You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Birgitte K. Ahring | -- | 3073 | 2023-04-19 00:22:18 | | | |
| 2 | Sirius Huang | Meta information modification | 3073 | 2023-04-19 03:06:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Harahap, B.M.; Ahring, B.K. Microbiology of Syngas-to-Acetate Fermentation. Encyclopedia. Available online: https://encyclopedia.pub/entry/43215 (accessed on 01 January 2026).
Harahap BM, Ahring BK. Microbiology of Syngas-to-Acetate Fermentation. Encyclopedia. Available at: https://encyclopedia.pub/entry/43215. Accessed January 01, 2026.
Harahap, Budi Mandra, Birgitte K. Ahring. "Microbiology of Syngas-to-Acetate Fermentation" Encyclopedia, https://encyclopedia.pub/entry/43215 (accessed January 01, 2026).
Harahap, B.M., & Ahring, B.K. (2023, April 18). Microbiology of Syngas-to-Acetate Fermentation. In Encyclopedia. https://encyclopedia.pub/entry/43215
Harahap, Budi Mandra and Birgitte K. Ahring. "Microbiology of Syngas-to-Acetate Fermentation." Encyclopedia. Web. 18 April, 2023.
Copy Citation
Biotransformation of lignocellulose-derived synthetic gas (syngas) into acetic acid is a promising way of creating biochemicals from lignocellulosic waste materials. Acetic acid has a growing market with applications within food, plastics and for upgrading into a wide range of biofuels and bio-products. This text described mesophilic and thermophilic microorganisms in axenic and culture that have the capability of syngas-to-acetate conversion, including their metabolic pathway.
lignocellulose
acetogens
acetic acid
syngas fermentation
1. Microorganism
Syngas fermentation for the production of biochemicals or biofuels predominantly involves autotrophic acetogens using the Wood–Ljungdahl pathway (WLP), also known as the reductive acetyl-coenzyme A (Acetyl-CoA) pathway [1]. Acetogens use C1 gases (CO and/or CO2) as electron acceptors, and CO and/or H2 as the electron donor for the reduction of CO2 into acetyl-CoA as an intermediate product for producing alcohols and various organic acids, including acetate. The acetate yield obtained during this fermentation is highly dependent on the type of microorganism used [2]. Thus, selecting the right microorganism is essential for obtaining high acetate yield and productivity.
1.1. Grow in Axenic Culture
The syngas fermentation by axenic culture can create reproducible data [3], and the process is also more easily controllable than mixed culture because a single type of microorganism in the axenic culture has consistent nutrient requirements, growth rate and other physiological characteristics of the cell [4]. Laboratory-scale research on syngas-to-acetate fermentation has, therefore, often used pure cultures. The fermentation by pure culture could use mesophilic or thermophilic bacteria. The classification of the different acetogens, along with their optimum growth conditions and by-products produced besides acetate, are shown in Table 1.
Table 1. Carboxydotrophic microorganisms.
| Microorganism | Opt. Temp. [°C] | Opt. pH [-] | Other Dominant Products | Ref. |
|---|---|---|---|---|
| Mesophilic bacteria | ||||
| Clostridium ljungdahlii | 37 | 6–7 | Ethanol, lactate, 2,3-butanediol | [5][6] |
| Clostridium carboxidivorans | 37 | 6 | Ethanol, butyrate, butanol, hexanoate, hexanol | [7][8] |
| Clostridium ragsdalei | 37 | 6 | Ethanol, lactate, 2,3-butanediol | [9][10] |
| Clostridium autoethanogenum | 37 | 6.5 | Ethanol, lactate, 2,3-butanediol | [11][12] |
| Clostridium aceticum | 30 | 8–8.5 | - | [13][14] |
| Clostridium drakei | 25–30 | 5.8–6.9 | Ethanol, butyrate | [15] |
| Acetobacterium woodii | 30 | 7.0 | - | [16] |
| Peptostreptococcus productus | 37 | 7.0 | - | [17][18] |
| Alkalibaculum bacchii | 37 | 8.0–8.8 | Ethanol | [19] |
| Butyribacterium methylotrophicum | 37 | 7.0 | Ethanol, butyrate, butanol | [20] |
| Eubacterium limosum | 37 | 7.0 | - | [21] |
| Oxobacter pfennigii | 36–38 | 7.3 | Butyrate | [22] |
| Thermophilic bacteria | ||||
| Moorella thermoacetica | 55 | 6.5–6.8 | - | [4] |
| Desulfotomaculum kuznetsovii | 60 | 7.0 | H2S | [23][24] |
| Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum | 55 | 7.0 | H2S | [24][25] |
| Mesophilic archaea | ||||
| Methanosarcina acetivorans | 37 | 7 | CH4, formate | [26] |
| Thermophilic archaea | ||||
| Archaeoglobus fulgidus | 83 | 6.4 | Formate, H2S | [27][28] |
Compared to thermophilic autotrophic bacteria, more mesophilic autotrophic bacteria have been isolated and further studied for their performance during the fermentation of C1 gases (CO and CO2) into various biochemical products (Table 1). The advantages of mesophilic acetogens for syngas-to-acetate fermentation over thermophilic acetogens are lower energy consumption for process heating and more genetic tools available for the genetic engineering of these strains. Most mesophilic acetogens described can both live autotrophically using inorganic compounds such as CO2 or CO as the carbon source or live heterotrophically on organic compounds such as Clostridium ljungdahlii, C. carboxidivorans, C. ragsdalei, C. autoethanogenum, C. drakei, Alkalibaculum bacchii, Butyribacterium methylotrophicum, and Oxobacter pfennigii. The products produced are either ethanol, butyrate, butanol, lactate, or 2,3-butanediol apart from acetate. For instance, C. carboxidivorans, shown in Figure 1, accumulated butyrate, butanol, and ethanol and then released these products outside the cell. The stoichiometric reaction and Gibbs free energy related to the production of these products can be seen in Table 2. As a consequence of the production of other products besides acetate, the yield of acetate will decline.
