Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raquel Abreu | -- | 1670 | 2023-04-21 11:51:25 | | | |
| 2 | Jessie Wu | Meta information modification | 1670 | 2023-04-23 03:51:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Important Pathogens in Poultry Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/43318 (accessed on 07 February 2026).
Abreu R, Semedo-Lemsaddek T, Cunha E, Tavares L, Oliveira M. Important Pathogens in Poultry Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/43318. Accessed February 07, 2026.
Abreu, Raquel, Teresa Semedo-Lemsaddek, Eva Cunha, Luís Tavares, Manuela Oliveira. "Important Pathogens in Poultry Production" Encyclopedia, https://encyclopedia.pub/entry/43318 (accessed February 07, 2026).
Abreu, R., Semedo-Lemsaddek, T., Cunha, E., Tavares, L., & Oliveira, M. (2023, April 21). Important Pathogens in Poultry Production. In Encyclopedia. https://encyclopedia.pub/entry/43318
Abreu, Raquel, et al. "Important Pathogens in Poultry Production." Encyclopedia. Web. 21 April, 2023.
Copy Citation
The presence of a wide variety of microorganisms is surveilled in several food producing animals due to their importance to public health, namely in broilers and laying hens. The pathogens most relevant in this industry, and associated with antimicrobial drug resistance, include Salmonella enterica, Campylobacter spp. (specially C. jejuni), Escherichia coli, Enterococcus spp. and methicillin-resistant Staphylococcus aureus (MRSA).
poultry production
microbiota
antibiotics
food safety
1. Salmonella enterica
Salmonella enterica causes foodborne enteric disease worldwide, representing the second most commonly reported zoonotic pathogen in the EU [1][2]. It is responsible for disease outbreaks associated with significant morbidity and mortality [3], and up to 25% of human Salmonella outbreaks, illnesses and hospitalizations are related to poultry sources [2][4].
Salmonella are gram-negative, facultative anaerobic bacteria belonging to the Enterobacteriaceae family, and are considered commensals of the gut microbiota of mammals, birds, reptiles, amphibians, fish and shellfish [5] (Figure 1).
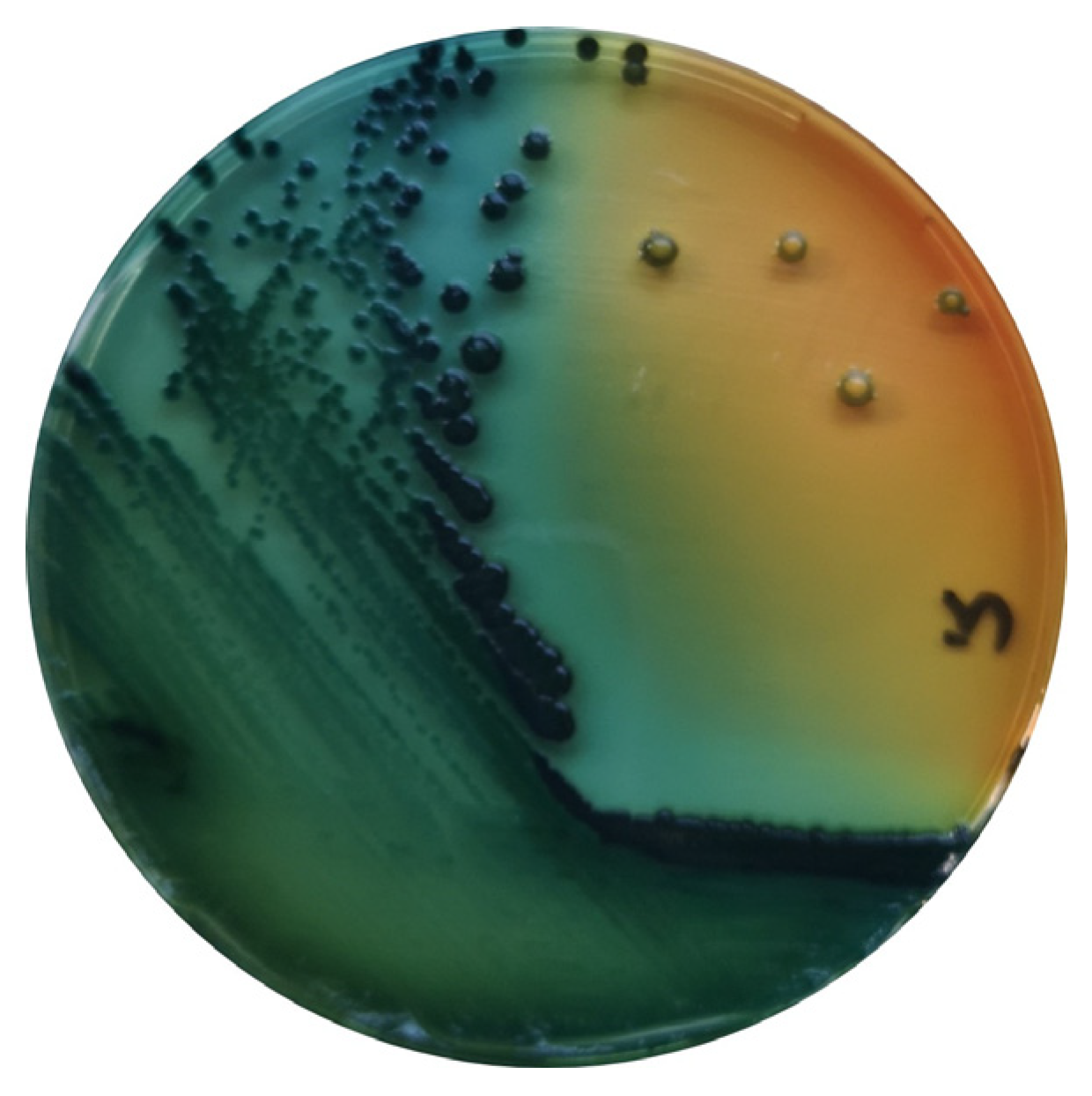
Figure 1. S. enterica in Hektoen agar (Oxoid, Hampshire, UK).
S. enterica includes more than 2650 serovars [6], in which several have been previously described as contaminants of poultry meat and eggs, representing a serious concern for public health [7].
