
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed Zayed | -- | 3770 | 2023-04-19 12:45:12 | | | |
| 2 | Lindsay Dong | Meta information modification | 3770 | 2023-04-20 07:41:35 | | | | |
| 3 | Lindsay Dong | -34 word(s) | 3736 | 2023-04-20 07:44:16 | | |
Video Upload Options
Fucoidan is a heterogeneous group of polysaccharides isolated from marine organisms, including brown algae and marine invertebrates. The physicochemical characteristics and potential bioactivities of fucoidan have attracted substantial interest in pharmaceutical industries. These polysaccharides are characterized by possessing sulfate ester groups that impart negatively charged surfaces, low/high molecular weight, and water solubility. In addition, various promising bioactivities have been reported, such as antitumor, immunomodulatory, and antiviral effects. Hence, the formulation of fucoidan has been investigated in diverse pharmaceutical dosage forms to be able to reach their site of action effectively. Moreover, they can act as carriers for various drugs in value-added drug delivery systems.
1. Introduction
2. Biopharmaceutical Properties of Fucoidan
2.1. Mucoadhesive Properties
2.2. pH Response
2.3. Temperature Response
2.4. Enzymatic Response
2.5. Targeting Ligand
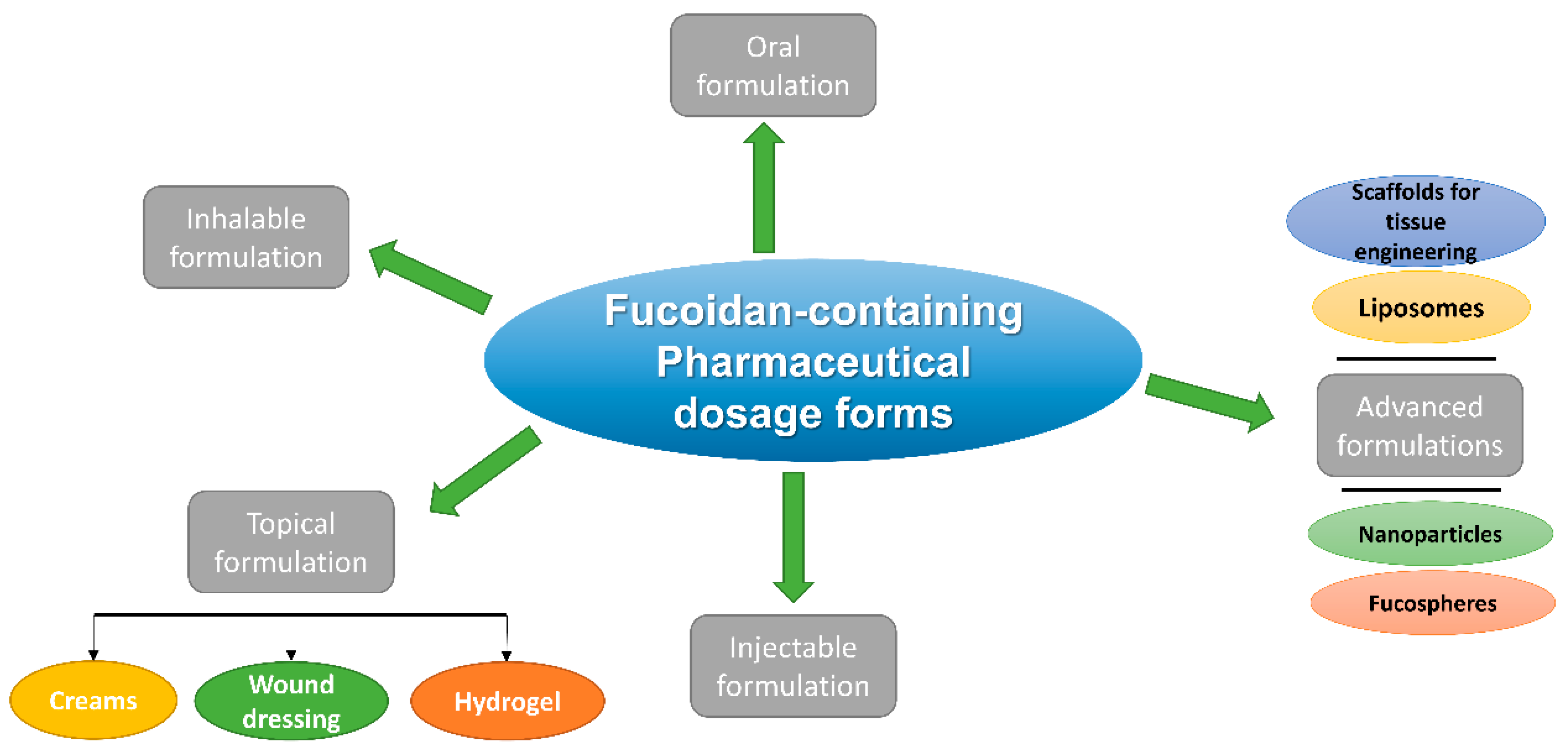
3. Pharmaceutical Dosage Forms of Fucoidan and Their Different Routes of Administration
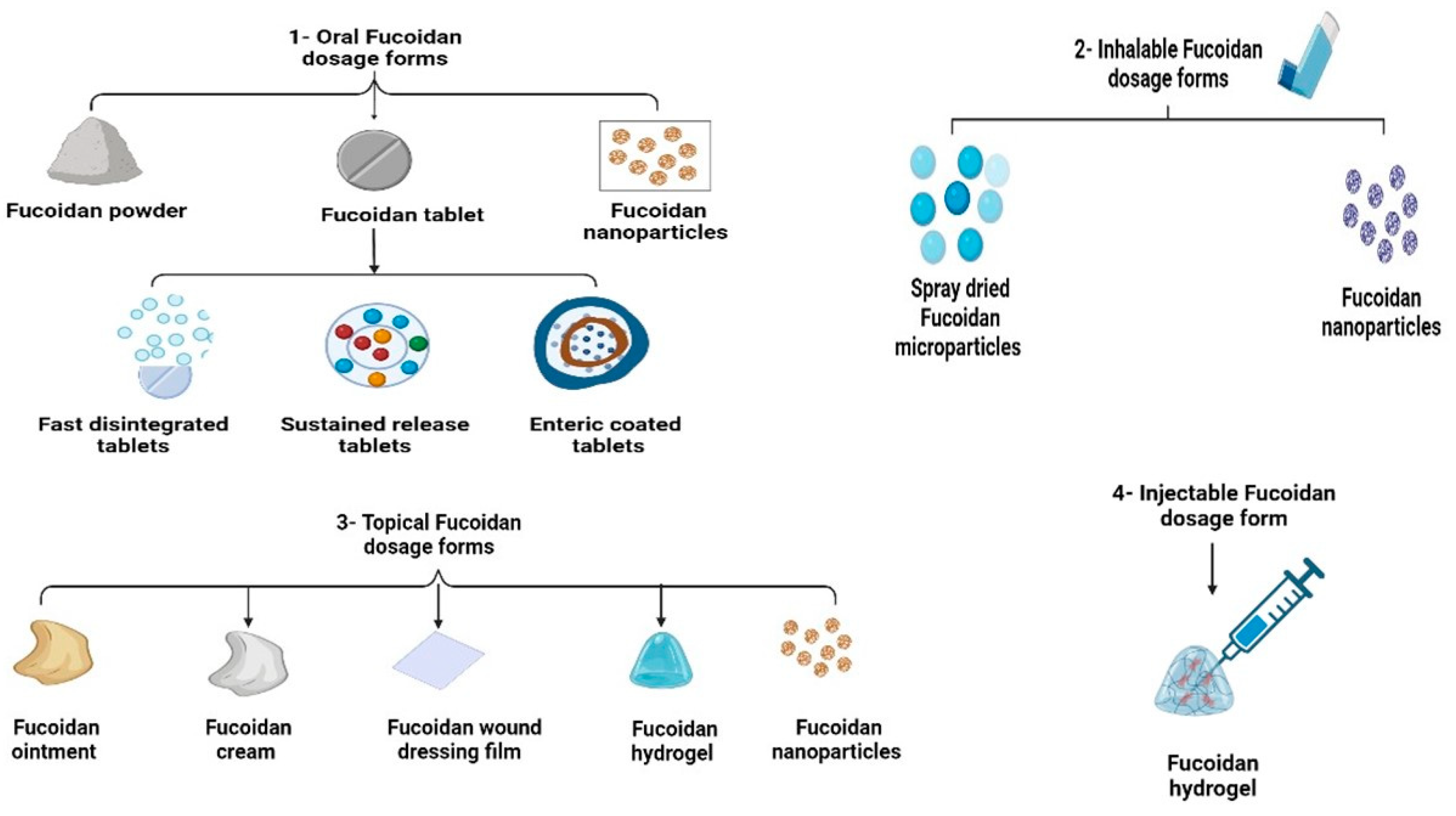
3.1. Oral Fucoidan Formulations
3.2. Inhalable Fucoidan Formulations
3.3. Topical Fucoidan Formulations
3.3.1. Fucoidan Creams
3.3.2. Fucoidan Wound Dressing Films
3.3.3. Fucoidan Topical Hydrogels
3.4. Injectable Fucoidan Formulations
3.5. Advanced Fucoidan Formulations
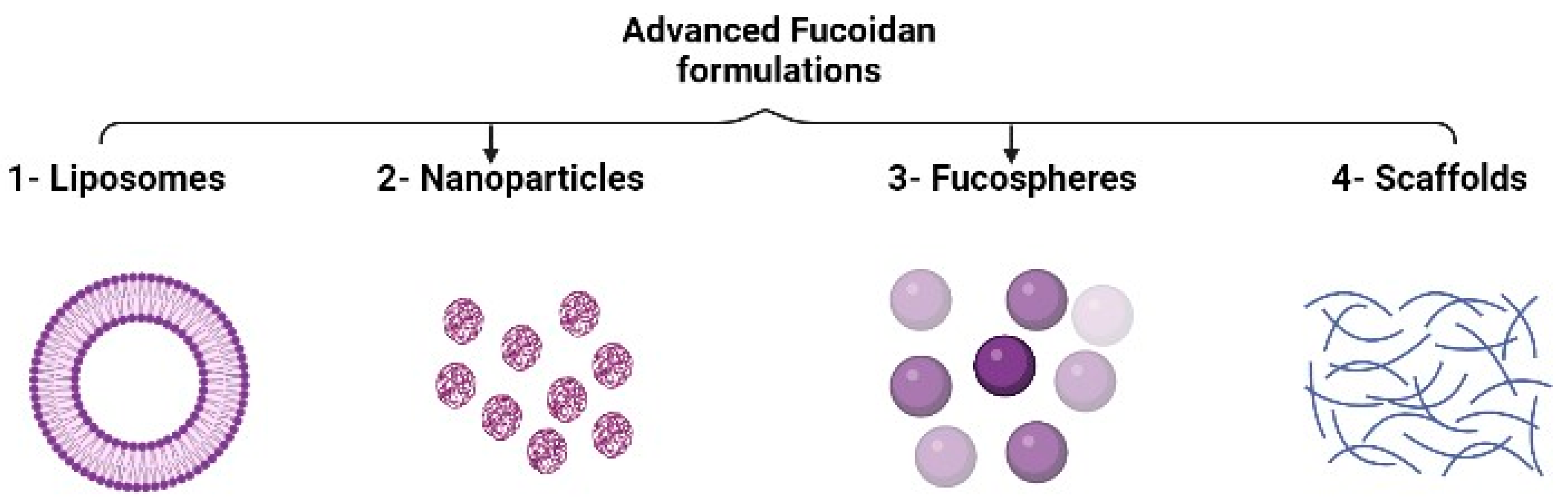
3.5.1. Liposomes
3.5.2. Nanoparticles
3.5.3. Fucospheres
3.5.4. Scaffolds for Tissue Engineering
4. Fucoidan Pharmacokinetics
References
- Zayed, A.; Dienemann, C.; Giese, C.; Krämer, R.; Ulber, R. An immobilized perylene diimide derivative for fucoidan purification from a crude brown algae extract. Process. Biochem. 2018, 65, 233–238.
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408.
- Contreras-esquivel, J.C.; Aguilar, O.; Ramos-de-la-pe, A.M. Structural and bioactive roles of fucoidan in nanogel delivery systems. A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100235.
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130.
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249.
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193.
- Benbow, N.L.; Karpiniec, S.; Krasowska, M.; Beattie, D.A. Incorporation of FGF-2 into Pharmaceutical Grade Fucoidan/Chitosan Polyelectrolyte Multilayers. Mar. Drugs 2020, 18, 531.
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628.
- Zayed, A.; Haggag, Y.; Ezzat, S.M.; Salem, M.A.; Ulber, R. Fucoidans as Nanoparticles: Pharmaceutical and Biomedical Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; ISBN 9780128223512.
- Zayed, A.; Finkelmeier, D.; Hahn, T.; Rebers, L.; Shanmugam, A.; Burger-Kentischer, A.; Ulber, R. Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds. Mar. Drugs 2023, 21, 48.
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42.
- Agarwal, S.; Aggarwal, S. Mucoadhesive Polymeric Platform for Drug Delivery; A Comprehensive Review. Curr. Drug Deliv. 2015, 12, 139–156.
- Coutinho, A.J.; Costa Lima, S.A.; Afonso, C.M.M.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 180–188.