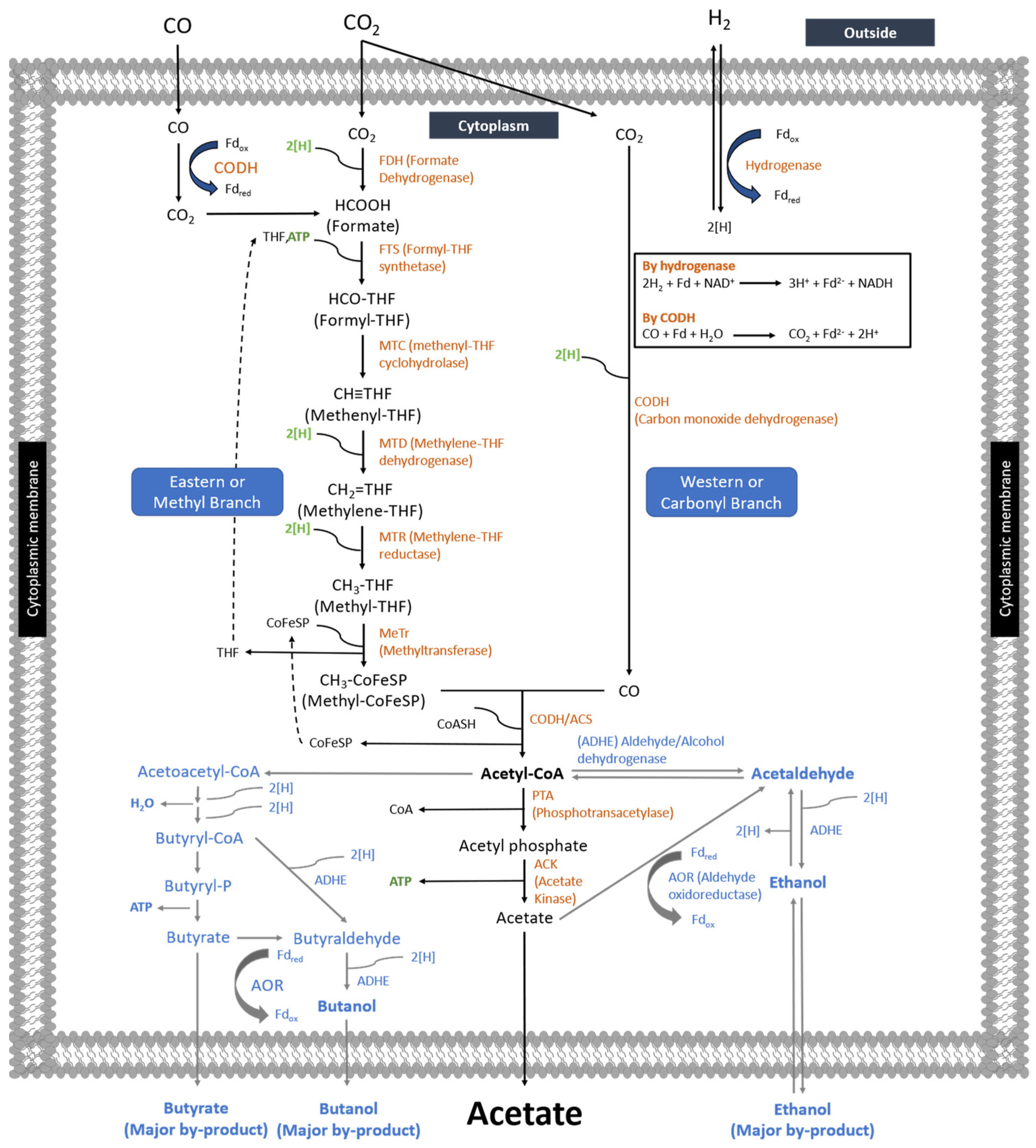
Figure 1. Wood–Ljungdahl Pathway of C. carboxidivorans using C1 gases (CO and CO2) [8].
Table 2. The potential reaction of acetic acid, ethanol, butyric acid, butanol, hexanoic acid, and hexanol [27][28].
| Product | Reactions | ∆G° [kJ/mol] |
|---|---|---|
| Acetic Acid | 4 CO + 2 H2O → CH3COOH + 2 CO2 | −154.6 |
| 4 H2 + 2 CO2 → CH3COOH + 2 H2O | −74.3 | |
| 2 CO + 2 H2 → CH3COOH | −114.5 | |
| Ethanol | 6 CO + 3 H2O → C2H5OH + 4 CO2 | −217.4 |
| 6 H2 + 2 CO2 → C2H5OH + 3 H2O | −97.0 | |
| 2 CO + 4 H2 → C2H5OH + H2 | −137.1 | |
| 3 CO + 3 H2 → C2H5OH + CO2 | −157.2 | |
| Butyric Acid | 10 CO + 4 H2O → CH3(CH2)2COOH + 6 CO2 | −420.8 |
| 10 H2 + 4 CO2 → CH3(CH2)2COOH + 6 H2O | −220.2 | |
| 6 CO + 4 H2 → CH3(CH2)2COOH + 2 CO2 | −317 | |
| Butanol | 12 CO + 5 H2O → C4H9OH + 8 CO2 | −486.4 |
| 12 H2 + 4 CO2 → C4H9OH + 7 H2O | −245.6 | |
| 6 CO + 6 H2 → C4H9OH + 2 CO2 + H2O | −373 | |
| 4 CO + 8 H2 → C4H9OH + 3 H2O | −334 | |
| Hexanoic Acid | 16 CO + 6 H2O → CH3(CH2)4COOH + 10 CO2 | −656 |
| 16 H2 + 6 CO2 → CH3(CH2)4COOH + 10 H2O | −341 | |
| 16 CO + 6 H2O → CH3(CH2)4COOH + 4 CO2 | 540 | |
| Hexanol | 18 CO + 7 H2O → C6H13OH + 12 CO2 | −753 |
| 18H2 + 6 CO2 → C6H13OH + 11 H2O | −395 | |
| 6 CO + 12 H2 → C6H13OH + 5 H2O | −514 |
A batch fermentation test of C. carboxidivorans using 2.2 atm CO gas maximally yielded 1.87 g/L acetate and formed several by-products such as butyrate (0.23 g/L), caproate (0.09 g/L), ethanol (0.19 g/L) and butanol (0.075 g/L) [29]. Meanwhile, batch fermentation by C. ljungdahlii maximally generated 2 g/L acetate using 2.0 H2/CO ratio, 0.35 g/L ethanol, and 0.5 g/L 2,3-butanediol using 0.5 H2/CO ratio [30]. The other mesophilic syngas bacteria, such as C. ragsdalei, C. autoethanogenum, C. drakei, A. bacchii, B. methylotrophicum, and O. pfennigii maximally produced acetate up to 12.3 g/L [31], 5.33 g/L [32], 0.5 g/L [33], 1.2 g/L [34], 1.3 g/L [35], and 0.16 g/L [36], respectively.