In poultry, diseases promoted by S. enterica are divided into three conditions: fowl typhoid (promoted by S. enterica subsp. enterica serovar Gallinarum biovar Gallinarum), pullorum disease (by S. enterica subsp. enterica serovar Gallinarum biovar Pullorum) and avian paratyphoid, which results from infection caused by other S. enterica serovars, including S. Enteriditis, S. Typhimurium and S. Infantis [8]. These bacteria can be transmitted via both vertical (from infected breeders) and horizontal (from other birds in a flock or from the environment) routes [3]. Young poultry are particularly susceptible to gastrointestinal tract (GIT) colonization by S. enterica. Its excretion in feces may result in the contamination of the environment and the infection of nearby birds [2][5]. Moreover, poultry meat contaminated with digesta during slaughter is a major risk to public health [2][9].
S. enterica can be transmitted from animals to humans through the consumption of contaminated animal derived products, such as meat, and of other foodstuffs contaminated with fecal matter, or through direct or indirect contact with colonized animals or contaminated water. This agent is considered moderately resistant to certain environmental conditions, such as freezing, acidic pH and dehydration, which contribute to its high transmissibility. When infecting humans, Salmonella attaches and colonizes the intestinal columnar epithelial cells, resulting in fever, nausea, diarrhea, vomiting and abdominal pain. The disease is usually self-limiting in healthy adults, but it can lead to septicemia and death in severe cases, especially in children, the elderly and immunocompromised patients [3][5]. In these cases, antimicrobial treatment is advised, and fluoroquinolones, macrolides and third-generation cephalosporins can be used to treat S. enterica infection [5].
Several studies comprising the evaluation of the antimicrobial drug resistance profiles of more than 4000 isolates of Salmonella spp. were revised by Saraiva et al., 2022 [8]. Higher frequencies of resistance were observed towards nalidixic acid, amoxicillin, ampicillin, erythromycin, penicillin G, sulfamethoxazole and tetracycline, while higher susceptibilities were associated with the aminoglycosides spectinomycin and gentamicin.
In the case of paratyphoid Salmonella, multidrug resistance is a concern because it can lead to treatment failure. The most common resistance patterns associated with this pathogen include important therapeutic antimicrobial classes used in human medicine, such as penicillins, tetracyclines, cephalosporins and fluoroquinolones, and this association represents a public health concern [8].
