
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mi Kyung Park | -- | 1581 | 2023-04-18 14:05:54 | | | |
| 2 | Peter Tang | Meta information modification | 1581 | 2023-04-19 03:08:49 | | |
Video Upload Options
Post-translational modification (PTM), the essential regulatory mechanisms of proteins, play essential roles in physiological and pathological processes. In addition, PTM functions in tumour development and progression. Zinc finger E-box binding homeobox (ZEB) family homeodomain transcription factors, such as ZEB1 and ZEB2, play a pivotal role in tumour progression and metastasis by induction epithelial-mesenchymal transition (EMT), with activation of stem cell traits, immune evasion and epigenetic reprogramming.
1. Introduction
2. ZEB1 and ZEB2 Proteins and Their Physiological Functions
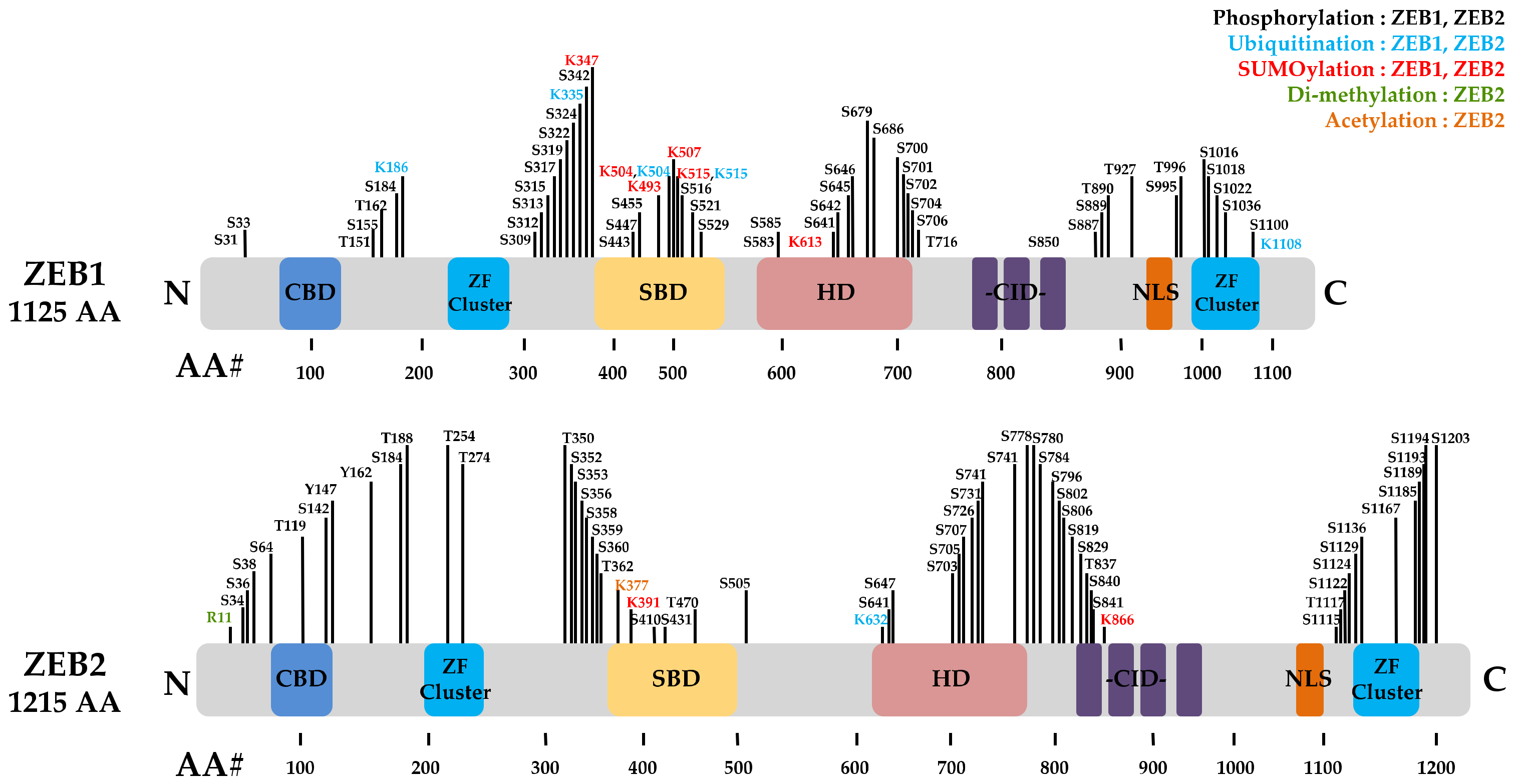
3. ZEB1 and ZEB2 in Cancer Progression
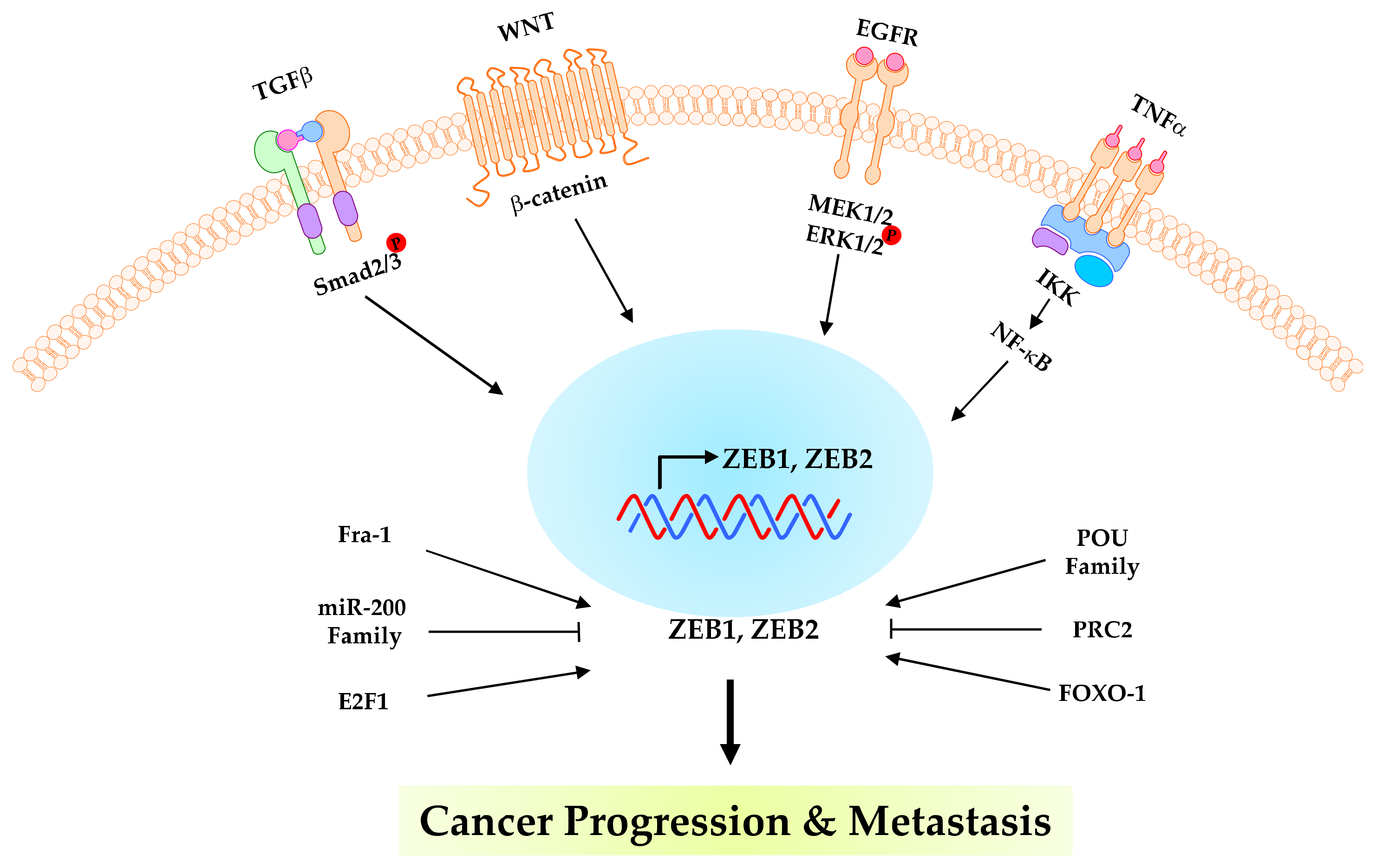
4. Post-Translational Modifications of ZEBs in Cancer Progression
|
PTMs Type |
PTM Sites |
Kinase/Enzyme |
Biological Function |
Cancer Type |
Ref. |
|---|---|---|---|---|---|
|
ZEB1 |
|||||
|
Phosphorylation |
Thr867 |
ERK |
Inhibition of the nuclear localisation of ZEB1 |