On the other hand, several mesophilic bacteria are categorized as autotrophic homoacetogens, such as C. aceticum, A. woodii, P. productus, and E. limosum, solely producing acetate with a maximum concentration of 7.2 g/L [37], 3.17 g/L [38], 3.2 g/L [39], and 0.5 g/L [40], respectively. These strains are, therefore, of special interest for large-scale acetate production.
In addition to acetogenic bacteria, methanogenic archaea have also been investigated for acetate production besides methane, formate, and methylated thiols from C1 gases. Methanosarcina acetivorans is, until now, the only acetate-producing archaea successfully identified, and the concentration of acetate produced was low (0.06 g/L) [41]. Rother [25] reported that acetate and formate are the primary metabolites of these archaea over methane. Acetate might, therefore, be produced by the multienzyme complex of CO dehydrogenase (CODH)/acetyl coenzyme A synthase (ACS), converting CO and CO2 into acetyl-CoA, an intermediate product for acetate formation and other metabolites [23]. The CO dissolved in the fermentation broth is oxidized to form CO2, and then the electron released from this oxidation step is used for CO2-to-a methyl group reduction and ultimately towards acetate synthesis [24].
Thermophilic microorganisms for syngas fermentation have recently attracted increased attention due to the higher growth rate of thermophiles compared to mesophiles. Furthermore, higher temperatures minimize the risk of contamination [42]. The thermophilic bacteria that have been reported to have the capability of syngas-to-acetate production include M. thermoacetica, previously known as Clostridium thermoaceticum, as well as Desulfotomaculum kuznetsovii.
The thermophilic homoacetogen of M. thermoacetica is classified as a gram-positive bacterium that can utilize both CO and CO2 to produce acetate [43][44]. Furthermore, unlike most mesophilic microorganisms that generate higher alcohols, acetate is the only product from syngas fermentation by M. thermoacetica [45]. This thermophilic bacterium was able to grow in various syngas blend compositions such as H2/CO2, 100% CO, and CO/CO2 fermentation [46], and the results of all these experiments showed that acetate could be produced (Table 3). For instance, Kerby and Zeikus reported that C. thermoacetium deposited by Fontaine et al. (currently named M. thermoacetica DSM 2955T or ATCC 35608) rapidly grew on H2/CO2 and needed adaptation to grow on CO [47]. This bacterium strain stoichiometrically required 4.44 mol H2 and 2.33 mol CO2 to produce 1 mol acetate or 3.64 mol CO to produce 1 mol acetate. Another strain (M. thermoacetica DSM 521T) can further oxidize CO to CO2, followed by CO2 reduction to acetate [8][48]. A mutant strain of M. thermoacetica (currently deposited as M. thermoacetica ATCC 49707 and DSM 6867) derived from Clostridium thermoaceticum ATCC 39289 has been described [49]. This Moorella thermoacetica strain generated up to 31 g/L acetic acid on 70% CO/30% CO2 at pH 6.0 in a bubble column bioreactor, a higher concentration as found with 40% CO/30% H2/30% CO2 fermentation of 26 g/L [50]. Genetic modification of M. thermoacetica is eased by the fact that the genome of this strain has been fully sequenced, as reported for M. thermoacetica DSM 521T [51] and DSM 2955T [51]. Hence, this bacterium is a good candidate for acetate production from syngas.
Another species of Moorella formerly named Clostridium thermoautotrophicum JW701/3 (M. thermoautotrophica ATCC 33924 and DSM 1974) isolated by Wiegel et al. could utilize CO2/H2 or CO for cell and acetate production [52]. Furthermore, Savage et al. reported that faster CO-dependent growth of this species of Moorella was reached by supplementing CO2 into the headspace [46]. However, M. thermoautotrophica is currently proposed to be on the list of rejected names because, based on phylogenetic and genomic analysis, M. thermoautotrophica and M. thermoacetica are closely related and show genetic similarities at the species level [53][54].
Table 3. Comparison of acetate production by several strains of M thermoacetica, their syngas fermentation conditions, and bioreactor type.
| Strain | Syngas Type and Composition [-] |
Additional Carbon Source [g/L] |
Temp. [°C] | pH [-] |
Bioreactor Type [-] |
Acetate Production | Ref. |
|---|---|---|---|---|---|---|---|
| DSM 2955 or ATCC 35608 | 20% CO2/80% H2 100% CO |
- | 55 | 7 | Bottle | 4.72 g/L 4.49 g/L |
[47] |
| DSM 521 | 100% CO2 20% CO2/80% H2 100% CO |
18 g/L glucose - 20 g/L glucose |
55 | 7 | Bottle | NA | [8][48] |
| DSM 6867 or ATCC 49707 | 40% CO/30% H2/30% CO2 70% CO/30% CO2 |
- | 60 | 6 | Bubble column bioreactor | 26 g/L 31 g/L |
[50] |
| DSM 1974 or ATCC 33924 | 20% CO2/80% H2 | - | 56–60 | 5.7 | Bottle | 3.78 g/L | [52] |
| ATCC 39073 | 0.4 L/min CO2 | 10 g/L glucose | 60 | 6.5 | Stirred tank bioreactor with continuous syngas flow | 9.3 g/L | [55] |
| JCM 9320 | 100% CO2 | - | 55 | 7.0 | Microbial electrosynthesis | 0.024 g/L/day | [56] |
Other potential thermophilic bacteria are D. kuznetsovii and D. thermobenzoicum subsp. thermosyntrophicum, capable of growing autotrophically on mixtures of gases of H2/CO2 [57][58]. Both bacteria can further reduce sulfate (SO4−)/sulfite (SO3) while utilizing CO and H2 as the electron donor to generate H2S [9]. Moreover, besides H2S, acetate will be produced up to 0.4 g/L for D. thermobenzoicum subsp. thermosyntrophicum and 0.44 g/L for D. kuznetsovii using CO. Hence, the use of this microorganism brings about two functions: acetate production as well as the removal of SO4−.
