Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | S. Ganguly | -- | 2660 | 2023-04-19 17:25:45 | | | |
| 2 | Sirius Huang | Meta information modification | 2660 | 2023-04-20 10:13:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ganguly, S.; Margel, S. Magneto-Fluorescent Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/43252 (accessed on 04 March 2026).
Ganguly S, Margel S. Magneto-Fluorescent Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/43252. Accessed March 04, 2026.
Ganguly, Sayan, Shlomo Margel. "Magneto-Fluorescent Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/43252 (accessed March 04, 2026).
Ganguly, S., & Margel, S. (2023, April 19). Magneto-Fluorescent Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/43252
Ganguly, Sayan and Shlomo Margel. "Magneto-Fluorescent Nanoparticles." Encyclopedia. Web. 19 April, 2023.
Copy Citation
Novel nanomaterials are of interest in biology, medicine, and imaging applications. Multimodal fluorescent-magnetic nanoparticles demand special attention because they have the potential to be employed as diagnostic and medication-delivery tools, which, in turn, might make it easier to diagnose and treat cancer, as well as a wide variety of other disorders.
nanoparticles
biomedical
magneto-fluorescent
multimodal imaging
1. Types of Magnetic and Fluorescent Nanoparticles
1.1. Superparamagnetic Nanoparticles
Unlike their bulk counterparts, magnetic NPs have completely distinct magnetic properties. A huge number of magnetic domains make up the majority of ferromagnetic materials, and each domain has parallel magnetic moments that are isolated by domain walls [1]. Domain walls arise when magnetostatic energy and domain-wall energy are in equilibrium [2][3]. The domain-wall energy is responsible for the larger interfacial area between domains, and the magnetostatic energy grows proportionately with the volume of the material [4]. The energy required to create domain barriers is substantially more than the magnetostatic energy required to maintain a single-domain NP; hence, there is a critical value below which a stable single-domain NP can be established when the size of ferromagnetic materials is lowered to the nanoscale level [5]. As illustrated in Figure 1, the critical size (rc) of an NP may be stated according to Equation (1) when the magnetostatic energy is equal to the domain-wall energy and the NP is transitioning from the multidomain state to the single-domain state [6].
where A represents the exchange constant, Keff is the anisotropy constant, 0 is the vacuum permeability, and M is the saturation magnetization. The critical diameter of magnetic NPs is generally between 10 and 100 nm.
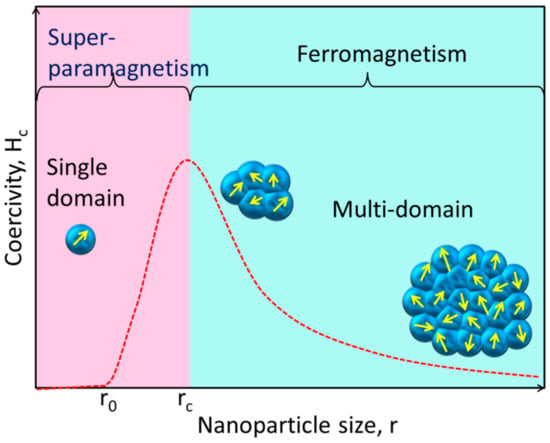
Figure 1. This simplified diagram shows how the magnetic coercivity (Hc, the magnetic field needed to decrease the magnetization to zero) of a magnetic NP varies with its physical dimensions. As an NP is shrunk to the critical size (rc), the domain wall vanishes and the Hc rises. The NP reaches the superparamagnetic domain and exhibits zero coercivity if its size is further reduced to r0, where the thermal agitation energy is greater than the magnetic anisotropy energy and the magnetic moment of the NP varies freely.
The magnetic spins of ferromagnetic NPs are linked and parallel-aligned inside a single domain, as was indicated before. When the magnetic moments are kept in a single domain of ferromagnetic NPs, the magnetic anisotropy energy [7] (ΔE) is calculated as follows:
Figure 2 shows how to calculate the volume of an NP using Equation (2).
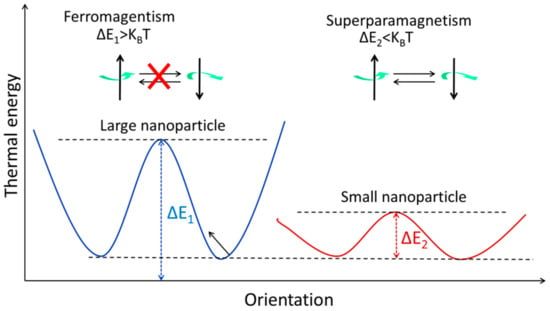
Figure 2. The energy levels of magnetic NPs with varying magnetic spin orientations. When it comes to stopping the magnetization from rotating, thermal energy (KBT) is the limiting factor. Both ferromagnetism and superparamagnetism may be seen in the large and small NPs, respectively.