- |
[64] |
|
Thr851, Ser852, Ser853 |
PKC |
Inhibition of the nuclear localisation of ZEB1 |
- |
[64] |
|
|
Ser585 |
ATM |
Promotes DDR and tumour radioresistance |
BC |
[14] |
|
|
SUMOylation |
- |
Senp1 |
Promotes migration and EMT. |
HCC |
[65] |
|
Ubiquitination |
- |
Siah |
Promotes cell proliferation and invasion |
BC |
[66] |
|
Deubiquitination |
N-terminal |
USP51 |
Promotes cell proliferation and invasion |
BC |
[67] |
|
CSN5 |
Promotes metastasis and EMT |
RCC |
[68] |
||
|
USP18 |
Promotes EMT |
ESCC |
[69] |
||
|
Acetylation |
Lys741, Lys774, Lys775 |
P/CAF |
Promotes the formation of a p300-SMAD transcriptional complex |
[22] |
|
|
N-terminal |
TIP60 |
Corepressor of the ZEB |
T lymphoma |
[70] |
|
|
Deacetylation |
HDAC1/2 |
Promotes EMT |
PAAD |
||
|
ZEB2 |
|||||
|
Phosphorylation |
Ser705, Tyr802 |
GSK-3β |
Promotes metastasis and chemoresistance |
CRC |
[73] |
|
SUMOylation |
Lys391, Lys866 |
Pc2 |
Promotes EMT |
[53] |
|
|
Ubiquitination |
Lys48 |
FBXO45 |
Promotes EMT initiation and cancer progression |
[74] |
|
|
FBXL14 |
Promotes EMT |
COAD |
[75] |
||
|
FBXW7 |
Promotes metastasis and chemoresistance |
CRC |
[73] |
||