Archaea such as Archaeoglobus fulgidus have also been reported to use CO, apart from CO2, as the carbon source for growth [59]. The presence of CO led to accumulation of CO2, acetate (18 mM), and formate (8.2 mM), while no H2 was detected. Another study also revealed that strains of this archaea genus effectively reduce SO4− with CO as an electron donor [60] but have less need for the sulfurous compound for growth [59].
1.2. Mixed Culture
Anaerobic fermentation by mixed cultures has further shown to be effective in producing acetate with high yields. Singla [61] mentioned that using mixed cultures has several benefits, such as no need for sterilization, more adaptive microorganisms that works synergistically together due to the microbial diversity along with the capability to utilize mixed substrates with lower operation costs, and long-term stability of growth. In addition, mixed cultures were found to tolerate higher variations in the syngas composition compared to pure cultures, where consistency often is a must [4]. Furthermore, acetate yields of up to 42 g/L have been reported by Wang [62] during mixed culture fermentation.
Syngas fermentation for acetate production by mixed cultures has been tested with both mesophilic and thermophilic inocula (Table 4) [63]. Wang reported that syngas fermentation with an inoculum containing thermophilic bacteria dominated by the genera Thermoanaerobacterium and Thermohydrogenium effectively produced acetate in both batch and continuous culture [62]. Similar studies were done with a mesophilic inoculum dominated by strains belonging to the Clostridium genus at temperatures from 35 to 37 °C [19][64][65][66]. Even though methanogens generally outcompete acetogens on CO2 and H2 under both mesophilic and thermophilic conditions, adjustment of the fermentation temperature and/or pH, or the addition of 2-Bromoethane sulfonic acid (BES) during start-up can inhibit the activity of methanogens and make acetogens prevail over methanogens [67].
Table 4. Acetate production from syngas by mixed culture inoculum.
| Inoculum Source | Dominant Microorganism |
Temp. [°C] | pH [-] |
Acetate Concentration [g/L] | Ref. |
|---|---|---|---|---|---|
| Sludge from a starch-containing biogas reactor | Thermoanaerobacterium and Thermohydrogenium | 55 | 6.0 | 42.4 | [62] |
| Mesophilic methane production reactor containing glucose and acetate | Clostridium spp. (C. ljungdahlii and C. drakei) | 35 | 4.5–4.8 | 12.5 | [65] |
| Mesophilic USAB reactor for cassava stillage waste treatment | Not applicable | 37 | 6.5–7.5 | 1.3 | [66] |
| Sewage sludge from a sewage treatment plant | Clostridium and Acetobacterium | 37 | 9.0 | 3.1 | [64] |
| Waste activated sludge | Natronincola and Desulfitispora | 37 | 9.0 | 8.1 | [19] |
2. C1 Metabolism by Acetogens
Homoacetogens are acetogens who can live solely metabolizing syngas (CO, CO2, and H2) through two branches of the Wood–Ljungdahl Pathway: the eastern or western branch to form acetyl-CoA as an intermediate ending as acetate (Figure 1). Furthermore, other products such as volatile fatty acids (butyrate and hexanoate), alcohol (ethanol, 2,3-butanediol, butanol, hexanol), and lactate can be produced. In general, one molecule of CO2 is reduced by six electrons with 1 ATP to form methyl tetrahydrofolate (CH3-THF) in the eastern branch, whereas the C1 substrate is directly converted into acetyl-CoA using 2 electrons in the western branch [68]. The acetyl-CoA formed is then converted to acetate while releasing ATP by substrate-level phosphorylation (SLP). The electron used during Wood–Ljungdahl Pathway comes from CO and H2 oxidation, whereas CO2 and CO serve as carbon sources.
2.1. CO Metabolism
Carbon monoxide (CO), a major gas generated from gasification, is generally a toxic gas for most microorganisms because this gas has a high affinity for metal-containing enzymes, causing the inactivation of these enzymes. However, some bacteria have high resistance to CO and are named carboxydotrophic acetogens (see Table 1). These microbes are capable of CO oxidation to generate electrons and provide reducing equivalents catalyzed by carbon monoxide dehydrogenase (CODH) while transferring the formed electron to Ferredoxin (Fd) or other electron acceptors [69].
In carboxydotrophic acetogen metabolism, CO can act as both a carbon source and an electron source. Meanwhile, H2 only serves as an electron donor and requires a carbon source obtained from CO and/or CO2 [70]. The electrons or reducing equivalents are generated from biological water gas shift reaction (BWGSR) of CO (see Equation (1)) or hydrogen oxidation (see Equation (2)) that will further be used for CO2 reduction through the eastern and western branches of Wood–Ljungdahl Pathway [27]. When fermentation is performed in the absence of H2, only half of the carbon in CO is transformed into acetic acid, whereas the remaining carbon of CO is converted into CO2 (see Equation (3)). On the other hand, when H2 is available in the fermentation media, two mol CO2 is required to form one mol acetic acid [71] (see Equation (4)). Such CO2 is obtained from CO oxidation or supplying CO2 gas into the fermentation system.
CO + H2O → CO2 + 2H+ + 2e−
H2 → 2H+ + 2e−
4 CO + 2 H2O → CH3COOH + 2CO2
2 CO2 + 4 H2 → CH3COOH + 2 H2O
CO-to-acetic acid conversion through Wood–Ljungdahl Pathway is catalyzed by oxidoreductase enzymes. For instance, the enzyme that plays an essential role in CO metabolism to acetate is carbon monoxide dehydrogenase (CODH). According to its role in the metabolic pathway, CODH is classified into a monofunctional CODH and a bifunctional CODH/acetyl CoA synthase (ACS). Monofunctional CODH plays a role in catalyzing reversible CO-to-CO2 oxidation, while bifunctional CODH, together with ACS, is involved in the reaction of CO, methyl-CoFeSP, and CoASH to form acetyl CoA [72][73]. The methyl-CoFeSP is formed by transferring the methyl group generated from the eastern branch of Wood–Ljungdahl Pathway to corrinoid/iron-sulfur protein (CoFeSP) catalyzed by a methyltransferase (MeTr); the mechanism can be seen in Figure 1. The catalytic reactions of both monofunctional CODH and bifunctional CODH/ACS are shown in Equations (11) and (12) of Table 5, respectively.
