
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rabia Aziz | -- | 4280 | 2023-04-19 16:55:45 | | | |
| 2 | Rita Xu | -73 word(s) | 4207 | 2023-04-20 05:31:39 | | |
Video Upload Options
As cardiac diseases, which mostly result in heart failure, are increasing rapidly worldwide, heart transplantation seems the only solution for saving lives. This practice is not always possible due to several reasons, such as scarcity of donors, rejection of organs from recipient bodies, or costly medical procedures. In the framework of nanotechnology, nanomaterials greatly contribute to the development of these cardiovascular scaffolds as they provide an easy regeneration of the tissues. Functional nanofibers can be used in the production of stem cells and in the regeneration of cells and tissues. The small size of nanomaterials, leads to changes in their chemical and physical characteristics that could alter their interaction and exposure to stem cells with cells and tissues.
1. Introduction
2. Natural Biodegradable Nanopolymers Used in Cardiac Tissue Engineering
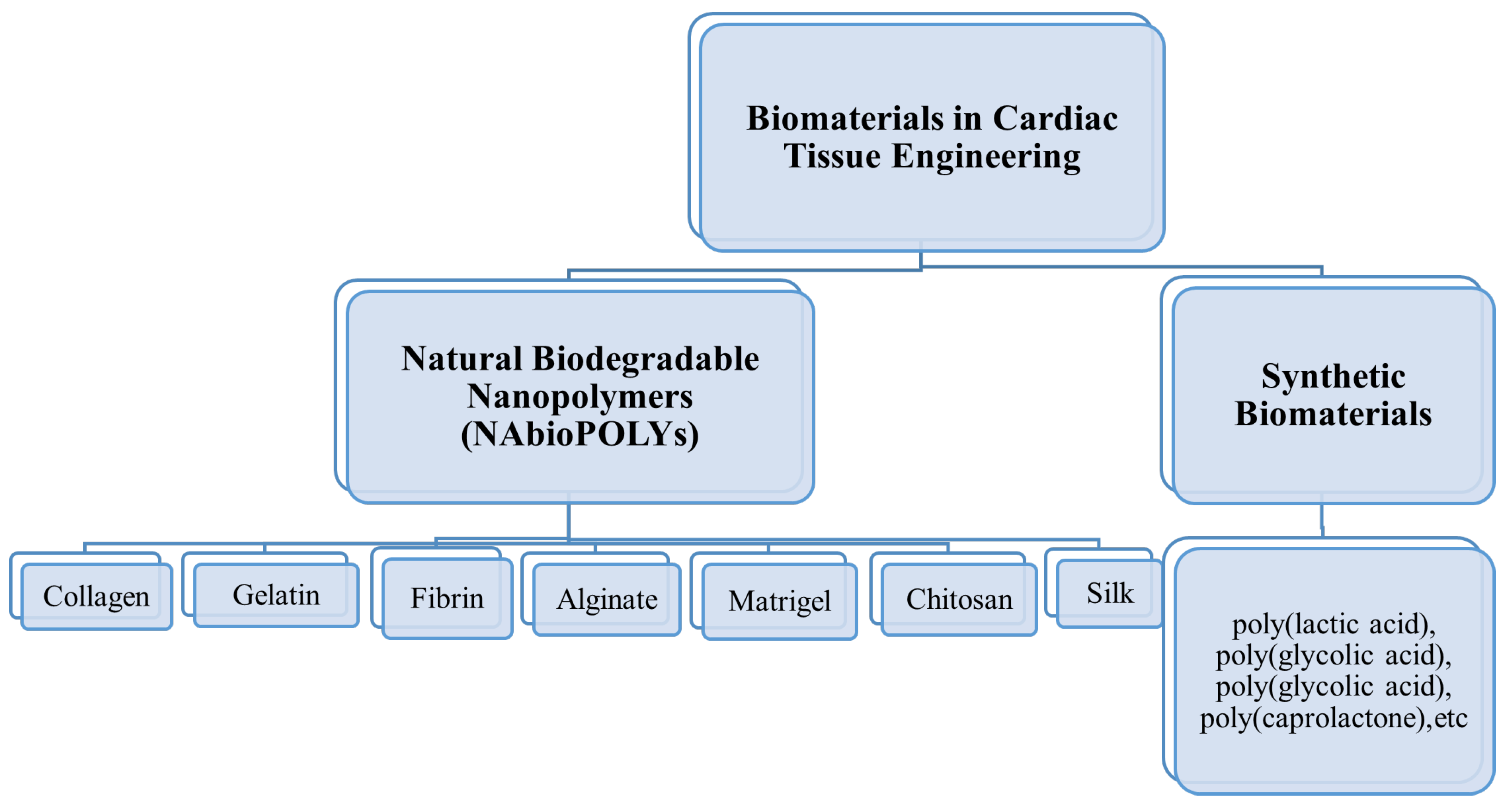
2.1. Collagen
2.2. Gelatin
2.3. Fibrin Gel
2.4. Alginate
2.5. Chitosan
2.6. Matrigel
2.7. Silk
References
- Huang, N.F.; Serpooshan, V.; Morris, V.B.; Sayed, N.; Pardon, G.; Abilez, O.J.; Nakayama, K.H.; Pruitt, B.L.; Wu, S.M.; Yoon, Y.S.; et al. Big bottlenecks in cardiovascular tissue engineering. Commun. Biol. 2018, 1, 199.