1.2. Fluorescent Nanoparticles
Over the course of the last ten years, research and development efforts have been heavily concentrated on fluorescent NPs [8][9][10][11][12][13]. These NPs include semiconductor NPs (quantum dots), metal NPs, silica NPs, polymer NPs, and many more [14][15][16]. In comparison to traditional fluorescent organic dyes, fluorescent NPs exhibit a more brilliant fluorescence, increased photostability, and enhanced biocompatibility. In addition, the NPs have a distinct set of chemical and optical characteristics [17][18]. Several different kinds of fluorescent NPs have emerged for bioimaging over the past few decades. These include organic-dye-doped silica NPs, organic polymer NPs, metallic NPs, carbon-based NPs (nanotubes and nanodots) [19], quantum dots (QDs) [20][21][22], and lanthanide-doped upconverting nanoparticles (UCNPs) [23][24][25]. In terms of their composition, fluorescent NPs employed in bioimaging may be roughly divided into two categories: organic and inorganic NPs. In order to be considered among the best fluorescent probes for imaging, fluorescent NPs should generally meet the following characteristics: high signal-to-background ratio, big strokes to prevent self-quenching, excellent stability under physiological settings, little cytotoxicity, and minimum disruption of biological activities, to name only a few. They also have limited fluorescence durations, typically around 10−9 s, which is insufficient for efficiently separating short-lived fluorescence interference from dispersed excitation light.
Optical biosensors based on fluorescence may be divided into two categories: those that use downconversion fluorescence and those that use upconversion fluorescence. Downconversion fluorescence, the foundation of most existing optical biosensors, works by transforming the energy of light, often in the ultraviolet to visible light range, into a more usable form. These probes excel in chemical, photochemical, and thermal stability, and their long fluorescence lifetimes make them ideal for biosensing applications. They are vulnerable to photobleaching and blinking, and their overlapping excitation and emission spectra are a challenge for multiplexing applications [26]. In addition, biological samples include endogenous fluorophores, such as hemoglobin, which strongly absorb and scatter light below 700 nm, resulting in a high background fluorescence that can only penetrate biological media superficially. These limitations of downconversion-based biosensors have severely stymied their use in biosensing and bioimaging. Nanoparticles based on upconversion fluorescence (UCNPs) have significant potential for use in biomedical settings due to their ability to convert near-infrared (NIR) light into visible light [27]. Due to its providing an optically clear window onto biological tissues, NIR light has attracted a lot of attention as an excitation source for biosensing and bioimaging in recent years [28].
1.3. Types of Noninvasive Imaging
Recently, a variety of noninvasive optical imaging techniques, including computed tomography (CT), magnetic resonance (MR), positron emission tomography (PET), single-photon emission CT (SPECT), ultrasound (US), and optical imaging (OI), as well as their variants and subcategories, have been described [29][30]. Each one is distinct from the others in respect of resolution and sensitivity complexity, the length of time required to obtain data, and cost. The selection of an imaging modality is largely determined by the precise question that needs answering, and various imaging modalities are often complimentary rather than in competition. Medical imaging with magnetic resonance (MRI) is another technique that has expanded greatly in recent years. MRI is particularly useful for diagnosing conditions affecting soft tissues [31]. Furthermore, the drawbacks of each approach, such as MRI’s low sensitivity and OI’s lack of anatomical background information, cancel each other out.
1.4. Magnetic Resonance Imaging (MRI)
The MRI method was developed from the foundations of nuclear magnetic resonance. NMR signals obtained from hydrogen nuclei placed in various physiological contexts throughout an organism are used to create tissue contrasts, which are then used for diagnostic purposes. The frequencies at which nuclear spins resonate when a specimen is immersed in a homogeneous, static magnetic field are proportional to the strength of the magnetic field. Specimens are activated by a radiofrequency pulse at their resonance frequency in order to alter their net magnetization once they have established an equilibrium magnetization. Three-dimensional pictures of the body are constructed by measuring the variations in generated electromagnetic signals in the presence of linear field gradients. MRI is a powerful diagnostic tool for detecting lesions in the brain and spinal cord due to its high tissue specificity. Figure 3 shows the main contributors to the overall signal and contrast levels obtained from a sample.