BC, breast cancer; HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; ESCC, oesophageal squamous cell carcinomas; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; PAAD, pancreatic adenocarcinoma; CRC, colorectal cancer; COAD, colon adenocarcinoma; ERK, extracellular signal-regulated kinase; PKC, protein kinase C; ATM, ataxia–telangiectasia mutated kinase; USP51, ubiquitin-specific peptidase 51; CSN5, COP9 signalosome subunit 5; USP18, ubiquitin-specific peptidase 18; PCAF, p300/CBP-associated factor; TIP60, tat-interacting protein of 60 kDa; HDAC1/2, histone deacetylase 1/2; GSK3β, glycogen synthase kinase 3 beta; FBXO45, F-box only protein 45; FBXL14, F-Box and leucine-rich repeat protein 14; FBXW7, F-box/WD repeat-containing protein 7.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Hay, E. An Overview of Epithelio-Mesenchymal Transformation. Acta Anat. 1995, 154, 8–20.
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890.
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428.
- Yu, J.-M.; Sun, W.; Hua, F.; Xie, J.; Lin, H.; Zhou, D.-D.; Hu, Z.-W. BCL6 induces EMT by promoting the ZEB1-mediated transcription repression of E-cadherin in breast cancer cells. Cancer Lett. 2015, 365, 190–200.
