
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianzhong Wang | -- | 3330 | 2023-04-20 02:28:57 | | | |
| 2 | Jianzhong Wang | Meta information modification | 3330 | 2023-04-20 02:36:24 | | | | |
| 3 | Rita Xu | -1316 word(s) | 2014 | 2023-04-20 05:51:07 | | |
Video Upload Options
Macrolide antibiotics are important drugs to combat infections. The pharmacokinetics (PK) of these drugs are essential for the determination of their optimal dose regimens, which affect antimicrobial pharmacodynamics and treatment success. For most drugs, the measurement of their concentrations in plasma/serum is the surrogate for drug concentrations in target tissues for therapy.
1. Introduction
2. ISF Concentrations of Macrolide Antibiotics in the Lower Respiratory Tract
3. Concentrations of Macrolide Antibiotics in Plasma/Serum, Airway Fluid, and Tissues
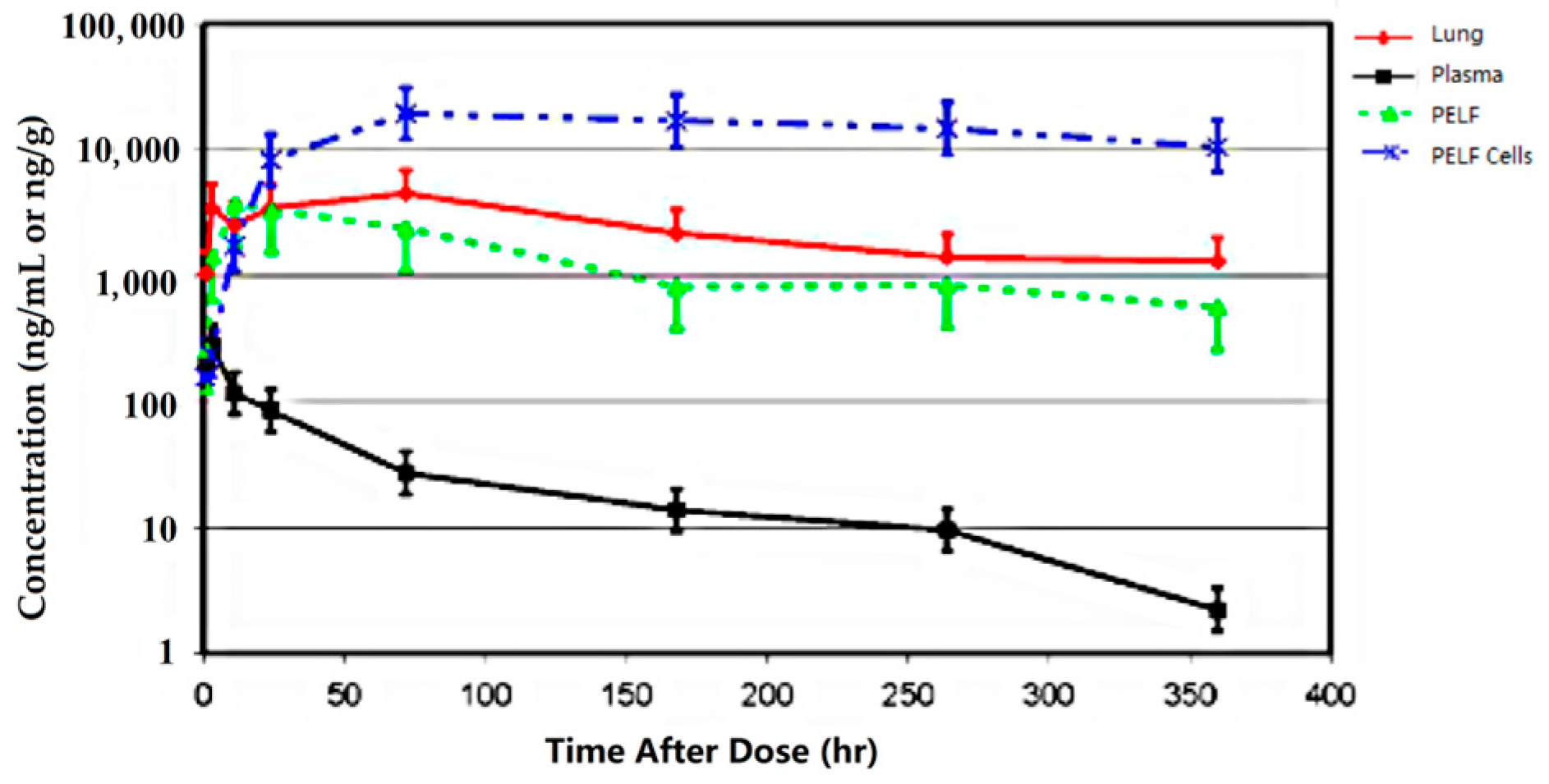
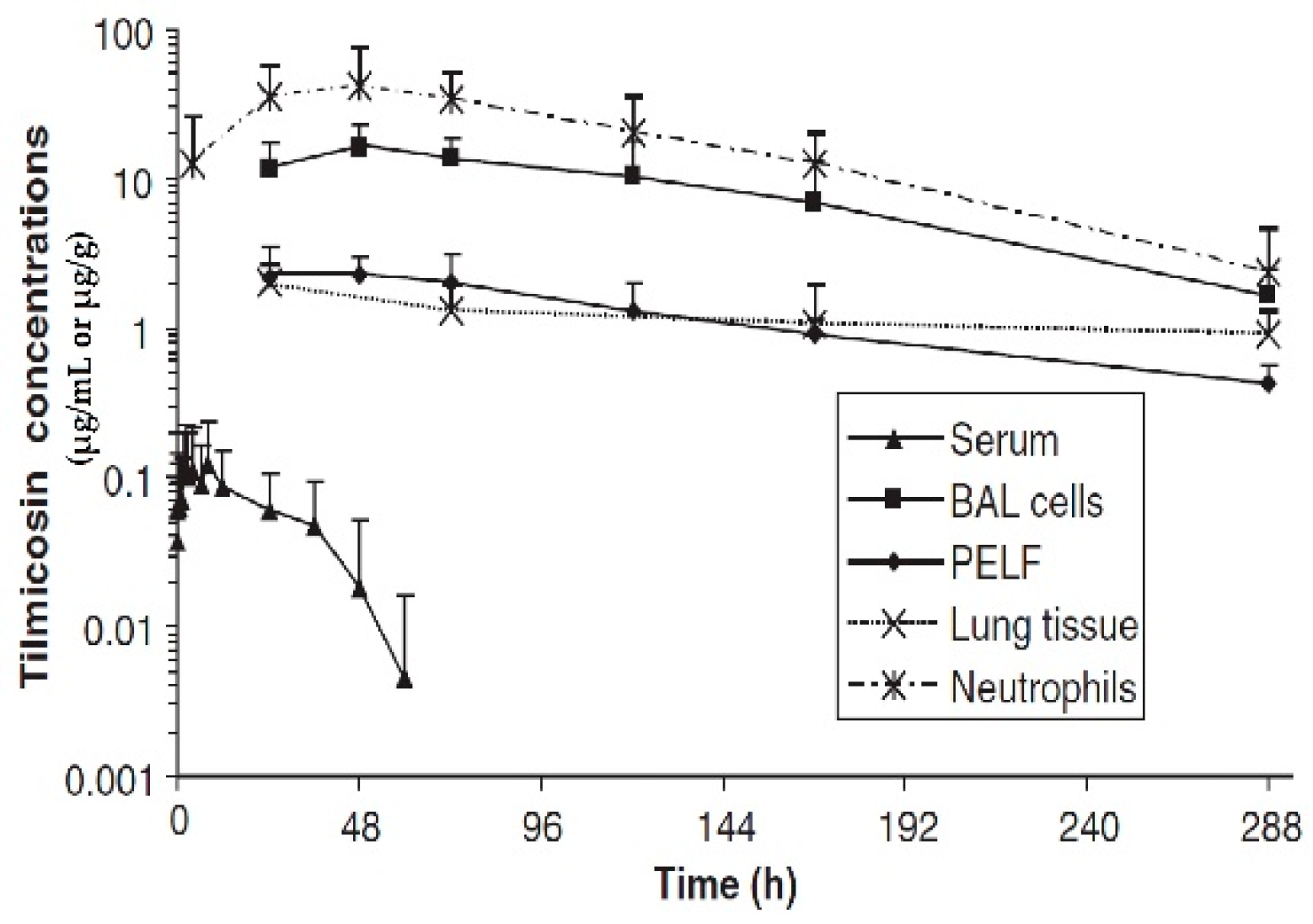
4. Conclusions
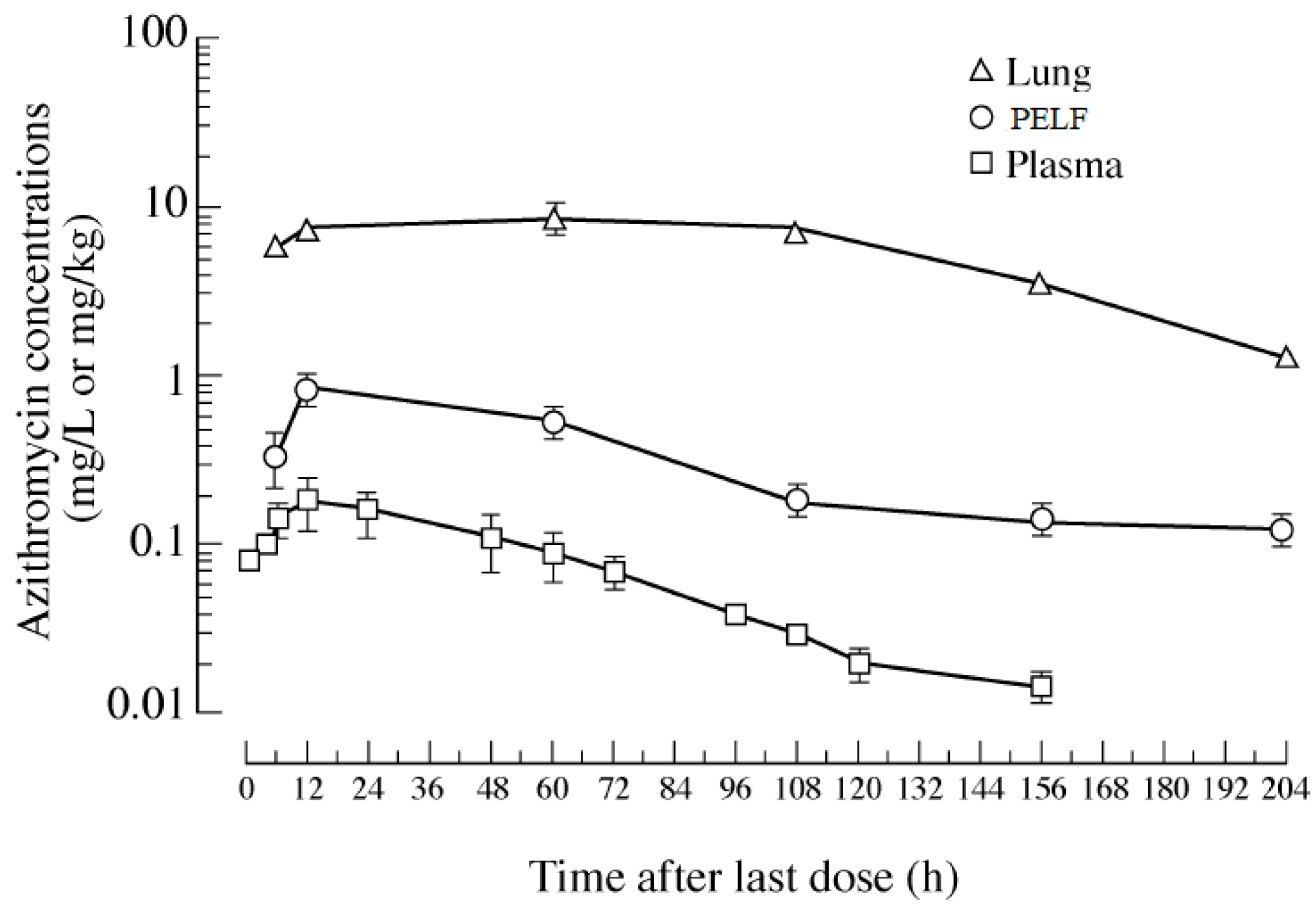
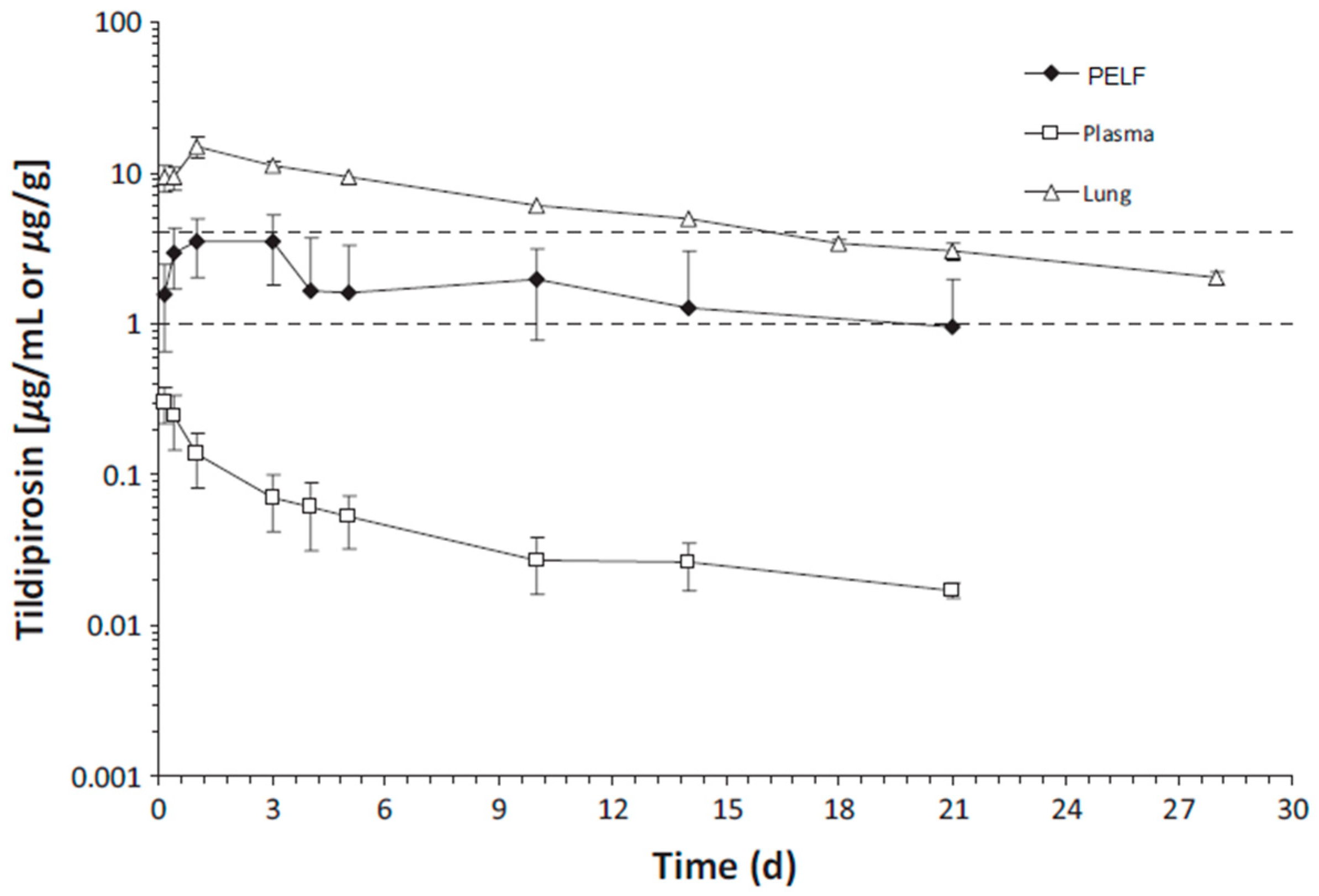