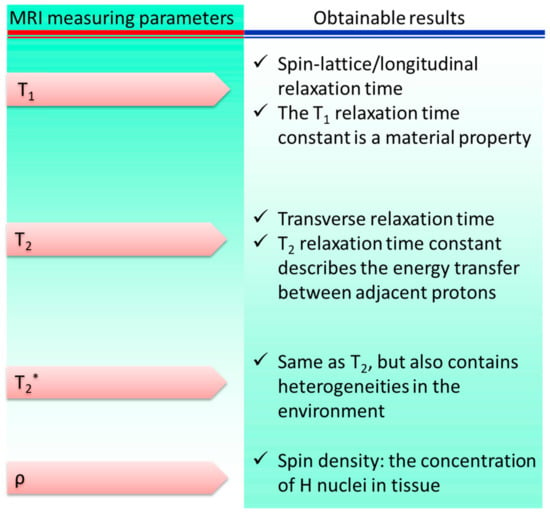
Figure 3. Parameters of magnetic resonance imaging: sources of signal variation. * represents a separate variable.
1.5. Computed Tomography (CT)
Over the past decade, there has been a meteoric surge in the number of papers published on the use of microcomputed tomography (CT) imaging in preclinical in vivo research [32][33]. Better spatial and temporal resolution has enabled researchers to acquire more precise anatomical pictures of small animals and track the development of illnesses in small-animal models. Although organs and tumors are not easily visible on CT images without the use of iodinated contrast agents, CT has poor soft-tissue contrast for malignancies and surrounding tissues [34][35][36]. At first, CT had great spatial resolution but low contrast in soft tissues. The noninvasive examination of high-contrast structures, such as bones and implants, was hence the primary emphasis of the earliest publications on the utilization of CT. There have been significant increases in the temporal and geometrical resolutions, as well as the readout speeds, of X-ray detectors as technology has developed. A µCT method with a spatial resolution of 1–100 µm is denoted as µCT [37]. µCT has the potential to supplant the laborious serial staining procedures needed for the histomorphometric examination of thin slices, and it might also be used to conduct longitudinal in vivo investigations in tiny animals. Given CT’s short imaging duration and great spatial resolution, it can be utilized to examine lung cancers and bone metastases. µCT has been employed in high-throughput phenotyping methods for a large number of transgenic mice, allowing for the detection of gross defects. To examine cell trafficking, tumor development, and response to treatment in vivo, µCT images showing tumor structural features were coregistered with bioluminescence pictures. This approach to image processing has the potential to be employed in evaluating hematological reconstitution after bone-marrow transplantation. CT is useful because of the high spatial resolution (12–50 µm) that is required to observe minute anatomical features. Functional imaging methods can be used with CT to reveal metabolic and dynamic details. Comparisons of in vivo trabecular structures and mineralization densities between mice strains were made in osseous disease investigations to account for any variations in experimental design and data collection [38]. Similar to CT angiography (CTA) in humans, contrast-agent injection is required for in vivo examination of vascular systems using CT in small animals. Vessel analysis in rats after sacrifice [39] was possible with the older, somewhat slow CT scanners, with scanning periods extending up to hours, employing perfusion with radiopaque polymerizing chemicals [40] or shock freezing of the contrast-agent-perfused material [41].
2. Synthesis and Fabrication of Magneto-Fluorescent Nanoparticles
Nanotechnology has grown rapidly over the past few decades as a new discipline that bridges previously separate scientific disciplines, such as biology, medicine, chemistry, materials engineering, quantum mechanics, and electronics. The benefits of nanomaterials, such as their high surface-to-volume ratios, unique optical characteristics, and nanoscale physical phenomena, have led to their widespread usage in scientific study and medical practice. Superparamagnetism at room temperature is a defining characteristic of MNPs (e.g., Fe3O4, γ-Fe2O3, or a combination of the two) when their size is small enough [42]. Molecular nanoparticles (MNPs) have the potential to be used in ferrofluid technology [43] and heterogeneous catalysis due to their extremely high surface-to-volume ratios and in the cleanup of polluted or contaminated environmental media [44]. Furthermore, it has recently been considered that MNPs might be used for a variety of biological applications (magnetic contrast agents, hyperthermia agents, magnetic vectors for drug administration, etc.). While MNPs have great potential for biomedical applications, they must first meet a number of requirements that are often contradictory. These include: (a) extremely low toxicity to the human body; (b) outstanding magnetic properties; (c) a relatively narrow size distribution; and (d) the ability to have their surfaces easily modified (through coating) in order to allow their functionalization for particular bioagents. Since magnetite (Fe3O4) and maghemite (γ-Fe2O3) NPs have been shown to be biocompatible, they are good choices to meet the first two requirements [42]. Additionally, MNPs with high stability, biocompatibility, and low toxicity may be created and employed in a wide range of biomedical applications after being modified by functional components [45].