Table 5. Detailed metabolic reactions of Wood–Ljungdahl Pathway (WLP).
| Eq. | Reaction | Enzymes | Ref. |
|---|---|---|---|
| The Eastern Branch of WLP | |||
| (5) | NADPH + CO2 + H+ ↔ NADP+ + HCOOH (formate) | Formate dehydrogenase (FDH) (EC 1.2.1.43) | [1] |
| (6) | HCOOH (formate) + ATP + H4folate ↔ 10-HCO-H4folate (formyl-THF) + ADP + Pi | Formyl-THF synthetase (FTS) (EC 6.3.4.3) | |
| (7) | 10-HCO-H4folate (formyl THF) + H+ ↔ 5,10-methenyl-H4folate (methenyl-THF) | Methenyl-THF cyclohydrolase (MTC) (EC 3.5.4.9) |
|
| (8) | NAD(P)H + 5,10-methenyl-H4folate (methenyl-THF) ↔ 5,10-methylene-H4folate (methylene-THF) + NAD(P) | Methylene-THF dehydrogenase (MTD) (EC 1.5.1.5, NADP; EC 1.5.1.15, NAD) |
|
| (9) | 2e− + H+ + 5,10-methylene-H4folate ↔ 5-methyl-H4folate (methyl-THF) | Methylene-THF reductase (MTR) (EC 1.1.99.15) |
|
| The Western Branch of WLP | |||
| (10) | CoFeSP (Corrinoid iron-sulfur protein) + 5-methyl-H4folate (methyl-THF)↔ methyl-CoFeSP + H4folate (THF) | Methyl-H4folate:CoFeSP methyltransferase (MeTR) (EC 2.1.1.X) | [72][73] |
| (11) | CO2 + 2H+ + 2e− ↔ CO + H2O | Monofunctional CO dehydrogenase (CODH) (EC 1.2.7.4) | |
| (12) | Methyl-CoFeSP + CO + CoASH → CH3COSCoA (Acetyl-CoA) + CoFeSP | Bifunctional CO dehydrogenase (CODH (EC 1.2.7.4)/Acetyl CoA synthase (ACS) (EC 6.2.1.1) |
|
| Acetyl CoA-to-Acetate Pathway | |||
| (13) | CH3COSCoA (Acetyl-CoA) + HPO42− → CH3CO2PO32− (Acetyl phosphate) + CoASH | Phosphotransacetylase (PTA) (EC 2.3.1.8) | [5] |
| (14) | CH3CO2PO32− (Acetyl phosphate) + ADP → CH3CO2PO32− (Acetate) + ATP + H+ | Acetate Kinase (ACK) (EC 2.7.2.1) | |
The enzymatic reaction mechanism of CODH was proposed by Can [74] as a ping-pong reaction. The metal center (Ni for most of the acetogens including M. thermoacetica) of CODH Cred1 in the C-cluster bind CO by releasing one H+ and forming Cred1-CO2, then reacting with H2O by releasing another H+ and CO2 to form Cred2. The electron produced is then transferred to ferredoxin to re-form Cred1, as shown in Figure 2.
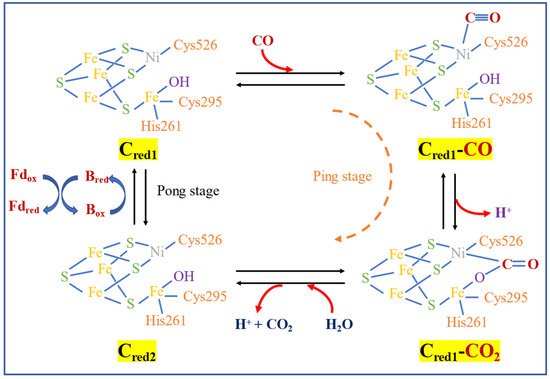
Figure 2. The ping pong reaction of CODH [74].
Some studies have successfully purified and characterized both enzymes from anaerobic carboxydotrophic acetogens and attempted to describe the structure [75]. The CODH in anaerobic acetogens is structured by a complex metal center consisting of nickel (Ni), iron (Fe), and sulfur (S), serving as CO and H2O reaction center, intermediary metal-carboxylate stabilization, and temporary storage of two electrons from the reaction [76]. On the other hand, aerobic carboxydotrophic bacteria employ Cu, Mo, and Fe-containing flavoenzymes [73]. Doukov [77] described the biofunctional CODH/ACS. In M. thermoacetica, this is composed of iron (Fe), sulfur (S), copper (Co), and nickel (Ni) metallocofactor. Another study mentioned that the active site of both enzymes, the C-cluster and A-cluster for CODH and ACS, respectively, has a [Fe4S4] cluster connected to Ni [75].
2.2. CO2 Metabolism
Chemolithoautotrophic acetogens utilize inorganic carbon (CO2) as the carbon source for product and cell formation through the eastern and western branches of WLP [1]. In the western branch, the conversion of CO2 into acetate involves several enzymes and proteins that are successfully identified: (1) CODH, (2) CODH/ACS, (3) corrinoid iron-sulfur protein (CoFeSP), two subunits, (4) MeTr, whereas the enzymes in eastern branch involve: (1) FDH, (2) FTS, (3) MTC, (4) MTD, and (5) MTR [72][73]. According to Figure 1, when acetogens grow with CO2 and H2 without CO in the fermentation media, CODH catalyzes the reduction of CO2 into CO in the western or carbonyl branch using the reducing equivalents produced from hydrogen oxidation. Meanwhile, CO2 is further reduced to form formate in the eastern branch. The formate formed in the last reaction is a precursor of the methyl groups of acetyl Co-A.
In the eastern branch, the formate formed from a two-electron reduction of CO2 is condensed with THF, involving ATP to form formyl-THF [1]. The formed formyl-THF subsequently undergoes cyclization to methenyl-THF catalyzed by MTC. Consecutive reduction then occurs of methenyl-THF to form methylene-THF, followed by methylene-THF to form methyl-THF. The CH3-THF is then transferred to the cobalt center of CoFeSP for CH3-CoFeSP formation. Together with CO, CH3-CoFeSP undergoes condensation to produce acetyl-CoA, then further reaction to form acetate. Finally, the formation of acetate is catalyzed by two enzymes, PTA and ACK, by generation ATP [5]. The detailed metabolic pathway and biochemical reaction of CO2 can be seen in Figure 1 and Table 5, respectively.
References
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl Pathway of CO2 Fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898.