2. Campylobacter spp.
Campylobacter spp. are ubiquitous bacteria that can be found in various environments, including soil and water, and as commensals of the GIT of poultry. Despite this, they can cause disease in animals and humans and constitute an important cause of foodborne diseases worldwide [10][11][12]. This bacterial genus can be responsible for acute bacterial diarrhea, which is mainly caused by C. jejuni and C. coli. Although other sources can be responsible for human infection, poultry products are considered the predominant source of human campylobacteriosis [3] (Figure 2).
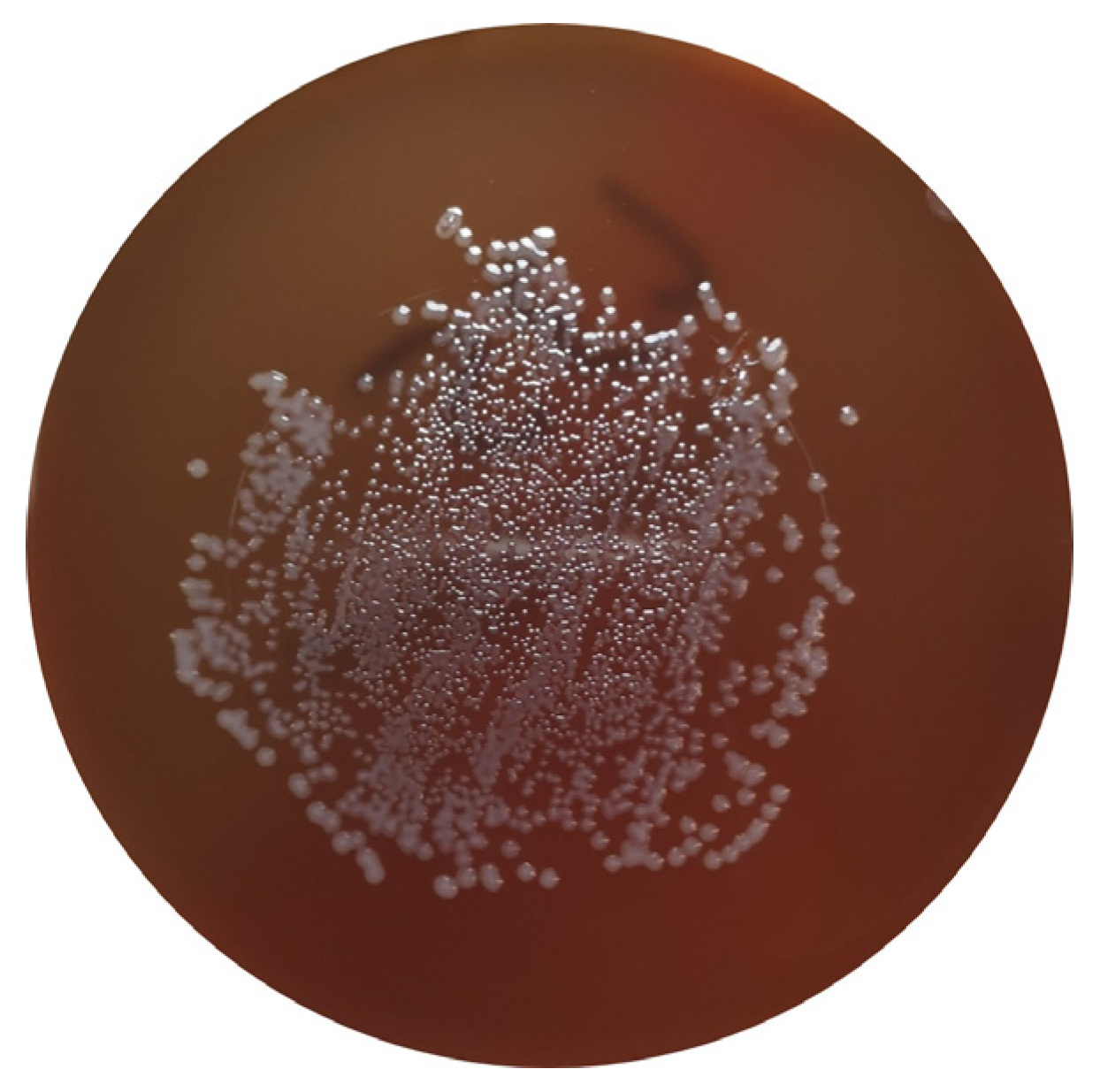
Figure 2. C. jejuni in Columbia Agar + 5% sheep blood (bioMérieux, Marcy-l’Etoile, France).