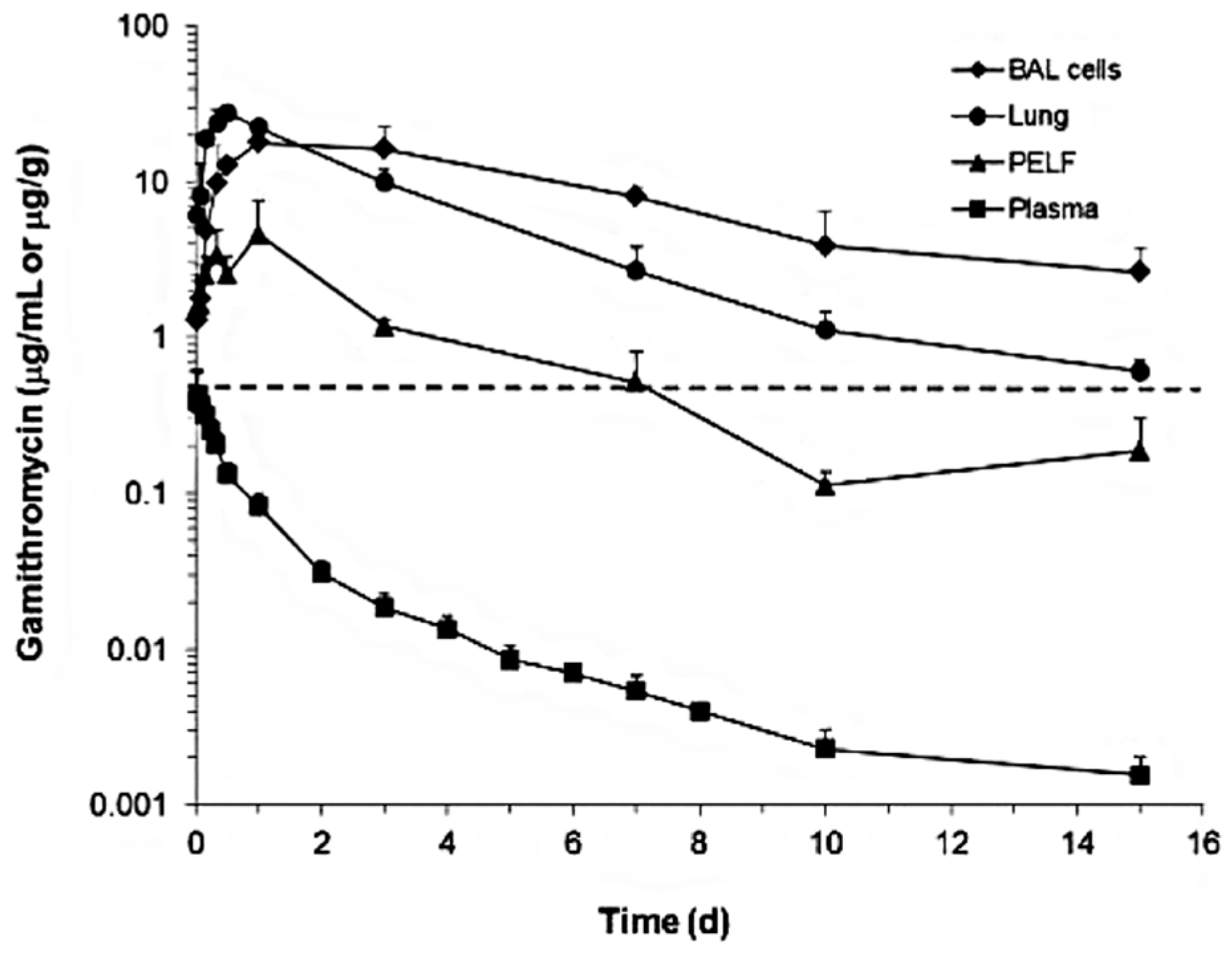
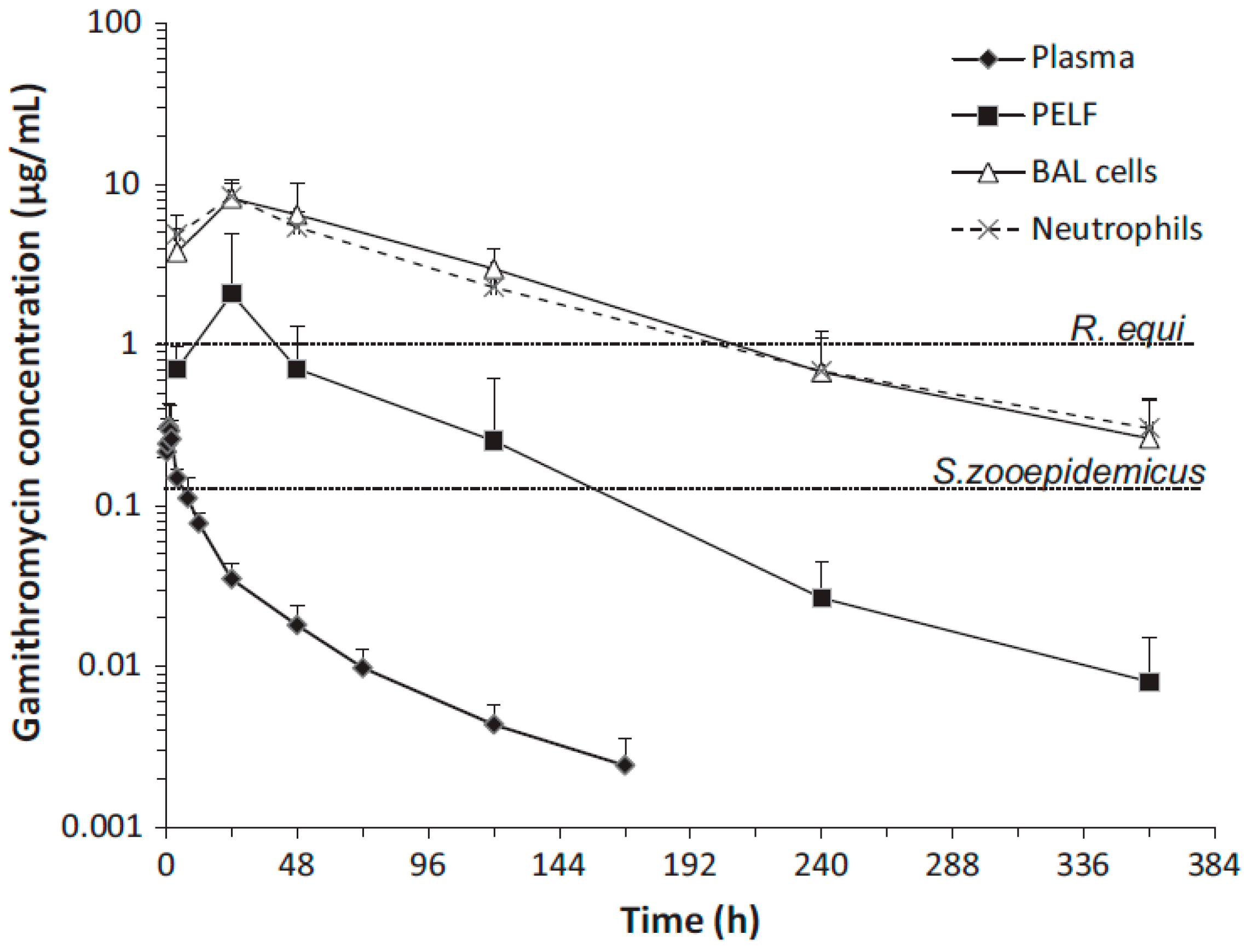
Although the change in plasma/serum concentration-time and the change in airway ISF-concentration-time of macrolide antibiotics are proportional, macrolide antibiotics in plasma/serum do not reflect the antibacterial activity of the airway ISF. Thus, if the ISF concentrations of a macrolide antibiotic is efficiently and precisely collected by the least invasive method, e.g., microdialysis, practical PK/PD parameters can be