- Bengelsdorf, F.R.; Straub, M.; Dürre, P. Bacterial Synthesis Gas (Syngas) Fermentation. Environ. Technol. 2013, 34, 1639–1651.
- Blaser, M.B.; Dreisbach, L.K.; Conrad, R. Carbon Isotope Fractionation of 11 Acetogenic Strains Grown on H2 and CO2. Appl. Environ. Microbiol. 2013, 79, 1787–1794.
- Esquivel-Elizondo, S.; Delgado, A.G.; Rittmann, B.E.; Krajmalnik-Brown, R. The Effects of CO2 and H2 on CO Metabolism by Pure and Mixed Microbial Cultures. Biotechnol. Biofuels 2017, 10, 220.
- Boynton, Z.L.; Bennett, G.N.; Rudolph, F.B. Cloning, Sequencing, and Expression of Clustered Genes Encoding β-Hydroxybutyryl-Coenzyme A (CoA) Dehydrogenase, Crotonase, and Butyryl-CoA Dehydrogenase from Clostridium Acetobutylicum ATCC 824. J. Bacteriol. 1996, 178, 3015–3024.
- Sathish, A.; Sharma, A.; Gable, P.; Skiadas, I.; Brown, R.; Wen, Z. A Novel Bulk-Gas-to-Atomized-Liquid Reactor for Enhanced Mass Transfer Efficiency and Its Application to Syngas Fermentation. Chem. Eng. J. 2019, 370, 60–70.
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical Factors Affecting the Integration of Biomass Gasification and Syngas Fermentation Technology. AIMS Bioeng. 2016, 3, 188–210.
- Huang, H.; Wang, S.; Moll, J.; Thauer, R.K. Electron Bifurcation Involved in the Energy Metabolism of the Acetogenic Bacterium Moorella Thermoacetica Growing on Glucose or H2 plus CO2. J. Bacteriol. 2012, 194, 3689–3699.
- Parshina, S.N.; Sipma, J.; Nakashimada, Y.; Henstra, A.M.; Smidt, H.; Lysenko, A.M.; Lens, P.N.L.; Lettinga, G.; Stams, A.J.M. Desulfotomaculum carboxydivorans Sp. Nov., a Novel Sulfate-Reducing Bacterium Capable of Growth at 100% CO. Int. J. Syst. Evol. Microbiol. 2005, 55, 2159–2165.
- Asimakopoulos, K.; Gavala, H.N.; Skiadas, I.V. Reactor Systems for Syngas Fermentation Processes: A Review. Chem. Eng. J. 2018, 348, 732–744.
- Xu, S.; Fu, B.; Zhang, L.; Liu, H. Bioconversion of H2/CO2 by Acetogen Enriched Cultures for Acetate and Ethanol Production: The Impact of PH. World J. Microbiol. Biotechnol. 2015, 31, 941–950.
- Zhang, L.; Hu, P.; Pan, J.; Yu, H.; Xu, J. Immobilization of Trophic Anaerobic Acetogen for Semi-Continuous Syngas Fermentation. Chin. J. Chem. Eng. 2021, 29, 311–316.
- Lee, M.; Yasin, M.; Jang, N.; Chang, I.S. A Simultaneous Gas Feeding and Cell-Recycled Reaction (SGCR) System to Achieve Biomass Boosting and High Acetate Titer in Microbial Carbon Monoxide Fermentation. Bioresour. Technol. 2020, 298, 122549.
- Gunes, B. A Critical Review on Biofilm-Based Reactor Systems for Enhanced Syngas Fermentation Processes. Renew. Sustain. Energy Rev. 2021, 143, 110950.
- Shen, N.; Dai, K.; Xia, X.Y.; Zeng, R.J.; Zhang, F. Conversion of Syngas (CO and H2) to Biochemicals by Mixed Culture Fermentation in Mesophilic and Thermophilic Hollow-Fiber Membrane Biofilm Reactors. J. Clean. Prod. 2018, 202, 536–542.
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A Comparison of Mass Transfer Coefficients between Trickle-Bed, Hollow Fiber Membrane and Stirred Tank Reactors. Bioresour. Technol. 2013, 133, 340–346.
- Mederos, F.S.; Ancheyta, J.; Chen, J. Review on Criteria to Ensure Ideal Behaviors in Trickle-Bed Reactors. Appl. Catal. A Gen. 2009, 355, 1–19.
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous Ethanol Production from Synthesis Gas by Clostridium Ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23.
- Rao, Y.; Wan, J.; Liu, Y.; Angelidaki, I.; Zhang, S.; Zhang, Y.; Luo, G. A Novel Process for Volatile Fatty Acids Production from Syngas by Integrating with Mesophilic Alkaline Fermentation of Waste Activated Sludge. Water Res. 2018, 139, 372–380.
- Schulte, M.J.; Wiltgen, J.; Ritter, J.; Mooney, C.B.; Flickinger, M.C. A High Gas Fraction, Reduced Power, Syngas Bioprocessing Method Demonstrated with a Clostridium ljungdahlii OTA1 Paper Biocomposite. Biotechnol. Bioeng. 2016, 113, 1913–1923.
- López, L.R.; Bezerra, T.; Mora, M.; Lafuente, J.; Gabriel, D. Influence of Trickling Liquid Velocity and Flow Pattern in the Improvement of Oxygen Transport in Aerobic Biotrickling Filters for Biogas Desulfurization. J. Chem. Technol. Biotechnol. 2016, 91, 1031–1039.
- Mathure, P.; Patwardhan, A. Comparison of Mass Transfer Efficiency in Horizontal Rotating Packed Beds and Rotating Biological Contactors. J. Chem. Technol. Biotechnol. 2005, 80, 413–419.
- Matschiavelli, N.; Oelgeschläger, E.; Cocchiararo, B.; Finke, J.; Rother, M. Function and Regulation of Isoforms of Carbon Monoxide Dehydrogenase/Acetyl Coenzyme A Synthase in Methanosarcina Acetivorans. J. Bacteriol. 2012, 194, 5377–5387.
- Lessner, D.J.; Li, L.; Li, Q.; Rejtar, T.; Andreev, V.P.; Reichlen, M.; Hill, K.; Moran, J.J.; Karger, B.L.; Ferry, J.G. An Unconventional Pathway for Reduction of CO2 to Methane in CO-Grown Methanosarcina Acetivorans Revealed by Proteomics. Proc. Natl. Acad. Sci. USA 2006, 103, 17921–17926.