The creation of specific magneto-fluorescent NPs has attracted a lot of attention in recent years. Although these nanocomposites have a wide range of compositions and morphologies, researchers have categorized the preparation techniques into two types of synthetic strategy: the coupling approach and the encapsulation method, based on reports from a variety of sources. Table 1 lists several common methods for synthesizing composite NPs and the various ways they might be employed.
Table 1. Synthetic tailored magneto-fluorescent multimodal nanoparticles (NPs) and potential uses.
| Nanoparticles | Method | Targeting Ligand | Application | Ref. |
|---|---|---|---|---|
| Fe3O4/anti-IgG/GQD/BSA | Coupling | Human IgG | Urine renal disease | [46] |
| Ab (anti-aflatoxin B1)–CdS–Fe3O4 bioconjugates | Coupling | Aflatoxin B1 | Detection of aflatoxin B1 B1 in corn samples | [47] |
| Dox-loaded carbon dot (CD)–4-carboxyphenylboronic acid (CBBA)–MnFe2O4 NPs [DCCM] | Coupling | Sialic acid | HeLa cells | [48] |
| Iron oxide superparamagnetic NPs-PEG-Cypher5E/folic acid | Coupling | Folic acid | MR imaging and fluorescence imaging | [49] |
| Fe3O4(MNP)-Cds(QDs)-folic acid | Coupling | Folic acid | As a delivery agent and an in vitro imaging diagnostic agent | [50] |
| MTX-PEG-CS-IONPs-Cy5.5 | Coupling | Folic acid | Dual-model imaging and synergistically self-targeted cancer therapy | [51] |
| Fe3O4-dopamine hydrobromide (DPA)-PEG-FA/FITC NPs | Coupling | Folic acid | Targeted imaging of various tumors | [52] |
| Fe3O4-CdTe-humanized monoclonal antibody CC49 (hCC49 antibody) | Coupling | Tumor-associated glycoprotein-72 (TAG-72) | Cancer cell imaging | [53] |
| Fe3O4@mSiO2–triphenylphospine (TPP)/CD | Coupling | Mitochondria | Mitochondrial diseases | [54] |
| BRCAA1 antibody-FMNPs (Fe3O4-CdTe) | Encapsulation | BRCAA1 protein | In vivo dual-model imaging of gastric cancer | [55] |
| FMN (flavin mononucleotide)-coated ultrasmall superparamagnetic iron oxide (FLUSPIO) | Encapsulation | Riboflavin (Rf) | Prostate cancer xenografts | [56] |
| MNPs@OPE[oligo(p-phenylene ethynylene)]-PEG-FA | Folate receptor | Targeted magnetic resonance and two-photon optical imaging in vitro and in vivo | [57] | |
| Fe3O4@SiO2/RhBITC-anti-HER2 antibody NPs | Encapsulation | Human epidermal growth factor receptor 2 (HER2) | Discrimination of HER2-positive breast cancer cells | [58] |
| Fe3O4@SiO2(FITC)-FA/AICAR(5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside)/DOX | Encapsulation | Folate receptor | Inhibition of cancer cell growth | [59] |
| PTX/Fe3O4 NPs/CuInS2/ZnS QDs@biotin–PEG–PCD [abiotin–poly(ethylene glycol)–poly (curcumin-dithiodipropionic acid) copolymer] | Encapsulation | Biotin receptor | Treatment of multidrug-resistant breast cancer at the cellular level | [60] |
| Trastuzumab-conjugated Lipo[MNP@m-SiO2(FITC)] | Encapsulation | Her2/neu | In vitro fluorescence and MR imaging of Her2/neu-positive breast cancer | [61] |
| Fe3O4/CuInS2(CIS)@SiO2(Gd–DTPA)–RGD (arginine-glycine-aspartic acid) | Encapsulation | αVβ3 integrin | MR and fluorescence imaging of pancreatic cancer | [62] |
There have been few attempts made to construct hybrid systems, despite the rising interest in and the relevance of SPIONs and SiQDs to the fabrication of biodegradable and biocompatible nanoprobes for biomedical use. Conjugation of the two systems and studies of their conjugated characteristics are scarce, and obstacles such as charge or energy transfer processes and iron oxide’s high absorption in the visible spectrum make even their simple fabrication by quenching QD/SiQDs PL difficult [63]. Long interparticular spacers are usually employed to avoid such disadvantages and ensure the flourishing of both luminous and magnetic characteristics [64]. Covalent binding and electrostatic absorption are used to couple the magnetic and fluorescent species shown in Figure 4 after they have been synthesized and functionalized