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275.
- Sarangi, M.K.; Rao, M.E.B.; Parcha, V.; Yi, D.K.; Nanda, S.S. Marine Polysaccharides for Drug Delivery in Tissue Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128170557.
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458.
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47.
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159.
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-responsive hydrogels: From recent progress to biomedical applications. Gels 2021, 7, 77.
- Wang, N.; Tian, J.; Wang, L.; Song, S.; Ai, C.; Janaswamy, S.; Wen, C. Fucoidan hydrogels induced by κ-carrageenan: Rheological, thermal and structural characterization. Int. J. Biol. Macromol. 2021, 191, 514–520.
- Reyes, B.A.S.; Dufourt, E.C.; Ross, J.; Warner, M.J.; Tanquilut, N.C.; Leung, A.B. Selected Phyto and Marine Bioactive Compounds: Alternatives for the Treatment of Type 2 Diabetes, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 55, ISBN 9780444640680.
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29.
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 20, 188.
- Hee, J.M.; Kyong, S.P.; Mi, J.K.; Myeong, S.L.; Seok, H.J.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Yong, H.L. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734.
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712.
- Bachelet, L.; Bertholon, I.; Lavigne, D.; Vassy, R.; Jandrot-Perrus, M.; Chaubet, F.; Letourneur, D. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. BBA-Gen. Subj. 2009, 1790, 141–146.
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224.
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R. International Journal of Biological Macromolecules Antibacterial ef fi cacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495.
- Biosci, I.J.; Phull, A.; Ali, A.; Ahmed, M.; Zia, M.; Haq, I.; Kim, S.J. In vitro antileishmanial, antibacterial, antifungal and anticancer activity of fucoidan from undaria pinnatifida. Int. J. Biosci. 2017, 6655, 219–227.
- Yang, J.Y.; Lim, S.Y. Fucoidans and bowel health. Mar. Drugs 2021, 19, 436.
- Lee, M.; Huang, Y. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol. 2019, 131, 949–958.
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946.
- Tran, P.H.L.; Lee, B.J.; Tran, T.T.D. Current developments in the oral delivery of fucoidan. Int. J. Pharm. 2021, 598, 120371.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Optimization of the Composition and Production Technology of Fucoidan Tablets and their Biopharmaceutical Evaluation. Pharm. Chem. J. 2020, 54, 509–513.
- Huang, Y.Y.; Wang, C.H. Pulmonary delivery of insulin by liposomal carriers. J. Control. Release 2006, 113, 9–14.
- Valente, S.A.; Silva, L.M.; Lopes, G.R.; Sarmento, B.; Coimbra, M.A.; Passos, C.P. Polysaccharide-based formulations as potential carriers for pulmonary delivery—A review of their properties and fates. Carbohydr. Polym. 2022, 277, 118784.
- Cunha, L.; Rodrigues, S.; da Costa, A.M.R.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable fucoidan microparticles combining two antitubercular drugs with potential application in pulmonary tuberculosis therapy. Polymers 2018, 10, 636.
- Cunha, L.; Rosa da Costa, A.M.; Lourenço, J.P.; Buttini, F.; Grenha, A. Spray-dried fucoidan microparticles for pulmonary delivery of antitubercular drugs. J. Microencapsul. 2018, 35, 392–405.
- Fireman, S.; Toledano, O.; Neimann, K.; Loboda, N.; Dayan, N. A look at emerging delivery systems for topical drug products. Dermatol. Ther. 2011, 24, 477–488.
- Sharadha, M.; Gowda, D.V.; Vishal Gupta, N.; Akhila, A.R. An overview on topical drug delivery system–updated review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385.
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64.
- Iwamoto, K.; Hiragun, T.; Takahagi, S.; Yanase, Y.; Morioke, S.; Mihara, S.; Kameyoshi, Y.; Hide, M. Fucoidan suppresses IgE production in peripheral blood mononuclear cells from patients with atopic dermatitis. Arch. Dermatol. Res. 2011, 303, 425–431.
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606.
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Formulation, optimization and in vivo evaluation of fucoidan-based cream with anti-inflammatory properties. Mar. Drugs 2021, 19, 643.
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059.
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114.
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90.
- Sezer, A.D.; Cevher, E. Topical drug delivery using chitosan nano- and microparticles. Expert Opin. Drug Deliv. 2012, 9, 1129–1146.
- Sezer, A.D.; Cevher, E.; Hatipoǧlu, F.; Oǧurtan, Z.; Baş, A.L.; Akbuǧa, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333.
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349.