- Woodruff, R.C.; Tong, X.; Jackson, S.L.; Loustalot, F.V.; Vaughan, A.S. Abstract 9853: Trends in National Death Rates from Heart Disease in the United States, 2010–2020. Circulation 2022, 146, A9853.
- Amezcua, R.; Shirolkar, A.; Fraze, C.; Stout, D.A. Nanomaterials for Cardiac Myocyte Tissue Engineering. Nanomaterials 2016, 6, 133.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175.
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell–derived cardiomyocytes. Circ. Res. 2014, 114, 511–523.
- Ye, L.; Chang, Y.H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761.
- Miki, K.; Uenaka, H.; Saito, A.; Miyagawa, S.; Sakaguchi, T.; Higuchi, T.; Shimizu, T.; Okano, T.; Yamanaka, S.; Sawa, Y. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl. Med. 2012, 1, 430–437.
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37.
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of cell composition and geometry on human induced pluripotent stem cells-derived engineered cardiac tissue. Sci. Rep. 2017, 7, 45641.
- Shachar, M.; Cohen, S. Cardiac Tissue Engineering, Ex-Vivo: Design Principles in Biomaterials and Bioreactors. Heart Fail. Rev. 2003, 8, 271–276.
- Saludas, L.; Pascual-Gil, S.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M. Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm. 2017, 523, 454–475.
- Rodrigues, I.C.P.; Kaasi, A.; Maciel Filho, R.; Jardini, A.L.; Gabriel, L.P. Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein 2018, 16, eRB4538.
- Kroeze, R.J.; Helder, M.N.; Govaert, L.E.; Smit, T.H. Biodegradable Polymers in Bone Tissue Engineering. Materials 2009, 2, 833–856.
- Aziz, R. Applications of Nanomaterials in Tissue Engineering and Regenerative Medicine. In Bio-Manufactured Nanomaterials: Perspectives and Promotion; Pal, K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 187–202.
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655.
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 57.
- Fleischer, S.; Feiner, R.; Dvir, T. Cutting-edge platforms in cardiac tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 23–29.
- R Amin, D.; Sink, E.; Narayan, S.P.; Abdel-Hafiz, M.; Mestroni, L.; Peña, B. Nanomaterials for Cardiac Tissue Engineering. Molecules 2020, 25, 5189.
- Garbayo, E.; Pascual-Gil, S.; Prosper, F.; Blanco-Prieto, M.J. 19—Bioresorbable Polymers for Next-Generation Cardiac Scaffolds; Woodhead Publishing: Sawston, UK, 2017; pp. 445–467.
- Doppalapudi, S.; Katiyar, S.; Domb, A.J.; Khan, W. Biodegradable Natural Polymers BT—Advanced Polymers in Medicine; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–66.
- Adamcova, M.; Baka, T.; Dolezelova, E.; Aziriova, S.; Krajcirovicova, K.; Karesova, I.; Stanko, P.; Repova, K.; Simko, F. Relations between markers of cardiac remodelling and left ventricular collagen in an isoproterenol-induced heart damage model. J. Physiol. Pharmacol. 2019, 70, 8.
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10, 25003.
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.A.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2000, 17, 587–591.
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604.