obtained for such a study. Nevertheless, the PK data of airway ISF, the site of bacterial infection, are more important in setting the optimal dose regimen of a macrolide than the PK data of plasma/serum.
References
- Spagnolo, P.; Fabbri, L.M.; Bush, A. Long-term macrolide treatment for chronic respiratory disease. Eur. Respir. J. 2013, 42, 239–251.
- Mazzei, T.; Mini, E.; Novelli, A.; Periti, P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31 (Suppl. C), 1–9.
- Bearden, D.T.; Rodvold, K.A. Penetration of macrolides into pulmonary sites of infection. Infect. Med. 1999, 16, 480A–484A.
- Drusano, G.L. Infection site concentrations: Their therapeutic importance and the macrolide and macrolide-like lass of antibiotics. Pharmacotherapy 2005, 25, 150S–158S.
- Barza, M. Anatomical barriers for antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12 (Suppl. S1), S31–S35.
- Barza, M. Pharmacokinetics of antibiotics in shallow and deep compartments. J. Antimicrob. Chemother. 1993, 31 (Suppl. D), 17–27.
- Landersdorfer, C.B.; Nation, R.L. Limitations of antibiotic MIC-based PK-PD metrics: Looking back to move forward. Front. Pharmacol. 2021, 12, 3024.
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, Tissue, and intracellular concentrations of azithromycin during and after end of therapy. Int. J. Antimicrob. Agents. 2013, 57, 1736–1742.
- Liu, P.; Müller, M.; Derendorf, H. Rational dosing of antibiotics: The use of plasma concentrations versus tissue concentrations. Int. J. Antimicrob. Agents 2002, 19, 285–290.