- Haggag, Y.; Abu Ras, B.; El-Tanani, Y.; Tambuwala, M.M.; McCarron, P.; Isreb, M.; El-Tanani, M. Co-delivery of a RanGTP inhibitory peptide and doxorubicin using dual-loaded liposomal carriers to combat chemotherapeutic resistance in breast cancer cells. Expert Opin. Drug Deliv. 2020, 17, 1655–1669.
- Qadir, S.A.; Kwon, M.C.; Han, J.G.; Ha, J.H.; Jin, L.; Jeong, H.S.; Kim, J.C.; You, S.G.; Lee, H.Y. Enhancement of immunomodulatory and anticancer activity of fucoidan by nano encapsulation. Food Sci. Biotechnol. 2008, 17, 1254–1260.
- Haggag, Y.A.; Yasser, M.; Tambuwala, M.M.; El Tokhy, S.S.; Isreb, M.; Donia, A.A. Repurposing of Guanabenz acetate by encapsulation into long-circulating nanopolymersomes for treatment of triple-negative breast cancer. Int. J. Pharm. 2021, 600, 120532.
- Zewail, M.B.; El-Gizawy, S.A.; Osman, M.A.; Haggag, Y.A. Preparation and In vitro characterization of a novel self-nano emulsifying drug delivery system for a fixed-dose combination of candesartan cilexetil and hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 61, 102320.
- Haggag, Y.A.; Abosalha, A.K.; Tambuwala, M.M.; Osman, E.Y.; El-Gizawy, S.A.; Essa, E.A.; Donia, A.A. Polymeric nanoencapsulation of zaleplon into PLGA nanoparticles for enhanced pharmacokinetics and pharmacological activity. Biopharm. Drug Dispos. 2021, 42, 12–23.
- Salem, M.A.; Manaa, E.G.; Osama, N.; Aborehab, N.M.; Ragab, M.F.; Haggag, Y.A.; Ibrahim, M.T.; Hamdan, D.I. Coriander (Coriandrum sativum L.) essential oil and oil-loaded nano-formulations as an anti-aging potentiality via TGFβ/SMAD pathway. Sci. Rep. 2022, 12, 1–15.
- Haggag, Y.A.; Osman, M.A.; El-Gizawy, S.A.; Goda, A.E.; Shamloula, M.M.; Faheem, A.M.; McCarron, P.A. Polymeric nano-encapsulation of 5-fluorouracil enhances anti-cancer activity and ameliorates side effects in solid Ehrlich Carcinoma-bearing mice. Biomed. Pharmacother. 2018, 105, 215–224.
- Haggag, Y.A.; Matchett, K.B.; Falconer, R.A.; Isreb, M.; Jones, J.; Faheem, A.; McCarron, P.; El-Tanani, M. Novel ran-RCC1 inhibitory peptide-loaded nanoparticles have anti-cancer efficacy in vitro and in vivo. Cancers 2019, 11, 222.
- Ibrahim, B.; Mady, O.Y.; Tambuwala, M.M.; Haggag, Y.A. PH-sensitive nanoparticles containing 5-fluorouracil and leucovorin as an improved anti-cancer option for colon cancer. Nanomedicine 2022, 17, 367–381.
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145.
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34.
- Sezer, A.D.; Akbuğa, J. Comparison on in vitro characterization of fucospheres and chitosan microspheres encapsulated plasmid DNA (pGM-CSF): Formulation design and release characteristics. AAPS PharmSciTech 2009, 10, 1193–1199.
- Suprunchuk, V.E. Low-molecular-weight fucoidan: Chemical modification, synthesis of its oligomeric fragments and mimetics. Carbohydr. Res. 2019, 485, 107806.
- Sezer, A.D.; Akbuǧa, J. Fucosphere-New microsphere carriers for peptide and protein delivery: Preparation and in vitro characterization. J. Microencapsul. 2006, 23, 513–522.
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide / Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, e1800221.
- Li, R.; McRae, N.L.; McCulloch, D.R.; Boyd-Moss, M.; Barrow, C.J.; Nisbet, D.R.; Stupka, N.; Williams, R.J. Large and Small Assembly: Combining Functional Macromolecules with Small Peptides to Control the Morphology of Skeletal Muscle Progenitor Cells. Biomacromolecules 2018, 19, 825–837.
- Zhang, W.; Sun, D.; Zhao, X.; Jin, W.; Wang, J.; Zhang, Q. Microanalysis and preliminary pharmacokinetic studies of a sulfated polysaccharide from Laminaria japonica. Chin. J. Oceanol. Limnol. 2016, 34, 177–185.
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557.