- Rother, M.; Metcalf, W.W. Anaerobic Growth of Methanosarcina Acetivorans C2A on Carbon Monoxide: An Unusual Way of Life for a Methanogenic Archaeon. Proc. Natl. Acad. Sci. USA 2004, 101, 16929–16934.
- Xu, D.; Tree, D.R.; Lewis, R.S. The Effects of Syngas Impurities on Syngas Fermentation to Liquid Fuels. Biomass Bioenergy 2011, 35, 2690–2696.
- Vega, J.L.; Prieto, S.; Harrison, S.B.; Clausen, E.C.; Gaddy, J.L. The Biological Production of Ethanol from Synthesis Gas. Appl. Biochem. Biotechnol. 1989, 20, 781–791.
- Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. H-B-E (Hexanol-Butanol-Ethanol) Fermentation for the Production of Higher Alcohols from Syngas/Waste Gas. J. Chem. Technol. Biotechnol. 2017, 92, 712–731.
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium Carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075.
- Jack, J.; Lo, J.; Maness, P.C.; Ren, Z.J. Directing Clostridium Ljungdahlii Fermentation Products via Hydrogen to Carbon Monoxide Ratio in Syngas. Biomass Bioenergy 2019, 124, 95–101.
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Ethanol Production during Semi-Continuous Syngas Fermentation in a Trickle Bed Reactor Using Clostridium Ragsdalei. Bioresour. Technol. 2016, 209, 56–65.
- Im, H.; An, T.; Kwon, R.; Park, S.; Kim, Y.K. Effect of Organic Nitrogen Supplements on Syngas Fermentation Using Clostridium Autoethanogenum. Biotechnol. Bioprocess Eng. 2021, 26, 476–482.
- Gößner, A.S.; Picardal, F.; Tanner, R.S.; Drake, H.L. Carbon Metabolism of the Moderately Acid-Tolerant Acetogen Clostridium Drakei Isolated from Peat. FEMS Microbiol. Lett. 2008, 287, 236–242.
- Liu, K.; Atiyeh, H.K.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Fermentative Production of Ethanol from Syngas Using Novel Moderately Alkaliphilic Strains of Alkalibaculum Bacchi. Bioresour. Technol. 2012, 104, 336–341.
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The Effect of Syngas Composition on the Growth and Product Formation of Butyribacterium Methylotrophicum. Enzym. Microb. Technol. 2007, 41, 362–367.
- Krumholz, L.R.; Bryant, M.P. Clostridium Pfennigii Sp. Nov. Uses Methoxyl Groups of Monobenzenoids and Produces Butyrate. Int. J. Syst. Bacteriol. 1985, 35, 454–456.
- Mayer, A.; Schädler, T.; Trunz, S.; Stelzer, T.; Weuster-Botz, D. Carbon Monoxide Conversion with Clostridium Aceticum. Biotechnol. Bioeng. 2018, 115, 2740–2750.
- Bertsch, J.; Müller, V. CO Metabolism in the Acetogen Acetobacterium Woodii. Appl. Environ. Microbiol. 2015, 81, 5949–5956.
- Lorowitz, W.H.; Bryant, M.P. Peptostreptococcus Productus Strain That Grows Rapidly with CO as the Energy Source. Appl. Environ. Microbiol. 1984, 47, 961–964.
- Kang, S.; Song, Y.; Jin, S.; Shin, J.; Bae, J.; Kim, D.R.; Lee, J.K.; Kim, S.C.; Cho, S.; Cho, B.K. Adaptive Laboratory Evolution of Eubacterium Limosum ATCC 8486 on Carbon Monoxide. Front. Microbiol. 2020, 11, 402.
- Oelgeschläger, E.; Rother, M. Carbon Monoxide-Dependent Energy Metabolism in Anaerobic Bacteria and Archaea. Arch. Microbiol. 2008, 190, 257–269.
- Talabardon, M.; Schwitzguébel, J.P.; Péringer, P. Anaerobic Thermophilic Fermentation for Acetic Acid Production from Milk Permeate. J. Biotechnol. 2000, 76, 83–92.
- Daniel, S.L.; Hsu, T.; Dean, S.I.; Drake, H.L. Characterization of the H2- and CO-Dependent Chemolithotrophic Potentials of the Acetogens Clostridium Thermoaceticum and Acetogenium Kivui. J. Bacteriol. 1990, 172, 4464–4471.
- Drake, H.L.; Daniel, S.L. Physiology of the Thermophilic Acetogen Moorella Thermoacetica. Res. Microbiol. 2004, 155, 869–883.
- Sakai, S.; Nakashimada, Y.; Inokuma, K.; Kita, M.; Okada, H.; Nishio, N. Acetate and Ethanol Production from H2 and CO2 by Moorella Sp. Using a Repeated Batch Culture. J. Biosci. Bioeng. 2005, 99, 252–258.
- Savage, M.D.; Wu, Z.G.; Daniel, S.L.; Lundie, L.L.; Drake, H.L. Carbon Monoxide-Dependent Chemolithotrophic Growth of Clostridium Thermoautotrophicum. Appl. Environ. Microbiol. 1987, 53, 1902–1906.
- Kerby, R.; Zeikus, J.G. Growth of Clostridium Thermoaceticum on H2/CO2 or CO as Energy Source. Curr. Microbiol. 1983, 8, 27–30.
- Diekert, G.B.; Thauer, R.K. Carbon Monoxide Oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J. Bacteriol. 1978, 136, 597–606.
- Parekh, S.R.; Cheryan, M. Production of Acetate by Mutant Strains of Clostridium Thermoaceticum. Appl. Microbiol. Biotechnol. 1991, 36, 384–387.
- Hu, P.; Rismani-Yazdi, H.; Stephanopoulos, G. Anaerobic CO2 Fixation by the Acetogenic Bacterium Moorella Thermoacetica. AIChE J. 2013, 59, 3176–3183.
- Poehlein, A.; Bengelsdorf, F.R.; Esser, C.; Schiel-Bengelsdorf, B.; Daniel, R.; Dürre, P. Complete Genome Sequence of the Acetogenic Bacterium Moorella thermoacetica DSM 2955T. Genome Announc. 2015, 3, 2–3.