Campylobacter spp. can be introduced in the production farms by wild animals, pests or humans. When infecting poultry, it colonizes the animal’s intestine, invades the intestinal epithelium and multiplies rapidly in the intestinal mucus, avoiding clearance and persisting in the animal’s GIT [12][13]. In this way, avian hosts constitute a natural reservoir for Campylobacter spp., namely C. jejuni and C. coli [12]. According to the European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC), the highest prevalence of Campylobacter is observed in fresh meat from broilers (37.5%) [1]. Although carriers of Campylobacter spp., chickens generally do not exhibit clinical signs [12]. Antibiotics have a limited role in the elimination of Campylobacter spp. by these animals, due to its high occurrence and commensal character in avian species, and can promote the emergence of resistant strains; therefore, biosecurity practices are the most important method for reducing Campylobacter infection at the production level [14].
Human infections are usually associated with the handling, preparation and consumption of contaminated poultry products, and occupational transmission has also been observed [10]. In humans, these pathogens cause gastroenteritis associated with diarrhea, abdominal pain, fever, nausea and vomiting, which usually occur between two and five days after infection. Symptoms are often mild and self-limiting. Antibiotic treatment is not usually required, but severe cases may be treated with macrolides, such as clarithromycin, azithromycin and erythromycin. Ciprofloxacin is not currently used, as resistance to quinolones is now considered to be too high for these antibiotics to be used as an empirical treatment [3][10][12][15]. Studies on the antimicrobial drug resistance profile of Campylobacter spp. isolated from broilers, laying hens, chicken carcasses and chicken meat revealed high frequencies of resistance to nalidixic acid, ampicillin, cephalexin, ciprofloxacin, erythromycin, gentamicin and tetracycline [8].
3. Escherichia coli
E. coli is a gram-negative bacillus belonging to the Enterobacteriaceae family [16]. It is an important bacterial species in the human–animal–environment triad, since it is a commensal inhabitant of the digestive tract of animals, including birds, being widely disseminated via fecal material [17]. This species is often studied as a marker of antimicrobial drug resistance, mainly due to its widespread distribution and capacity to harbor several genes in mobile genetic elements, serving as a source of antimicrobial drug resistance determinants to other bacteria [18].
Most E. coli are nonpathogenic; however, certain pathogenic serotypes may induce disease. There are several E. coli pathotypes, which can be divided into extraintestinal E. coli (ExPEC) and diarrhoeagenic E. coli (DEC). Avian pathogenic E. coli (APEC), an ExPEC, may induce colibacillosis in domestic birds, a disease characterized as a local or systemic syndrome that can be transmitted by oral or vertical routes or through inhalation. E. coli-associated infections are widely distributed among poultry of all ages. Birds can be asymptomatic until sudden death or present various forms of disease, such as septicemia, coligranuloma (Hjarre’s disease), air sac disease (chronic respiratory disease), swollen-head syndrome, venereal colibacillosis, cellulitis, peritonitis, salpingitis, orchitis, osteomyelitis/synovitis, panophthalmitis, omphalitis/yolk sac infection and enteritis [19]. Colibacillosis constitutes the most frequent infectious bacterial disease found in poultry, being responsible for significant economic losses due to the loss of productivity, increased mortality and condemnations of carcasses [19][20].
Other ExPEC pathotypes, such as uropathogenic (UPEC), neonatal meningitis (NMEC) and sepsis-associated E. coli (SEPEC), have already been identified in poultry and can promote disease in humans (Figure 3) [21]. E. coli isolated from poultry may be resistant to aminoglycosides, β-lactam groups (penicillins and cephalosporins) and fluoroquinolones [8].