independently. In this procedure, the coupling strategy for magnetic and fluorescent NPs is determined by the modifications made to their surfaces. Thiol, carboxyl, and amino groups are all examples of functional moieties that may be found in the coupling agents [65]. The formation of core–shell structures on the surfaces of magnetic materials often involves loading fluorescent elements onto the top of a magnetic material by either bond formation or charge attraction. After that, conjugation of specific ligands onto the surfaces of the magneto-fluorescent NPs is often performed. In another method, which is illustrated in Figure 4, the cores of new composite micro- or nanospheres are formed by encasing prefabricated fluorescent NPs and magnetic NPs in various materials, such as silica or polymer beads, protein, chitosan, and liposomes. This results in the formation of new NPs. In order to create these composite nanospheres, two different approaches have been utilized. These materials give the NPs favorable features, such as strong biocompatibility and stability and facile functionalization, to enable the inclusion of ligands so that the NPs may be selectively targeted physiologically. These materials also make the incorporation of ligands easier [66]. Since the existing coupling techniques are straightforward and provide a number of important benefits, a wide variety of magneto-fluorescent NPs have been fabricated and put to use in a variety of subfields of biomedicine. However, the stability of NPs generated in this manner frequently shifts in response to varied settings; as a result, their applications in biomedicine are restricted. When compared to magneto-fluorescent NPs created using the coupling approach, those prepared using the encapsulation method provide a number of benefits, including the ones listed below:
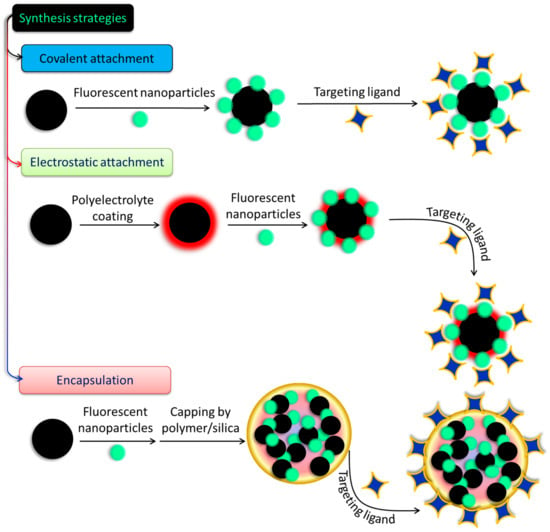
Figure 4. Different approaches to prepare fluorescent-nanoparticle-decorated magnetic nanostructures.
- (a)
-
NPs can be covalently modified with diverse targeted ligands using the surface functionalities of silica or polymer nanospheres without a stable structure.
- (b)
-
However, controlling the proportion of MNPs to luminescent NPs is difficult, making the production of well-dispersed, homogeneous, multimodal NPs complicated.
- (c)
-
Additionally, the outer layer of silica or polymer, which may serve as a screen, may play a role in preventing unwanted particles from entering.
References
- Chen, D.-X.; Sanchez, A.; Taboada, E.; Roig, A.; Sun, N.; Gu, H.-C. Size determination of superparamagnetic nanoparticles from magnetization curve. J. Appl. Phys. 2009, 105, 083924.
- Manukyan, K.V.; Chen, Y.-S.; Rouvimov, S.; Li, P.; Li, X.; Dong, S.; Liu, X.; Furdyna, J.K.; Orlov, A.; Bernstein, G.H. Ultrasmall α-Fe2O3 superparamagnetic nanoparticles with high magnetization prepared by template-assisted combustion process. J. Phys. Chem. C 2014, 118, 16264–16271.
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic targeting of growth factors using iron oxide nanoparticles. Nanomaterials 2018, 8, 707.
- Lukawska, A.; Jagoo, Z.; Kozlowski, G.; Turgut, Z.; Kosai, H.; Sheets, A.; Bixel, T.; Wheatley, A.; Abdulkin, P.; Knappett, B. Ac magnetic heating of superparamagnetic Fe and Co nanoparticles. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2013; pp. 159–167.
- Chalise, D.; Cahill, D.G. Highly Sensitive and High-Throughput Magnetic Resonance Thermometry of Fluids Using Superparamagnetic Nanoparticles. Phys. Rev. Appl. 2023, 19, 014055.
- Melo, L.G.; Soares, T.R.; Neto, O.P.V. Analysis of the magnetostatic energy of chains of single-domain nanomagnets for logic gates. IEEE Trans. Magn. 2017, 53, 1–10.
- Nozaki, T.; Yamamoto, T.; Miwa, S.; Tsujikawa, M.; Shirai, M.; Yuasa, S.; Suzuki, Y. Recent progress in the voltage-controlled magnetic anisotropy effect and the challenges faced in developing voltage-torque MRAM. Micromachines 2019, 10, 327.