- Mohammadi Nasr, S.; Rabiee, N.; Hajebi, S.; Ahmadi, S.; Fatahi, Y.; Hosseini, M.; Bagherzadeh, M.; Ghadiri, A.M.; Rabiee, M.; Jajarmi, V.; et al. Biodegradable Nanopolymers in Cardiac Tissue Engineering: From Concept Towards Nanomedicine. Int. J. Nanomed. 2020, 15, 4205–4224.
- Ou, L.; Li, W.; Zhang, Y.; Wang, W.; Liu, J.; Sorg, H.; Furlani, D.; Gäbel, R.; Mark, P.; Klopsch, C.; et al. Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J. Cell. Mol. Med. 2011, 15, 1310–1318.
- Di Felice, V.; Serradifalco, C.; Rizzuto, L.; De Luca, A.; Rappa, F.; Barone, R.; Di Marco, P.; Cassata, G.; Puleio, R.; Verin, L.; et al. Silk fibroin scaffolds enhance cell commitment of adult rat cardiac progenitor cells. J. Tissue Eng. Regen. Med. 2015, 9, 51–64.
- Majid, Q.A.; Fricker, A.T.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 192.
- Yaacob, A.; Jamaludin, N.S. Biodegradable Polymers for Cardiac Tissue Engineering BT—Handbook of Biodegradable Materials; Springer International Publishing: Cham, Switzerland, 2023; pp. 979–1013.
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-based tissue engineering strategies for vascular medicine. Front. Bioeng. Biotechnol. 2019, 7, 166.
- Lin, Y.L.; Chen, C.P.; Lo, C.M.; Wang, H.S. Stiffness-controlled three-dimensional collagen scaffolds for differentiation of human Wharton’s jelly mesenchymal stem cells into cardiac progenitor cells. J. Biomed. Mater. Res. Part A 2016, 104, 2234–2242.
- Ricard-Blum, S. The collagen family. Cold Spring Harbor Perspect. Biol. 2011, 3, a004978.
- Mirsadraee, S.; Wilcox, H.E.; Watterson, K.G.; Kearney, J.N.; Hunt, J.; Fisher, J.; Ingham, E. Biocompatibility of acellular human pericardium. J. Surg. Res. 2007, 143, 407–414.
- Jang, Y.; Park, Y.; Kim, J. Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue. Coatings 2020, 10, 925.
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 45003.
- Gotenstein, J.R.; Koo, C.C.; Ho, T.W.; Chisholm, A.D. Genetic Suppression of Basement Membrane Defects in Caenorhabditis elegans by Gain of Function in Extracellular Matrix and Cell-Matrix Attachment Genes. Genetics 2018, 208, 1499–1512.
- Pang, Y.; Wang, X.; Lee, D.; Greisler, H.P. Dynamic quantitative visualization of single cell alignment and migration and matrix remodeling in 3-D collagen hydrogels under mechanical force. Biomaterials 2011, 32, 3776–3783.
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899.
- Wu, W.Q.; Peng, S.; Song, Z.Y.; Lin, S. Collagen biomaterial for the treatment of myocardial infarction: An update on cardiac tissue engineering and myocardial regeneration. Drug Deliv. Transl. Res. 2019, 9, 920–934.
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72.
- Mohamed, M.A.; Islas, J.F.; Schwartz, R.J.; Birla, R.K. Electrical Stimulation of Artificial Heart Muscle: A Look into the Electrophysiologic and Genetic Implications. ASAIO J. 2017, 63, 333–341.
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Masoli, O.H.; Barisani, J.L.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): One year follow-up. Cell Transplant. 2007, 16, 927–934.
- Punnoose, A.M.; Elamparithi, A.; Kuruvilla, S. Electrospun Type 1 Collagen Matrices Using a Novel Benign Solvent for Cardiac Tissue Engineering. J. Cell. Physiol. 2015, 231, 744.
- C Echave, M.; S Burgo, L.; L Pedraz, J.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584.
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301.
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180.
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177.
- Echave, M.C.; Sánchez, P.; Pedraz, J.L.; Orive, G. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017, 42, 63–74.
- Echave, M.C.; Hernáez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779.
- Askari, E.; Naghib, S.M.; Zahedi, A.; Seyfoori, A.; Zare, Y.; Rhee, K.Y. Local delivery of chemotherapeutic agent in tissue engineering based on gelatin/graphene hydrogel. J. Mater. Res. Technol. 2021, 12, 412–422.
