods and are safe for the environment; the FDA has authorized Arthrospira extract as a “safe” food ingredient.
Table 1. Potential application of microalgae as natural cosmetics.
| Species | Ingredient | Activity/Uses | References |
|---|---|---|---|
| Anabaena virabilis, Nostoc punctiforme, Gloeocapsa sp. |
Mycosporine-like amino acids (MAAs) | Photoprotection | [8][9] |
| Arthrospira platensis, Dunaliella salina, Chlorella vulgaris, and Nannochloropsis oculata |
Sporopollenin, scytonemin, mycosporine-like amino acids (MAAs) |
Photoprotection | [10] |
| Odontella aurita | Polyunsaturated fatty acids (PUFAs) |
Prevents oxidative stress on skin | [11] |
| Porphyra sp., Porphyridium sp. |
Phycoerythrin | Cosmetics (face powder, eye shadow) | [12] |
| Arthrospira sp. | Phycocyanin | Cosmetics (eye shadow) | [13] |
| Dunaliella, Nannochloropsis |
Carotenoids | Wrinkle reduction, collagen formation and tissue regeneration | [14] |
| Arthrospira sp. | Phycobiliprotein | Improves moisture balance of the skin, skin complexion, strengthens skin’s immunity and reduces wrinkle |
[15] |
| Dunaliella salina | β-carotene | Stimulates cell proliferation |
[14] |
| Arthrospiraplatensis | γ-linoleic acid | Prevents early skin aging and wrinkle formation |
[15] |
| Asterocapsa nidulans | Alguronic acid | Strengthens skin immunity | [16] |
| Porphyridium sp. |
Sulfated polysaccharide | Strengthens skin immunity |
[16] |
| Botryococcus braunii | Squalene | Improves skin elasticity and moisture retention, prevents age spots and hyperpigmentation |
[17] |
| Haematococcus lacustris |
Astaxanthin | Photoprotection | [18][19] |
| Nostoc commune, Anabaena variabilis, Aphanothece halophytica |
Mycosporine-2- glycine | Inhibition of advanced glycation end products | [16] |
| Olisthodiscus luteus, Microchloropsis salina | Fucosterol | Decrease matrix metalloproteinases expression and increase collagen production | [10] |
| Dunaliella bardawil | β-carotene | Improves bioavailability and antioxidant properties on skin |
[20] |
Researchers have discovered that compounds derived from microalgae can be utilized as the primary active ingredient in cosmetics, but some of these compounds also have characteristics that allow for them to be employed as excipients, such as stabilizers, dyes, or thickening agents [5][21]. Typically, personal care items, including face lotion, cream, shampoo, body soap, and colorants for cosmetics, are formulated using their extracts or bioactive compounds [6][22][23]. Their sterols can also be utilized in moisturizing lotions [24]. Moreover, their pigments, like the keto carotenoid astaxanthin and β-carotene, are utilized in skin care, anti-aging, and anti-irritant treatments [24]. In addition, β-carotene with non-modified β-ionone rings such as β-carotenes are the precursor molecules for vitamin A [23]. Fucoxanthin protects against UV-B-induced skin damage by reducing intracellular ROS, like astaxanthin, an orange-colored pigment with antioxidant and provitamin A properties. Fucoxanthin has anti-pigmentary action in UV-B-induced melanogenesis in addition to its sunscreen properties [24][25]. Fucoxanthin could be an active component of cosmeceuticals and nutraceuticals utilized in the defense of the skin from photoaging [24][26][27]. Microalgae are also a source of phenolic compounds which are valuable antioxidants and photoprotective compounds [28][29]. The green microalga Lobosphaera incisa accumulates triacylglycerols (TAGs) with exceptionally high levels of long-chain polyunsaturated fatty acids (LC-PUFA) and arachidonic acids (ARA) under nitrogen (N)-deprived conditions [30]. The cosmetic industry uses extracts from microalgae which are high in pigments, PUFAs, phycobiliproteins and carbohydrates which can be used to make lotions, creams and ointments. Mycosporine-like amino acids (MAAs) are small <400 Da, water-soluble, colorless UV-absorbing compounds synthesized by marine microbes as an adaptation for their high sunlight environments. They have a unique absorption spectrum between 309 and 362 nm. Structurally, MAAs are divided into two groups: (i) the mycosporines, which have a single modified amino acid residue connected to a cyclohexenone core, and (ii) MAAs, which have two such amino acid substituents [31][32]. MAAs have good antioxidant properties. MAAs produced from Dunaliella, Arthrospira, and Chlorella have the potential to act as sunscreens to reduce the damage induced by ultraviolet rays. Microalgae Odontella aurita also showed potential free radical scavenging activity to maintain youthful skin.