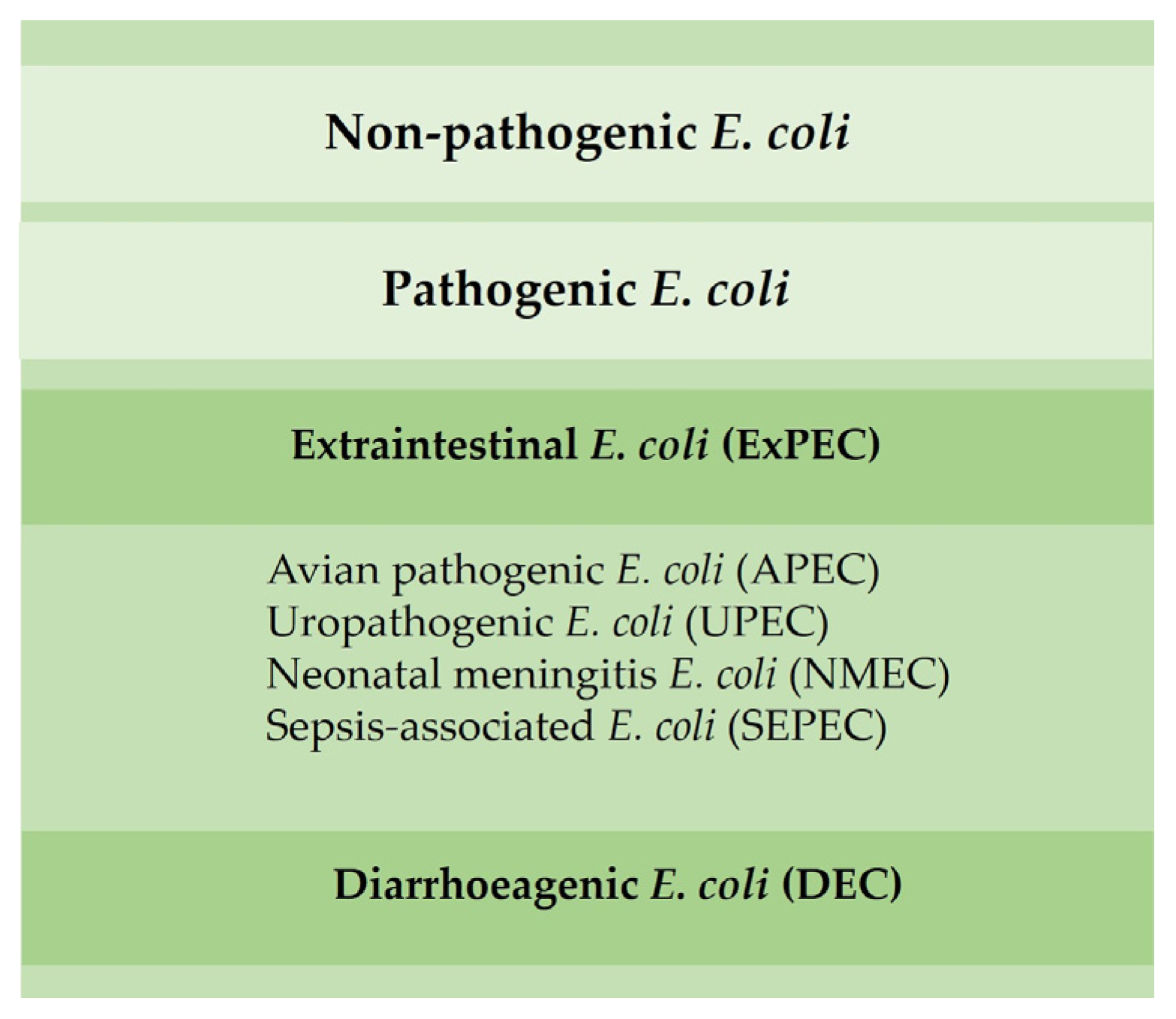
Figure 3. Main E. coli pathotypes found in poultry.
4. Enterococcus spp.
Enterococcus species are ubiquitous and are commensals of the gastrointestinal microbiota of both humans and animals [22]. Some enterococcal strains have been used as probiotics [23], while others are known to be pathogenic, including in birds [24].
The transmission of enterococci can occur via vertical and horizontal routes. E. cecorum and E. faecalis are the most important species associated with avian disease. Pathogenic strains of E. cecorum have been associated with free thoracic vertebra (FTV) osteomyelitis in broilers [25], resulting in the paralysis of the posterior limbs, and with septicemia related to pericarditis or hepatitis, which can lead to death. In turn, E. faecalis can cause omphalitis and yolk sacculitis, which can lead to sepsis and the death of chicks in the first week of life. Surviving animals may develop chronic diseases, such as valve endocarditis, which can also lead to death [8].