- Eger, A.; Aigner, K.; Sonderegger, S.; Dampier, B.; Oehler, S.; Schreiber, M.; Berx, G.; Cano, A.; Beug, H.; Foisner, R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005, 24, 2375–2385.
- Schmalhofer, O.; Brabletz, S.; Brabletz, T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009, 28, 151–166.
- Krebs, A.M.; Mitschke, J.; Lasierra Losada, M.; Schmalhofer, O.; Boerries, M.; Busch, H.; Boettcher, M.; Mougiakakos, D.; Reichardt, W.; Bronsert, P.; et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19, 518–529.
- Wu, C.-Y.; Tsai, Y.-P.; Wu, M.-Z.; Teng, S.-C.; Wu, K.-J. Epigenetic reprogramming and post-transcriptional regulation during the epithelial–mesenchymal transition. Trends Genet. 2012, 28, 454–463.
- Gheldof, A.; Hulpiau, P.; van Roy, F.; De Craene, B.; Berx, G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell. Mol. Life Sci. 2012, 69, 2527–2541.
- Wu, L.; Zhou, Z.; Han, S.; Chen, J.; Liu, Z.; Zhang, X.; Yuan, W.; Ji, J.; Shu, X. PLAGL2 promotes epithelial–mesenchymal transition and mediates colorectal cancer metastasis via β-catenin-dependent regulation of ZEB1. Br. J. Cancer 2020, 122, 578–589.
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487.
- Zhang, P.; Wei, Y.; Wang, L.; Debeb, B.G.; Yuan, Y.; Zhang, J.; Yuan, J.; Wang, M.; Chen, D.; Sun, Y.; et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat. Cell Biol. 2014, 16, 864–875.
- Wang, H.; Xiao, Z.; Zheng, J.; Wu, J.; Hu, X.-L.; Yang, X.; Shen, Q. ZEB1 Represses Neural Differentiation and Cooperates with CTBP2 to Dynamically Regulate Cell Migration during Neocortex Development. Cell Rep. 2019, 27, 2335–2353.e2336.
- Chang, R.; Zhang, P.; You, J. Post-translational modifications of EMT transcriptional factors in cancer metastasis. Open Life Sci. 2016, 11, 237–243.
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal Transduct. Target. Ther. 2020, 5, 90.
- Hill, L.; Browne, G.; Tulchinsky, E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int. J. Cancer 2013, 132, 745–754.
- Williams, T.M.; Montoya, G.; Wu, Y.; Eddy, R.L.; Byers, M.G.; Shows, T.B. The TCF8 gene encoding a zinc finger protein (Nil-2-a) resides on human chromosome 10p11.2. Genomics 1992, 14, 194–196.
- Wakamatsu, N.; Yamada, Y.; Yamada, K.; Ono, T.; Nomura, N.; Taniguchi, H.; Kitoh, H.; Mutoh, N.; Yamanaka, T.; Mushiake, K.; et al. Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat. Genet. 2001, 27, 369–370.
- Postigo, A.A. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J. 2003, 22, 2443–2452.
- Postigo, A.; Depp, J.L.; Taylor, J.J.; Kroll, K.L. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003, 22, 2453–2462.
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428.
- Wellner, U.; Brabletz, T.; Keck, T. ZEB1 in Pancreatic Cancer. Cancers 2010, 2, 1617–1628.
- Chung, D.-W.D.; Frausto, R.F.; Ann, L.B.; Jang, M.S.; Aldave, A.J.; Rosales, M.A.B.; Silva, K.C.; Duarte, D.A.; Rossato, F.A.; de Faria, J.B.L.; et al. Functional Impact of ZEB1 Mutations Associated With Posterior Polymorphous and Fuchs’ Endothelial Corneal Dystrophies. Investig. Opthalmol. Vis. Sci. 2014, 55, 6159–6166.
- Vandewalle, C.; van Roy, F.; Berx, G. The role of the ZEB family of transcription factors in development and disease. Cell. Mol. Life Sci. 2009, 66, 773–787.
- Scott, C.L.; Omilusik, K.D. ZEBs: Novel Players in Immune Cell Development and Function. Trends Immunol. 2019, 40, 431–446.