- Toutain, P.L.; Bousquet-Melou, A. Free drug fraction vs. free drug concentration: A matter of frequent confusion. J. Vet. Pharmacol. Ther. 2002, 25, 460–463.
- Toutain, P.L.; del Castillo, J.R.E.; Bousquet-Mélou, A. The pharmacokinetic–pharmacodynamic approach to a rational regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114.
- Gonzalez, D.; Schmidt, S.; Derendorf, H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin. Microbiol. Rev. 2013, 26, 274–288.
- Rose, M.; Menge, M.; Bohland, C.; Zschiesche, E.; Wilhelm, C.; Kilp, S.; Metz, W.; Allan, M.; Ropke, R.; Nurnberger, M. Pharmacokinetics of tildipirosin in porcine plasma, lung tissue, and bronchial fluid and effects of test conditions on in vitro activity against reference strains and field isolates of Actinobacillus pleuropneumoniae. J. Vet. Pharmacol. Ther. 2013, 36, 140–153.
- Huang, R.A.; Letendre, L.T.; Banav, N.; Fischer, J.; Somerville, B. Pharmacokinetics of gamithromycin in cattle with comparison of plasma and lung tissue concentrations and plasma antibacterial activity. J. Vet. Pharmacol. Ther. 2010, 33, 227–237.
- Giguere, S.; Tessman, R.K. Rational dosing of antimicrobial agents for bovine respiratory disease: The use of plasma versus tissue concentrations in predicting efficacy. Int. J. Appl. Res. Vet. M 2009, 9, 342–355.
- Ball, P.; Baquero, F.; Cars, O.; File, T.; Garau, J.; Klugman, K.; Low, D.E.; Rubinstein, E.; Wise, R. Antibiotic therapy of community respiratory tract infections: Strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 2002, 49, 31–40.
- Togami, K.; Chono, S.; Morimoto, K. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lining fluid and alveolar macrophages. Biopharm. Drug Dispos. 2011, 32, 389–397.
- Di, L.; Kerns, E.H. Chapter 37—Pharmacokinetic Methods. In Drug-Like Properties, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 455–461.
- Maglio, D.; Capitano, B.; Banevicius, M.A.; Geng, Q.; Nightingale, C.H.; Nicolau, D.P. Differential efficacy of clarithromycin in lung versus thigh infection models. Chemotherapy 2004, 50, 63–66.
- Bachtold, K.A.; Alcorn, J.M.; Boison, J.O.; Matus, J.L.; Woodbury, M.R. Pharmacokinetics and lung and muscle concentrations of tulathromycin following subcutaneous administration in white-tailed deer (Odocoileus virginianus). J. Vet. Pharmacol. Ther. 2016, 39, 292–298.
- Romanet, J.; Smith, G.W.; Leavens, T.L.; Baynes, R.E.; Wetzlich, S.E.; Riviere, J.E.; Tell, L.A. Pharmacokinetics and tissue elimination of tulathromycin following subcutaneous administration in meat goats. Am. J. Vet. Res. 2012, 73, 1634–1640.
- Kobuchi, S.; Kabata, T.; Maeda, K.; Ito, Y.; Sakaeda, T. Pharmacokinetics of macrolide antibiotics and transport into the interstitial fluid: Comparison among erythromycin, clarithromycin, and azithromycin. Antibiotics 2020, 9, 199.
- Okamoto, H.; Miyazaki, S.; Tateda, K.; Ishii, Y.; Yamaguchi, K. In vivo efficacy of telithromycin (HMR3647) against Streptococcus pneumoniae and Haemophilus influenzae. Antimicrob. Agents Chemother. 2001, 45, 3250–3252.
- Ambrose, P.G.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Drusano, G.L. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: Look before you leap! Clin. Infect. Dis. 2010, 51, S103–S110.
- Kanoh, S.; Rubin, B.K. Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin. Microbiol. Rev. 2010, 23, 590–615.
- Danesi, R.; Lupetti, A.; Barbara, C.; Ghelardi, E.; Chella, A.; Malizia, T.; Senesi, S.; Alberto Angeletti, C.; Del Tacca, M.; Campa, M. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 2003, 51, 939–945.
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739.
- Kong, F.Y.; Rupasinghe, T.W.; Simpson, J.A.; Vodstrcil, L.A.; Fairley, C.K.; McConville, M.J.; Hocking, J.S. Pharmacokinetics of a single 1g dose of azithromycin in rectal tissue in men. PLoS ONE 2017, 12, e0174372.