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796.
- Parameswaranpillai, J.; Das, P.; Ganguly, S. Introduction to Quantum Dots and Their Polymer Composites. In Quantum Dots and Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–19.
- Parameswaranpillai, J.; Das, P.; Ganguly, S. Quantum Dots and Polymer Nanocomposites: Synthesis, Chemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2022.
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031.
- Das, P.; Ganguly, S.; Maity, P.P.; Srivastava, H.K.; Bose, M.; Dhara, S.; Bandyopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Converting waste Allium sativum peel to nitrogen and sulphur co-doped photoluminescence carbon dots for solar conversion, cell labeling, and photobleaching diligences: A path from discarded waste to value-added products. J. Photochem. Photobiol. B Biol. 2019, 197, 111545.
- Das, P.; Bose, M.; Das, A.K.; Banerjee, S.; Das, N.C. One-step synthesis of fluorescent carbon dots for bio-labeling assay. Macromol. Symp. 2018, 382, 1800077.
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally Derived Carbon Dots In Situ Confined Self-Healing and Breathable Hydrogel Monolith for Anomalous Diffusion-Driven Phytomedicine Release. ACS Appl. Bio Mater. 2022, 5, 5617–5633.
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir 2021, 37, 3508–3520.
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790.
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly (norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050.
- Das, P.; Ganguly, S.; Mondal, S.; Ghorai, U.K.; Maity, P.P.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Banerjee, S.; Das, N.C. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV-active marker and fluorescent polymer composite. Luminescence 2018, 33, 1136–1145.
- Ahmed, S.R.; Sherazee, M.; Srinivasan, S.; Rajabzadeh, A.R. Nanozymatic detection of thiocyanate through accelerating the growth of ultra-small gold nanoparticles/graphene quantum dots hybrids. Food Chem. 2022, 379, 132152.
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853.
- Das, P.; Ganguly, S.; Maity, P.P.; Bose, M.; Mondal, S.; Dhara, S.; Das, A.K.; Banerjee, S.; Das, N.C. Waste chimney oil to nanolights: A low cost chemosensor for tracer metal detection in practical field and its polymer composite for multidimensional activity. J. Photochem. Photobiol. B Biol. 2018, 180, 56–67.
- Ganguly, S.; Das, P.; Das, T.K.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.K.; Das, N.C. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer-mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochem. 2020, 60, 104797.
- Wu, F.; Su, H.; Zhu, X.; Wang, K.; Zhang, Z.; Wong, W.-K. Near-infrared emissive lanthanide hybridized carbon quantum dots for bioimaging applications. J. Mater. Chem. B 2016, 4, 6366–6372.
- Martinić, I.; Eliseeva, S.V.; Petoud, S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J. Lumin. 2017, 189, 19–43.
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dot Biopolymer-Based Flexible Functional Films for Antioxidant and Food Monitoring Applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340.
- Dempsey, G.T.; Bates, M.; Kowtoniuk, W.E.; Liu, D.R.; Tsien, R.Y.; Zhuang, X. Photoswitching mechanism of cyanine dyes. J. Am. Chem. Soc. 2009, 131, 18192–18193.
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711.
- Li, C.; Liu, J.; Alonso, S.; Li, F.; Zhang, Y. Upconversion nanoparticles for sensitive and in-depth detection of Cu2+ ions. Nanoscale 2012, 4, 6065–6071.
- Mahmood, U.; Weissleder, R. Some Tools for Molecular Imaging. Acad. Radiol. 2002, 9, 629–631.
- Plewes, D.B.; Kucharczyk, W. Physics of MRI: A primer. J. Magn. Reson. Imaging 2012, 35, 1038–1054.
- Santra, S.; Bagwe, R.P.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and Characterization of Fluorescent, Radio-Opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv. Mater. 2005, 17, 2165–2169.
- Sarma, A.; Heilbrun, M.E.; Conner, K.E.; Stevens, S.M.; Woller, S.C.; Elliott, C.G. Radiation and chest CT scan examinations: What do we know? Chest 2012, 142, 750–760.
- Ghosh, S.; Ganguly, S.; Maruthi, A.; Jana, S.; Remanan, S.; Das, P.; Das, T.K.; Ghosh, S.K.; Das, N.C. Micro-computed tomography enhanced cross-linked carboxylated acrylonitrile butadiene rubber with the decoration of new generation conductive carbon black for high strain tolerant electromagnetic wave absorber. Mater. Today Commun. 2020, 24, 100989.