- Verschueren, K.; Remacle, J.E.; Collart, C.; Kraft, H.; Baker, B.S.; Tylzanowski, P.; Nelles, L.; Wuytens, G.; Su, M.-T.; Bodmer, R.; et al. SIP1, a Novel Zinc Finger/Homeodomain Repressor, Interacts with Smad Proteins and Binds to 5′-CACCT Sequences in Candidate Target Genes. J. Biol. Chem. 1999, 274, 20489–20498.
- Remacle, J.E.; Kraft, H.; Lerchner, W.; Wuytens, G.; Collart, C.; Verschueren, K.; Smith, J.; Huylebroeck, D. New mode of DNA binding of multi-zinc finger transcription factors: Delta EF1 family members bind with two hands to two target sites. EMBO J. 1999, 18, 5073–5084.
- Kropivšek, K.; Pickford, J.; Carter, D.A. Postnatal Dynamics of Zeb2 Expression in Rat Brain: Analysis of Novel 3′ UTR Sequence Reveals a miR-9 Interacting Site. J. Mol. Neurosci. 2014, 52, 138–147.
- Bronsert, P.; Kohler, I.; Timme, S.; Kiefer, S.; Werner, M.; Schilling, O.; Vashist, Y.; Makowiec, F.; Brabletz, T.; Hopt, U.T.; et al. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery 2014, 156, 97–108.
- Zhang, G.-J.; Zhou, T.; Tian, H.-P.; Liu, Z.-L.; Xia, S.-S. High expression of ZEB1 correlates with liver metastasis and poor prognosis in colorectal cancer. Oncol. Lett. 2013, 5, 564–568.
- Okugawa, Y.; Toiyama, Y.; Tanaka, K.; Matsusita, K.; Fujikawa, H.; Saigusa, S.; Ohi, M.; Inoue, Y.; Mohri, Y.; Uchida, K.; et al. Clinical significance of zinc finger E-box binding homeobox 1 (ZEB1) in human gastric cancer. J. Surg. Oncol. 2012, 106, 280–285.
- Jia, B.; Liu, H.; Kong, Q.; Li, B. Overexpression of ZEB1 associated with metastasis and invasion in patients with gastric carcinoma. Mol. Cell. Biochem. 2012, 366, 223–229.
- Zhang, J.; Lu, C.; Zhang, J.; Kang, J.; Cao, C.; Li, M. Involvement of ZEB1 and E-cadherin in the invasion of lung squamous cell carcinoma. Mol. Biol. Rep. 2013, 40, 949–956.
- Matsubara, D.; Kishaba, Y.; Yoshimoto, T.; Sakuma, Y.; Sakatani, T.; Tamura, T.; Endo, S.; Sugiyama, Y.; Murakami, Y.; Niki, T. Immunohistochemical analysis of the expression of E-cadherin and ZEB1 in non-small cell lung cancer. Pathol. Int. 2014, 64, 560–568.
- Larsen, J.; Nathan, V.; Osborne, J.K.; Farrow, R.K.; Deb, D.; Sullivan, J.P.; Dospoy, P.D.; Augustyn, A.; Hight, S.K.; Sato, M.; et al. ZEB1 drives epithelial-to-mesenchymal transition in lung cancer. J. Clin. Investig. 2016, 126, 3219–3235.
- Spoelstra, N.S.; Manning, N.G.; Higashi, Y.; Darling, D.; Singh, M.; Shroyer, K.R.; Broaddus, R.R.; Horwitz, K.B.; Richer, J.K. The Transcription Factor ZEB1 Is Aberrantly Expressed in Aggressive Uterine Cancers. Cancer Res. 2006, 66, 3893–3902.
- Zhou, Y.-M.; Cao, L.; Li, B.; Zhang, R.-X.; Sui, C.-J.; Yin, Z.-F.; Yang, J.-M. Clinicopathological Significance of ZEB1 Protein in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2012, 19, 1700–1706.