Enterococcus spp. can easily acquire resistance determinants and, therefore, play a central role in AMR dissemination. Vancomycin-resistant Enterococcus (VRE) has been associated with economic losses in animal production and healthcare and associated with infections in humans [26]. Humans are exposed to enterococci from a variety of sources, including other humans, the environment and foods contaminated with animal’s intestinal microbiota. Certain species, such as E. faecalis and E. faecium, are a prominent cause of opportunistic infections in hospitalized humans, causing mild to fatal diseases, such as endocarditis, urinary tract infections or septicemia [22][24]. Studies previously performed have identified high levels of resistance against aminoglycosides (streptomycin), tetracyclines (doxycycline and tetracycline) and quinolones (ciprofloxacin and enrofloxacin) in enterococci isolated from poultry [8]. Vancomycin resistance, which is reported as infrequent, can be higher in isolates from chickens affected with FTV.
5. Methicillin-Resistant Staphylococcus aureus
S. aureus is considered the most common and pathogenic staphylococcal species isolated from poultry. Staphylococci are natural inhabitants of the skin and mucous membranes of healthy birds, being ubiquitous in the poultry environment [8][20]. The presence of S. aureus that is resistant to antimicrobials in production animals is a global health concern affecting both humans and animals [27]. Staphylococcal infections caused by S. aureus are a worldwide problem in poultry production, causing economic losses due to decreased production, increased mortality and the condemnation of carcasses. Infections caused by S. aureus include arthritis, synovitis, chondronecrosis, osteomyelitis, gangrenous dermatitis, subdermal abscesses (bumblefoot) and septicemia [8][20]. Moreover, some enterotoxin-producing strains can cause food poisoning in humans. Poultry-associated food poisoning can occur due to the contamination of carcasses with S. aureus at the processing phase, especially with enterotoxin-producing strains [28]. Regarding the presence of S. aureus in poultry, the major concern is the emergence of MRSA strains. Although they are infrequently isolated from poultry, MRSA can still be transmitted to humans through direct contact or through meat consumption [29]. Staphylococci from poultry can be resistant to amoxicillin, amoxicillin-clavulanic acid, ampicillin, cefoxitin, kanamycin, penicillin and tetracycline [8].
References
- European, T.; One, U.; Report, Z. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971.
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Torres, J.; Marin, C.; Sevilla-Navarro, S. Microencapsulated Bacteriophages Incorporated in Feed for Salmonella Control in Broilers. Vet. Microbiol. 2022, 274, 109579.
- Ricke, S.C. Strategies to Improve Poultry Food Safety, a Landscape Review. Annu. Rev. Anim. Biosci. 2021, 9, 379–400.
- Chai, S.J.; Cole, D.; Nisler, A.; Mahon, B.E. Poultry: The Most Common Food in Outbreaks with Known Pathogens, United States, 1998–2012. Epidemiol. Infect. 2017, 145, 316–325.
- Cosby, D.E.; Cox, N.A.; Harrison, M.A.; Wilson, J.L.; Jeff Buhr, R.; Fedorka-Cray, P.J. Salmonella and Antimicrobial Resistance in Broilers: A Review. J. Appl. Poult. Res. 2015, 24, 408–426.
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; De Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.X. Supplement 2008–2010 (No. 48) to the White-Kauffmann-Le Minor Scheme. Res. Microbiol. 2014, 165, 526–530.
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 110–121.
- de Saraiva, M.M.S.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Neto, O.C.F.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial Resistance in the Globalized Food Chain: A One Health Perspective Applied to the Poultry Industry. Braz. J. Microbiol. 2022, 53, 465–486.
- Alali, W.Q.; Hofacre, C.L. Preharvest Food Safety in Broiler Chicken Production. In Preharvest Food Safety; Thakur, S., Kniel, K.E., Eds.; ASM Press: Washington, DC, USA, 2018; pp. 69–86.
- Veterinary Medicines Directorate. UK One Health Report—Joint Report on Antibiotic Use and Antibiotic Resistance, 2013–2017; Veterinary Medicines Directorate: Addlestone, UK, 2019. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/447319/One_Health_Report_July2015.pdf (accessed on 20 December 2022).