Adequate research is currently lacking in order to apply the findings of in vitro experiments on model organisms and the application of efficient compounds to human skin. Additionally, patients and researchers must understand that compliance is crucial when using natural cosmetics since they work more slowly than traditional cosmetics made of synthetic substances. Important research on genetic toxicity, photo genotoxicity, phototoxicity, toxicokinetics, and carcinogenicity should be carried out in order to choose substances as safe cosmetics. To specify compounds as a harmless cosmetic, certain crucial studies such genetic toxicity, photogenotoxicity, phototoxicity, toxicokinetics, and carcinogenicity should be performed [33]. Using genetic toxicity studies, such as the Ames Salmonella test, one may determine the carcinogenic potential of bio compounds produced from microalgae [34]. Moreover, the 3T3 neutral red uptake (3T3 NRU) experiment, the 3-dimensional (3D) epidermis model, and the erythrocyte photohemolysis test can be used to determine the phototoxicity of microalgal bioactive compounds. Nevertheless, as natural products have long been used to promote health and wellbeing, they may have promised nutritional and therapeutic benefits with minimal-to-no negative effects.
2. Photoprotection Prospects of Microalgal Products
Organic carbon molecules and oxygen, which are necessary for life on Earth, are produced by cyanobacteria, algae, and plants, which also turn solar energy into chemical energy. UV rays hit the Earth’s surface more intensely as a result of ozone layer depletion and the rising emission of atmospheric contaminants. The ozone layer absorbs UV-C radiation, which ranges in wavelength from 100 to 280 nm. The ozone layer blocks most of the ultraviolet-B (UV-B) radiation (280–315 nm) that reaches the Earth’s surface, but some do get through. As the ozone layer thins, UV-B radiation intensity rises. However, the ozone layer has little effect on ultraviolet-A (UV-A) light, which ranges in wavelength from 315 to 400 nm (320 to 400 nm). The primary kind of solar radiation that enters our atmosphere is UV-B, which harms living things exposed to the sun by causing DNA strand breaks, membrane rupture, enzyme deactivation, the production of cytotoxic DNA lesions, and other extremely hazardous effects. Because native DNA molecules cannot absorb UV-A radiation, ROS are created, which cause indirect DNA damage [35]. As a result of these consequences, UV-A and UV-B have mutagenic and carcinogenic effects on humans, speed up the skin’s aging process, and cause photodermatitis. In order to defend themselves from UV radiation, microalgae have developed a number of defenses, including (i) the expression of DNA-repair enzymes; (ii) the creation of antioxidant enzymes; (iii) the avoidance of the UV; and (iv) the production and accumulation of UV filter metabolites [36]. These mechanisms, along with the manufacture of UV filter metabolites, commonly known as “microbial sunscreens”, make microalgae potential candidates for the cosmetic sector to be employed in sunscreens produced from natural sources [35][37].
To defend themselves from sun radiation, microalgae produce a variety of UV filter substances, including sporopollenin, scytonemin, mycosporine-like amino acids, β-carotene, and other substances such as biopterin glucoside, lycopene, and ectoine for UV protection and photoaging [10]. These bioactive substances shield the body from skin cancer, sunburn, and other diseases by inhibiting the manufacture of melanin, among other things.
The skin is shielded from UV damage by lutein, which is generated by Chlorella protothecoides, Scenedesmus almeriensis, Muriellopsis sp., Neospongiococcus gelatinosum, Chlorococcum citriforme, Chlorella zofingiensis, D. salina, and Galdieria sulphuraria [5][21][38]. The chemicals in microalgal extracts or extracts from microalgae help shield the skin from UV damage [5]. The most significant and extensively researched compounds that are utilized in sunscreens made by microalgae are scytonemin and mycosporine-like amino acids. Cyanobacteria produce scytonemin—a lipophilic, extracellular yellow-brown pigment—in their sheath when exposed to intense sun radiation in order to shield themselves from UV-A radiation with an absorption of up to 90% [36][38][39]. Scytonemin has a maximal absorption range of 252–386 nm [36][40][41]. Coelastrin A and Coelastrin B, two novel MAAs from Coelastrella rubescens, exhibit photoprotective properties [42]. Klebsormidin A and klebsormidin B, the newly identified MAAs from Klebsormidium, showed that their biosynthesis and intracellular enrichment is strongly induced by UVR exposure, supporting the function of these compounds as natural UV-sunscreens [43].
Scytonemin is produced when the gene responsible for its production is activated by UV-A, and it then builds up in the body. Scytonemin and derivatives of scytonemin can be produced by a number of cyanobacterial species such as Anabaena, Calothrix, Chlorogloeopsis, Diplocolon, Gloeocapsa, Hapalosiphon, Lyngbya, Nostoc, Phormidium, Pleurocapsa, Rivularia, Schizothrix, Scytonema, and Tolypothrix [35].