- Daikuara, L.Y.; Yue, Z.; Skropeta, D.; Wallace, G.G. In vitro characterisation of 3D printed platelet lysate-based bioink for potential application in skin tissue engineering. Acta Biomater. 2021, 123, 286–297.
- Zhang, Z.; Rong, Z.; Wu, G.; Wang, Y.; Tan, Z.; Zheng, J.; Jin, Y.; Liang, Z.; Liu, C.; Guo, J.; et al. Gelatin-CaO2/SAP/PLGA composite scaffold enhances the reparation of critical-sized cranial defects by promoting seed cell survival. Appl. Mater. Today 2021, 22, 100960.
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128.
- Elamparithi, A.; Ravi, M.; Balachandran, C.; Rao, S.; Paul, S.F. Biocompatibility Evaluation of Electrospun Collagen, Gelatin, Polycaprolactone and their Composite Matrices in Rattus Norvegicus. Indian Vet. J. 2016, 93, 49–51.
- Alonzo, M.; AnilKumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. J. Lab. Clin. Med. 2019, 211, 64–83.
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76.
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690.
- Barsotti, M.C.; Felice, F.; Balbarini, A.; Di Stefano, R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 301–310.
- Shaikh, F.M.; Callanan, A.; Kavanagh, E.G.; Burke, P.E.; Grace, P.A.; McGloughlin, T.M. Fibrin: A natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 2008, 188, 333–346.
- Mi, F.L.; Sung, H.W.; Shyu, S.S. Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr. Polym. 2002, 48, 61–72.
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76.
- Sondermeijer, H.P.; Witkowski, P.; Seki, T.; van der Laarse, A.; Itescu, S.; Hardy, M.A. The Use of Biocompatible Alginate Scaffolds Covalently Modified with Cyclic RGDfK Peptides to Improve Survival of Transplanted Cells and Angiogenesis in Damaged Myocardium. Tissue Eng. Part A 2017.
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485.
- Deng, C.; Li, F.; Griffith, M.; Ruel, M.; Suuronen, E.J. Application of Chitosan-Based Biomaterials for Blood Vessel Regeneration. Macromol. Symp. 2010, 297, 138–146.
- Xu, B.; Li, Y.; Deng, B.; Liu, X.; Wang, L.; Zhu, Q.L. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp. Ther. Med. 2017, 13, 588–594.
- Sobczak-Kupiec, A.; Iqbal, M.J.; Qureshi, M.Z.; Mansoor, Q.; Nabavi, S.M.; Purenovic, J.; Yaylim, I.; Farooqi, A.A.; Ismail, M. Role of TRAIL and miR-34a as Therapeutic Agents in Prostate Cancer: Increasing the Armory of Micro-Musketeers. In Molecular Oncology: Underlying Mechanisms and Translational Advancements; Farooqi, A.A., Ismail, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 237–245.
- Chae, M.P.; Hunter-Smith, D.J.; Murphy, S.V.; Findlay, M.W. 15—3D bioprinting adipose tissue for breast reconstruction. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 305–353.
- Zhang, L.; Li, X.; Yu, X.; Li, Y.; Sun, A.; Huang, C.; Xu, F.; Guo, J.; Sun, Y.; Zhang, X.; et al. Construction of vascularized pacemaker tissues by seeding cardiac progenitor cells and endothelial progenitor cells into Matrigel. Life Sci. 2017, 179, 139–146.
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416.
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 940–952.
- Stoppel, W.L.; Hu, D.; Domian, I.J.; Kaplan, D.L.; Black, L.D.R. Anisotropic silk biomaterials containing cardiac extracellular matrix for cardiac tissue engineering. Biomed. Mater. 2015, 10, 34105.
- Song, Y.; Wang, H.; Yue, F.; Lv, Q.; Cai, B.; Dong, N.; Wang, Z.; Wang, L. Silk-Based Biomaterials for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e2000735.
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117.
- Liang, Y.; Mitriashkin, A.; Lim, T.T.; Goh, J.C.H. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterials 2021, 276, 121008.
- Tian, D.; Yu, D.N.; Xu, Y.M.; Ding, X.Y.; Zhang, Z.Y.; Wan, C.L.; He, J.H. Electrospun Mussel-derived Silk Fibers. Recent Patents Nanotechnol. 2020, 14, 14–20.