- Ghosh, S.; Das, P.; Ganguly, S.; Remanan, S.; Das, T.K.; Bhattacharyya, S.K.; Baral, J.; Das, A.K.; Laha, T.; Das, N.C. 3D-enhanced, high-performing, super-hydrophobic and electromagnetic-interference shielding fabrics based on silver paint and their use in antibacterial applications. ChemistrySelect 2019, 4, 11748–11754.
- Bartling, S.H.; Budjan, J.; Aviv, H.; Haneder, S.; Kraenzlin, B.; Michaely, H.; Margel, S.; Diehl, S.; Semmler, W.; Gretz, N. First multimodal embolization particles visible on x-ray/computed tomography and magnetic resonance imaging. Investig. Radiol. 2011, 46, 178–186.
- Aviv, H.; Bartling, S.; Grinberg, I.; Margel, S. Synthesis and characterization of Bi2O3/HSA core-shell nanoparticles for X-ray imaging applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 131–138.
- Schladitz, K. Quantitative micro-CT. J. Microsc. 2011, 243, 111–117.
- Jiang, Y.; Zhao, J.; White, D.; Genant, H. Micro CT and Micro MR imaging of 3D architecture of animal skeleton. J. Musculoskelet Neuron. Interact 2000, 1, 45–51.
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259.
- Abruzzo, T.; Tumialan, L.; Chaalala, C.; Kim, S.; Guldberg, R.E.; Lin, A.; Leach, J.; Khoury, J.C.; Morgan, A.E.; Cawley III, C.M. Microscopic computed tomography imaging of the cerebral circulation in mice: Feasibility and pitfalls. Synapse 2008, 62, 557–565.
- Dorr, A.; Sled, J.G.; Kabani, N. Three-dimensional cerebral vasculature of the CBA mouse brain: A magnetic resonance imaging and micro computed tomography study. Neuroimage 2007, 35, 1409–1423.
- Riani, P.; Napoletano, M.; Canepa, F. Synthesis, characterization and ac magnetic analysis of magnetite nanoparticles. J. Nanoparticle Res. 2011, 13, 7013–7020.
- Raj, K.; Moskowitz, B.; Casciari, R. Advances in ferrofluid technology. J. Magn. Magn. Mater. 1995, 149, 174–180.
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691.
- Takahashi, M.; Mohan, P.; Nakade, A.; Higashimine, K.; Mott, D.; Hamada, T.; Matsumura, K.; Taguchi, T.; Maenosono, S. Ag/FeCo/Ag core/shell/shell magnetic nanoparticles with plasmonic imaging capability. Langmuir 2015, 31, 2228–2236.
- Jiang, D.; Ni, D.; Liu, F.; Zhang, L.; Liu, L.; Pu, X. A fluorescent imaging assay of cast in renal disease based on graphene quantum dots and Fe3O4 nanoparticles. Clin. Chim. Acta 2016, 454, 94–101.
- Liu, Y.; Zhang, X.; Fang, F.; Kuang, G.; Wang, G. Sandwich immunoassays of multicomponent subtrace pathogenic DNA based on magnetic fluorescent encoded nanoparticles. BioMed Res. Int. 2016, 2016, 7324384.
- Fahmi, M.Z.; Chen, J.-K.; Huang, C.-C.; Ling, Y.-C.; Chang, J.-Y. Phenylboronic acid-modified magnetic nanoparticles as a platform for carbon dot conjugation and doxorubicin delivery. J. Mater. Chem. B 2015, 3, 5532–5543.
- Chen, Y.-C.; Chang, W.-H.; Wang, S.-J.; Hsieh, W.-Y. Fluorescent magnetic nanoparticles with specific targeting functions for combinded targeting, optical imaging and magnetic resonance imaging. J. Biomater. Sci. Polym. Ed. 2012, 23, 1903–1922.
- Wen, C.-Y.; Xie, H.-Y.; Zhang, Z.-L.; Wu, L.-L.; Hu, J.; Tang, M.; Wu, M.; Pang, D.-W. Fluorescent/magnetic micro/nano-spheres based on quantum dots and/or magnetic nanoparticles: Preparation, properties, and their applications in cancer studies. Nanoscale 2016, 8, 12406–12429.
- Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Zhou, S.; Xie, L.; Luo, F.; Lin, C.; Hou, Z. Drug/dye-loaded, multifunctional PEG–chitosan–iron oxide nanocomposites for methotraxate synergistically self-targeted cancer therapy and dual model imaging. ACS Appl. Mater. Interfaces 2015, 7, 11908–11920.