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. As a Foodborne Pathogen: A Review. Front. Microbiol. 2011, 2, 200.
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Sahin, O.; Kassem, A.I.I.; Shen, B.Z.; Lin, A.J.; Rajashekara, C.G.; et al. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015, 59, 185–200.
- Van Deun, K.; Pasmans, F.; Ducatelle, R.; Flahou, B.; Vissenberg, K.; Martel, A.; Van den Broeck, W.; Van Immerseel, F.; Haesebrouck, F. Colonization Strategy of Campylobacter jejuni Results in Persistent Infection of the Chicken Gut. Vet. Microbiol. 2008, 130, 285–297.
- Hermans, D.; Pasmans, F.; Messens, W.; Martel, A.; Van Immerseel, F.; Rasschaert, G.; Heyndrickx, M.; Van Deun, K.; Haesebrouck, F. Poultry as a Host for the Zoonotic Pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 2012, 12, 89–98.
- Public Health England. Summary of Antimicrobial Prescribing Guidance: Managing Common Infections. 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/994444/Common_Infect_PHE_context_references_and_rationale_May_2021_Bites_and_Eczema__1_.pdf (accessed on 20 January 2023).
- Daneshmand, A.; Kermanshahi, H.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M. Antimicrobial Peptide, CLF36, Affects Performance and Intestinal Morphology, Microflora, Junctional Proteins, and Immune Cells in Broilers Challenged with E. coli. Sci. Rep. 2019, 9, 14176.
- Islam, M.S.; Nayeem, M.M.H.; Sobur, M.A.; Ievy, S.; Islam, M.A.; Rahman, S.; Kafi, M.A.; Ashour, H.M.; Rahman, M.T. Virulence Determinants and Multidrug Resistance of Escherichia coli Isolated from Migratory Birds. Antibiotics 2021, 10, 190.
- Bass, L.; Liebert, C.A.; Lee, M.D.; Summers, A.O.; White, D.G.; Thayer, S.G.; Maurer, J.J. Incidence and Characterization of Integrons, Genetic Elements Mediating Multiple-Drug Resistance, in Avian Escherichia coli. Antimicrob. Agents Chemother. 1999, 43, 2925–2929.
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.; Abdul-aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 751–805.
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872.
- Mitchell, N.M.; Johnson, J.R.; Johnston, B.; Curtiss, R.; Mellata, M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl. Environ. Microbiol. 2015, 81, 1177–1187.
- Dea, M.O.; Sahibzada, S.; Jordan, D.; Laird, T.; Lee, T.; Hewson, K.; Pang, S.; Abraham, R.; Coombs, G.W.; Harris, T.; et al. Genomic, Antimicrobial Resistance, and Public Health Insights into Enterococcus spp. from Australian Chickens. J. Clin. Microbiol. 2019, 57, e00319.
- Kabir, S.M.L. The Role of Probiotics in the Poultry Industry. Int. J. Mol. Sci. 2009, 10, 3531–3546.
- Souillard, R.; Laurentie, J.; Kempf, I.; Le Caër, V.; Le Bouquin, S.; Serror, P.; Allain, V. Increasing Incidence of Enterococcus-Associated Diseases in Poultry in France over the Past 15 Years. Vet. Microbiol. 2022, 269, 109426.
- Devriese, L.A.; Cawerts, K.; Hermans, K.; Wood, A.M. Enterococcus cecorum Septicaemia as a Cause of Bone and Joint Lesions Resulting in Lameness in Broiler Chickens. Vlaams Diergeneeskd. Tijdschr. 2002, 71, 219–221.
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial Infections and Their Control Strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514.
- Lee, G.Y.; Lee, S.I.; Do Kim, S.; Park, J.H.; Kim, G.-B.; Yang, S.-J. Clonal Distribution and Antimicrobial Resistance of Methicillin-Susceptible and -Resistant Staphylococcus aureus Strains Isolated from Broiler Farms, Slaughterhouses, and Retail Chicken Meat. Poult. Sci. 2020, 101, 102070.
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Food and Food Products of Poultry Origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157.
- Verkade, E.; Kluytmans, J. Livestock-Associated Staphylococcus aureus CC398: Animal Reservoirs and Human Infections. Infect. Genet. Evol. 2014, 21, 523–530.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