The hydrophilic and colorless mycosporine-like amino acids (MAAs) are produced by marine organisms such as cyanobacteria [44][45], microalgae, macroalgae, fungi, etc., that act as an antioxidant by preventing ROS-induced DNA damage as well as a photoprotectant by shielding cells from UV-B and UV-A radiation by absorbing radiation and dissipating excess heat energy into the cell and surroundings [46][47][48]. Only a small percentage of the physical and chemical filters on the market, referred to as “broad-spectrum sunscreen”, can effectively block both UV-A and UV-B rays [49]. Therefore, it is crucial to include MAAs as a UV filter agent in sunscreens since they have a high capacity to absorb UV between 309 and 362 nm, making them a broad-spectrum sunscreen [37]. They can be a very stable cosmetic product because they are also very photostable and very resistant to heat, pH fluctuations, and different solvents [50]. The first sunscreen product, named Helioguard 365, was created by the Swiss company Mibelle AG Biotechnology using a natural UV screening substance called an MAA containing a certain proportion of Porphyra-334 and shinorine obtained from red algae Porphyra umbilicalis [51][52].
3. Microalgal Compounds as Anti-Aging Therapies
The formation of AGE (advanced glycation end products), the impact of ROS, and matrix metalloproteinases (MMPs) are the most important processes among theories about the aging process. Pharmaceutical companies have recently become interested in substances that are preventing AGE. Recently described as an inhibitor of AGE formation, mycosporine-2-glycine (M2G), a very uncommon mycosporine-like amino acid, has been proposed as a key component in anti-aging therapies [53]. Nostoc commune, Anabaena variabilis, and Aphanothece halophytica have all been found to be capable of producing mycosporine-2-glycine. The key strategy to delay the aging of the skin is moisturization. It can support skin elasticity and beauty maintenance and increase environmental harm prevention. Hydroxy acid (HA) benefits the skin and has been utilized in cosmetic goods to moisturize skin. Plants are capable of producing hydroxy acids, but because plant output is restricted, interest in algal polysaccharides is growing. According to studies, Pediastrum duplex extract has a significant number of polysaccharides and can be used to preserve and moisturize skin [5]. Salicylic acid, α-HA, and β-HA are different types of HA. Due to the hydroxyl group connected to the carbon atom adjacent to the carboxyl group, α-HA is also known as 2-hydroxy acid. Lactic and glycolic acids are cosmetics’ two most widely used 2-hydroxy acids. As a result of the hydroxyl group linked to the carbon atom that comes in second place when counting, starting from the carboxyl group, β-HA is also known as a 3-hydroxy acid. Citric acid is the most well-known 3-hydroxy acid utilized in the cosmetic formulation [54]. A. variabilis, Anacystis nidulans, Chlorella pyrenoidosa, Chlamydomonas reinhardtii, Cyanidium caldarium, Phormidium foveolarum, and Oscillatoria species have also been shown to create 2-hydroxy acids and 3-hydroxy acids, and their extracts can act as antioxidants [55][56].
4. Microalgal Product’s Potential as Skin Whitening Agents
The pigment melanin is what gives hair, skin, and eyes their pigmentation, and it is created to protect the skin from UV damage. Nevertheless, melanin overproduction gives the skin a distinct color [5][57]. Tyrosinase is a crucial enzyme that starts the manufacture of melanin, and tyrosinase inhibitors can stop pigmentation in the skin [58]. Tyrosinase inhibitors contain phenolic structures or metal chelating groups, which result in two mechanisms: inhibiting interactions between the substrate and the enzyme and chelating copper within the active site of the enzyme. Finding novel, naturally derived alternatives to these synthetic tyrosinase inhibitors is important due to their high toxicity, low stability, insufficient action, and poor skin penetration, and microalgae can serve as a promising possibility [59]. S. plantensis extracts can be employed as tyrosinase inhibitors [59]. According to Oh et al. (2015), Pavlova lutheri inhibits melanogenesis [60]. Tyrosinase inhibitory activity was also demonstrated by Oscillapeptin G from Oscillatoria agardhii, asthaxanthin from Haematococcus pluvialis, and zeaxanthin from N. oculata [61]. In addition to inhibiting the tyrosinase enzyme, vitamins C and E can inhibit the skin’s synthesis of melanosomes. A well-known NADH (Nicotinamide Adenine Dinucleotide Hydrate)-based process that protects mammalian skin from UV radiation damage is the self-acting, synergistic combination of vitamins E and C. Pediastrum cruentum’s high concentration of vitamins E and C make it a potential candidate as a cosmetic to prevent melanoma [62].
References
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232.
- Kumar, M.; Enamala, S.; Chavali, M.; Donepudi, J. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68.
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172.
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications-A review. Energy Procedia 2019, 160, 752–760.