- Majd, M.H.; Barar, J.; Asgari, D.; Valizadeh, H.; Rashidi, M.R.; Kafil, V.; Shahbazi, J.; Omidi, Y. Targeted fluoromagnetic nanoparticles for imaging of breast cancer mcf-7 cells. Adv. Pharm. Bull. 2013, 3, 189.
- Ahmed, S.R.; Dong, J.; Yui, M.; Kato, T.; Lee, J.; Park, E.Y. Quantum dots incorporated magnetic nanoparticles for imaging colon carcinoma cells. J. Nanobiotechnol. 2013, 11, 28.
- Zhang, Y.; Shen, Y.; Teng, X.; Yan, M.; Bi, H.; Morais, P.C. Mitochondria-targeting nanoplatform with fluorescent carbon dots for long time imaging and magnetic field-enhanced cellular uptake. ACS Appl. Mater. Interfaces 2015, 7, 10201–10212.
- Wang, K.; Ruan, J.; Qian, Q.; Song, H.; Bao, C.; Zhang, X.; Kong, Y.; Zhang, C.; Hu, G.; Ni, J. BRCAA1 monoclonal antibody conjugated fluorescent magnetic nanoparticles for in vivo targeted magnetofluorescent imaging of gastric cancer. J. Nanobiotechnol. 2011, 9, 23.
- Jayapaul, J.; Arns, S.; Bunker, M.; Weiler, M.; Rutherford, S.; Comba, P.; Kiessling, F. In vivo evaluation of riboflavin receptor targeted fluorescent USPIO in mice with prostate cancer xenografts. Nano Res. 2016, 9, 1319–1333.
- Yin, C.; Hong, B.; Gong, Z.; Zhao, H.; Hu, W.; Lu, X.; Li, J.; Li, X.; Yang, Z.; Fan, Q. Fluorescent oligo (p-phenyleneethynylene) contained amphiphiles-encapsulated magnetic nanoparticles for targeted magnetic resonance and two-photon optical imaging in vitro and in vivo. Nanoscale 2015, 7, 8907–8919.
- Li, J.; An, Y.-L.; Zang, F.-C.; Zong, S.-F.; Cui, Y.-P.; Teng, G.-J. A dual mode targeting probe for distinguishing HER2-positive breast cancer cells using silica-coated fluorescent magnetic nanoparticles. J. Nanoparticle Res. 2013, 15, 1980.
- Daglioglu, C.; Okutucu, B. Synthesis and characterization of AICAR and DOX conjugated multifunctional nanoparticles as a platform for synergistic inhibition of cancer cell growth. Bioconjugate Chem. 2016, 27, 1098–1111.
- Wang, S.; Li, W.; Yuan, D.; Song, J.; Fang, J. Quantitative detection of the tumor-associated antigen large external antigen in colorectal cancer tissues and cells using quantum dot probe. Int. J. Nanomed. 2016, 11, 235.
- Jang, M.; Yoon, Y.I.; Kwon, Y.S.; Yoon, T.-J.; Lee, H.J.; Hwang, S.I.; La Yun, B.; Kim, S.M. Trastuzumab-conjugated liposome-coated fluorescent magnetic nanoparticles to target breast cancer. Korean J. Radiol. 2014, 15, 411–422.
- Shen, J.; Li, Y.; Zhu, Y.; Yang, X.; Yao, X.; Li, J.; Huang, G.; Li, C. Multifunctional gadolinium-labeled silica-coated Fe3O4 and CuInS 2 nanoparticles as a platform for in vivo tri-modality magnetic resonance and fluorescence imaging. J. Mater. Chem. B 2015, 3, 2873–2882.
- Sathe, T.R.; Agrawal, A.; Nie, S. Mesoporous Silica Beads Embedded with Semiconductor Quantum Dots and Iron Oxide Nanocrystals: Dual-Function Microcarriers for Optical Encoding and Magnetic Separation. Anal. Chem. 2006, 78, 5627–5632.
- You, X.; He, R.; Gao, F.; Shao, J.; Pan, B.; Cui, D. Hydrophilic high-luminescent magnetic nanocomposites. Nanotechnology 2007, 18, 035701.
- Shen, M.; Jia, W.; Lin, C.; Fan, G.; Jin, Y.; Chen, X.; Chen, G. Facile synthesis of folate-conjugated magnetic/fluorescent bifunctional microspheres. Nanoscale Res. Lett. 2014, 9, 1–8.
- Margulis-Goshen, K.; Netivi, H.D.; Major, D.T.; Gradzielski, M.; Raviv, U.; Magdassi, S. Formation of organic nanoparticles from volatile microemulsions. J. Colloid Interface Sci. 2010, 342, 283–292.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
20 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