- Wiegel, J.; Braun, M.; Gottschalk, G. Clostridium thermoautotrophicum Species Novum, a Thermophile Producing Acetate from Molecular Hydrogen and Carbon Dioxide. Curr. Microbiol. 1981, 5, 255–260.
- Redl, S.; Poehlein, A.; Esser, C.; Bengelsdorf, F.R.; Jensen, T.; Jendresen, C.B.; Tindall, B.J.; Daniel, R.; Dürre, P.; Nielsen, A.T. Genome-Based Comparison of All Species of the Genus Moorella, and Status of the Species Moorella thermoacetica and Moorella thermoautotrophica. Front. Microbiol. 2020, 10, 3070.
- Kimura, Z.I.; Hoshino, T.; Murakami, K. The Status of the Species Moorella thermoautotrophica Wiegel et Al. 1981. Request for an Opinion. Int. J. Syst. Evol. Microbiol. 2016, 66, 3249–3251.
- Rabemanolontsoa, H.; Van Nguyen, D.; Jusakulvjit, P.; Saka, S. Effects of Gas Condition on Acetic Acid Fermentation by Clostridium thermocellum and Moorella thermoacetica (C. thermoaceticum). Appl. Microbiol. Biotechnol. 2017, 101, 6841–6847.
- Ha, B.N.; Pham, D.M.; Masuda, D.; Kasai, T.; Katayama, A. Humin-Promoted Microbial Electrosynthesis of Acetate from CO2 by Moorella thermoacetica. Biotechnol. Bioeng. 2022, 119, 3487–3496.
- Plugge, C.M.; Balk, M.; Stams, A.J.M. Thermosyntrophicum Subsp. Nov., a Spore-Forming Bacterium. Int. J. Syst. Evol. Microbiol. 2002, 391–399.
- Visser, M.; Worm, P.; Muyzer, G.; Pereira, I.A.C.; Schaap, P.J.; Plugge, C.M.; Kuever, J.; Parshina, S.N.; Nazina, T.N.; Ivanova, A.E.; et al. Genome Analysis of Desulfotomaculum kuznetsovii Strain 17t Reveals a Physiological Similarity with Pelotomaculum thermopropionicum Strain Sit. Stand. Genom. Sci. 2013, 8, 69–87.
- Henstra, A.M.; Dijkema, C.; Stams, A.J.M. Archaeoglobus Fulgidus Couples CO Oxidation to Sulfate Reduction and Acetogenesis with Transient Formate Accumulation. Environ. Microbiol. 2007, 9, 1836–1841.
- Klenk, H.; Clayton, R.A.; Tomb, J.; Dodson, R.J.; Gwinn, M.; Hickey, E.K.; Fleischmann, R.D.; Quackenbush, J.; Lee, N.H.; Dougherty, B.A.; et al. The Complete Genome Sequence of the Hyperthermophilic, Sulphate-Reducing Archaeon. Nature 1998, 394, 6342–6349.
- Singla, A.; Verma, D.; Lal, B.; Sarma, P.M. Enrichment and Optimization of Anaerobic Bacterial Mixed Culture for Conversion of Syngas to Ethanol. Bioresour. Technol. 2014, 172, 41–49.
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y.; Zeng, R.J. Enhancement of Acetate Productivity in a Thermophilic (55 °C) Hollow-Fiber Membrane Biofilm Reactor with Mixed Culture Syngas (H2/CO2) Fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627.
- Kolter, K.; Dashevsky, A.; Irfan, M.; Bodmeier, R. Polyvinyl Acetate-Based Film Coatings. Int. J. Pharm. 2013, 457, 470–479.
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The Effects of PH and Temperature on the Acetate Production and Microbial Community Compositions by Syngas Fermentation. Fuel 2018, 224, 537–544.
- Zhang, F.; Ding, J.; Shen, N.; Zhang, Y.; Ding, Z.; Dai, K.; Zeng, R.J. In Situ Hydrogen Utilization for High Fraction Acetate Production in Mixed Culture Hollow-Fiber Membrane Biofilm Reactor. Appl. Microbiol. Biotechnol. 2013, 97, 10233–10240.
- Luo, G.; Jing, Y.; Lin, Y.; Zhang, S.; An, D. A Novel Concept for Syngas Biomethanation by Two-Stage Process: Focusing on the Selective Conversion of Syngas to Acetate. Sci. Total Environ. 2018, 645, 1194–1200.
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of PH, Substrate Loading, Oxygen, and Methanogens Inhibitors on Volatile Fatty Acid (VFA) Production from Citrus Waste by Anaerobic Digestion. Bioresour. Technol. 2020, 302, 122800.
- Fernández-Naveira, Á.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Production of Chemicals from C1 Gases (CO, CO2) by Clostridium Carboxidivorans. World J. Microbiol. Biotechnol. 2017, 33, 43.
- Ragsdale, S.W.; Kumar, M. Nickel-Containing Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. Chem. Rev. 1996, 96, 2515–2540.
- Hurst, K.M.; Lewis, R.S. Carbon Monoxide Partial Pressure Effects on the Metabolic Process of Syngas Fermentation. Biochem. Eng. J. 2010, 48, 159–165.
- Menon, S.; Ragsdale, S.W. Unleashing Hydrogenase Activity in Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase and Pyruvate:Ferredoxin Oxidoreductase. Biochemistry 1996, 35, 15814–15821.
- Lindahl, P.A. The Ni-Containing Carbon Monoxide Dehydrogenase Family: Light at the End of the Tunnel? Biochemistry 2002, 41, 2097–2105.
- Ragsdale, S.W. Life with Carbon Monoxide. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 165–195.
- Can, M.; Armstrong, F.A.; Ragsdale, S.W. Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase. Chem. Rev. 2014, 114, 4149–4174.
- Drennan, C.L.; Doukov, T.I.; Ragsdale, S.W. The Metalloclusters of Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase: A Story in Pictures. J. Biol. Inorg. Chem. 2004, 9, 511–515.
- Jeoung, J.H.; Dobbek, H. Structural Basis of Cyanide Inhibition of Ni, Fe-Containing Carbon Monoxide Dehydrogenase. J. Am. Chem. Soc. 2009, 131, 9922–9923.
- Doukov, T.I.; Iverson, T.M.; Seravalli, J.; Ragsdale, S.W.; Drennan, C.L. A Ni-Fe-Cu Center in a Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. Science 2002, 298, 567–572.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