- Zuckerman, J.M. Macrolides and ketolides: Azithromycin, clarithromycin, telithromycin. Infect. Dis. Clin. N. Am. 2004, 18, 621–649.
- Muller-Serieys, C.; Soler, P.; Cantalloube, C.; Lemaitre, F.; Gia, H.P.; Brunner, F.; Andremont, A. Bronchopulmonary disposition of the ketolide telithromycin (HMR 3647). Antimicrob. Agents Chemother. 2001, 45, 3104–3108.
- Menge, M.; Rose, M.; Bohland, C.; Zschiesche, E.; Kilp, S.; Metz, W.; Allan, M.; Ropke, R.; Nurnberger, M. Pharmacokinetics of tildipirosin in bovine plasma, lung tissue, and bronchial fluid (from live, nonanesthetized cattle). J. Vet. Pharmacol. Ther. 2012, 35, 550–559.
- Torres, F.; Santamaria, R.; Jimenez, M.; Menjón, R.; Ibanez, A.; Collell, M.; Azlor, O.; Fraile, L. Pharmacokinetics of tildipirosin in pig tonsils. J. Vet. Pharmacol. Ther. 2016, 39, 199–201.
- Giguere, S.; Huang, R.; Malinski, T.J.; Dorr, P.M.; Tessman, R.K.; Somerville, B.A. Disposition of gamithromycin in plasma, pulmonary epithelial lining fluid, bronchoalveolar cells, and lung tissue in cattle. Am. J. Vet. Res. 2011, 72, 326–330.
- Berghaus, L.J.; Giguère, S.; Sturgill, T.L.; Bade, D.; Malinski, T.J.; Huang, R. Plasma pharmacokinetics, pulmonary distribution, and in vitro activity of gamithromycin in foals. J. Vet. Pharmacol. Ther. 2012, 35, 59–66.
- Cox, S.R.; McLaughlin, C.; Fielder, A.E.; Yancey, M.; Bowersock, T.; Garcia-Tapia, D.; Bryson, L.; Lucas, M.J.; Robinson, J.A.; Nanjiani, I.; et al. Rapid and prolonged distribution of tulathromycin into lung homogenate and pulmonary epithelial lining fluid of holstein calves following a single subcutaneous administration of 2.5 mg/kg body weight. Int. J. Appl. Res. Vet. Med. 2010, 8, 129–137.
- Leventhal, H.R.; McKenzie, H.C.; Estell, K.; Council-Troche, M.; Davis, J.L. Pharmacokinetics and pulmonary distribution of Draxxin® (tulathromycin) in healthy adult horses. J. Vet. Pharmacol. Ther. 2021, 44, 714–723.
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. Understanding the pharmacokinetics of tulathromycin: A pulmonary perspective. J. Vet. Pharmacol. Ther. 2014, 37, 211–221.
- Villarino, N.; Lesman, S.; Fielder, A.; García-Tapia, D.; Cox, S.; Lucas, M.; Robinson, J.; Brown, S.A.; Martín-Jiménez, T. Pulmonary pharmacokinetics of tulathromycin in swine. Part 2: Intra-airways compartments. J. Vet. Pharmacol. Ther. 2013, 36, 340–349.
- Villarino, N.; Lesman, S.; Fielder, A.; García-Tapia, D.; Cox, S.; Lucas, M.; Robinson, J.; Brown, S.A.; Martín-Jiménez, T. Pulmonary pharmacokinetics of tulathromycin in swine. Part I: Lung homogenate in healthy pigs and pigs challenged intratracheally with lipopolysaccharide of Escherichia coli. J. Vet. Pharmacol. Ther. 2013, 36, 329–339.
- Womble, A.; Giguère, S.; Murthy, Y.V.S.N.; Cox, C.; Obare, E. Pulmonary disposition of tilmicosin in foals and in vitro activity against Rhodococcus equi and other common equine bacterial pathogens. J. Vet. Pharmacol. Ther. 2006, 29, 561–568.
- Javsicas, L.; Giguère, S.; Womble, A.Y. Disposition of oral telithromycin in foals and in vitro activity of the drug against macrolide-susceptible and macrolide-resistant Rhodococcus equi isolates. J. Vet. Pharmacol. Ther. 2010, 33, 383–388.