- Wang, H.D.; Chen, C.; Huynh, P.; Chang, J. Exploring the Potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362.
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487.
- Alencar-Silva, T.; Braga, M.C.; Santana, G.O.S.; Saldanha-Araujo, F.; Pogue, R.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018, 36, 2019–2031.
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656.
- Garciapichel, F.; Wingard, C.E.; Castenholz, R.W. evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176.
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100.
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648.
- Arad, S.M.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Tech. 1992, 3, 92–97.
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439.
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106.
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. potential applications of blue green algae. J. Sci. Ind. Res. 2012, 71, 13–20.
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304.
- Achitouv, E.; Metzger, P.; Rager, M.N.; Largeau, C. C-31-C-34 methylated squalenes from a bolivian strain of Botryococcus braunii. Phytochemistry 2004, 65, 3159–3165.
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216.
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47.
- Walker, T.L.; Purton, S.; Becker, D.K.; Collet, C. Microalgae as bioreactors. Plant Cell Rep. 2005, 24, 629–664.
- Levasseur, W.; Perr’e, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545.
- Rizwan, M.; Mujtaba, G.; Ahmed, S.; Lee, K.; Rashid, N. Exploring the Potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404.
- Tiwari, O.N.; Gayen, K.; Kumar, T. Food and bioproducts processing downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84.
- Balić, A.; Mokos, M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants 2019, 8, 259.
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108.
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Mar. Drugs 2022, 20, 222.
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, 183.
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342.
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262.
- Kokabi, K.; Gorelova, O.; Zorin, B.; Didi-Cohen, S.; Itkin, M.; Malitsky, S.; Solovchenko, A.; Boussiba, S.; Khozin-Goldberg, I. Lipidome Remodeling and Autophagic Respose in the Arachidonic-Acid-Rich Microalga Lobosphaera incisa Under Nitrogen and Phosphorous Deprivation. Front. Plant Sci. 2020, 11, 614846.
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63.
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683.
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25.
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and methods for in vitro toxicity. In In Vitro Toxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563.
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332.
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326.
- Milito, A.; Castellano, I.; Damiani, E. From sea to skin: Is there a future for natural photoprotectants? Mar. Drugs 2021, 19, 379.
- Santiesteban-Romero, B.; Martínez-Ruiz, M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M. Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology. Mar. Drugs 2022, 20, 487.
- Pathak, J.; Ahmed, H.; Rajneesh; Singh, S.P.; Hader, D.P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172.
- Rajneesh; Pathak, J.; Richa; Hader, D.P.; Sinha, R.P. Impacts of ultraviolet radiation on certain physiological and biochemical processes in cyanobacteria inhabiting diverse habitats. Environ. Exp. Bot. 2019, 161, 375–387.
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601.
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal Interfilum and Klebsormidium species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499.
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112.
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalized sunscreens. Mar. Drugs 2019, 17, 638.
- Fernanda, P.M. Algae and aquatic macrophytes responses to cope to ultraviolet radiation—A Review. Emir. J. Food Agric. 2012, 24, 527–545.
- Singh, A.; Tyagi, M.B.; Kumar, A. Cyanobacteria growing on tree barks possess high amount of sunscreen compound mycosporine-like amino acids (MAAs). Plant Physiol. Biochem. 2017, 119, 110–120.
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2- glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)- stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39.
- Kumari, N.; Pandey, A.; Gupta, A.; Mishra, S.; Sinha, R.P. Characterization of UV-screening pigment scytonemin from cyanobacteria inhabiting diverse habitats of Varanasi, India. Biologia 2023, 78, 319–330.
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Phcog. Rev. 2011, 5, 138–146.
- Siezen, R.J. Microbial sunscreens. Microb. Biotechnol. 2011, 4, 1–7.
- Schmid, B.D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Cosmetics 2006, 9, 1–4.
- Tarasuntisuk, S.; Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett. Appl. Microbiol. 2018, 67, 314–320.
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Effects of cosmetic formulations containing hydroxyacids on sun-exposed skin: Current applications and future developments. Dermatol. Res. Pract. 2012, 2012, 710893.
- Matsumoto, G.I.; Nagashima, H. Occurrence of 3-hydroxy acids in microalgae and cyanobacteria and their geochemical significance. Geochem. Cosmochim. Acta 1984, 48, 1683–1687.
- Matsumoto, G.I.; Shioya, M.; Nagashima, H. Occurrence of 2-hydroxy acids in microalgae. Phytochemistry 1984, 23, 1421–1423.
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425.
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26.
- Sahin, S.C. The Potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. South Afr. J. Bot. 2018, 119, 236–243.
- Oh, G.; Ko, S.; Heo, S.; Nguyen, V.; Jung, W. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process Biochem. 2015, 50, 1318–1326.
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851.
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.8K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