- Contreras, H.; Orellana-Serradell, O.; Herrera, D.; Castellón, E. The transcription factor ZEB1 promotes chemoresistance in prostate cancer cell lines. Asian J. Androl. 2019, 21, 460–467.
- Wang, H.; Huang, B.; Li, B.M.; Cao, K.Y.; Mo, C.Q.; Jiang, S.J.; Pan, J.C.; Wang, Z.R.; Lin, H.Y.; Wang, D.H.; et al. ZEB 1-mediated vasculogenic mimicry formation associates with epithelial–mesenchymal transition and cancer stem cell phenotypes in prostate cancer. J. Cell. Mol. Med. 2018, 22, 3768–3781.
- Wu, C.; Li, J.; Tian, C.; Shi, W.; Jiang, H.; Zhang, Z.; Wang, H.; Zhang, Q.; Sun, W.; Sun, P.; et al. Epigenetic dysregulation of ZEB1 is involved in LMO2-promoted T-cell acute lymphoblastic leukaemia leukaemogenesis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 2511–2525.
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S.; Walenkamp, A.M.E.; Boddeke, E.; Balasubramanyian, V.; Wagemakers, M.; et al. TGF-β is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 2014, 5, e1443.
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230.
- Kalra, R.S.; Chaudhary, A.; Yoon, A.-R.; Bhargava, P.; Omar, A.; Garg, S.; Yun, C.-O.; Kaul, S.C.; Wadhwa, R. CARF enrichment promotes epithelial–mesenchymal transition via Wnt/β-catenin signaling: Its clinical relevance and potential as a therapeutic target. Oncogenesis 2018, 7, 39.
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172.
- Han, Y.; Luo, Y.; Wang, Y.; Chen, Y.; Li, M.; Jiang, Y. Hepatocyte growth factor increases the invasive potential of PC-3 human prostate cancer cells via an ERK/MAPK and Zeb-1 signaling pathway. Oncol. Lett. 2016, 11, 753–759.
- Sinh, N.D.; Endo, K.; Miyazawa, K.; Saitoh, M. Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2, dependent on ERK1/2 activity, in breast cancer cells. Cancer Sci. 2017, 108, 952–960.
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724.
- Bakiri, L.; Macho-Maschler, S.; Custic, I.; Niemiec, J.; Guío-Carrión, A.; Hasenfuss, S.C.; Eger, A.; Müller, M.; Beug, H.; Wagner, E.F. Fra-1/AP-1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression. Cell Death Differ. 2015, 22, 336–350.
- Wang, T.; Chen, X.; Qiao, W.; Kong, L.; Sun, D.; Li, Z. Transcription factor E2F1 promotes EMT by regulating ZEB2 in small cell lung cancer. BMC Cancer 2017, 17, 719.
- Katoh, M. Integrative genomic analyses of ZEB2: Transcriptional regulation of ZEB2 based on SMADs, ETS1, HIF1α, POU/OCT, and NF-κB. Int. J. Oncol. 2009, 34, 1737–1742.
- Long, J.; Zuo, D.; Park, M. Pc2-mediated Sumoylation of Smad-interacting Protein 1 Attenuates Transcriptional Repression of E-cadherin. J. Biol. Chem. 2005, 280, 35477–35489.
- Dong, T.; Zhang, Y.; Chen, Y.; Liu, P.; An, T.; Zhang, J.; Yang, H.; Zhu, W.; Yang, X. FOXO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget 2017, 8, 1703–1713.
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop—A motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677.
- Takagi, T.; Moribe, H.; Kondoh, H.; Higashi, Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 1998, 125, 21–31.
- Liu, Y.; El-Naggar, S.; Darling, D.S.; Higashi, Y.; Dean, D.C. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 2008, 135, 579–588.
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83.
- Molina-Ortiz, P.; Villarejo, A.; MacPherson, M.; Santos, V.; Montes, A.; Souchelnytskyi, S.; Portillo, F.; Cano, A. Characterization of the SNAG and SLUG Domains of Snail2 in the Repression of E-Cadherin and EMT Induction: Modulation by Serine 4 Phosphorylation. PLoS ONE 2012, 7, e36132.
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939.
- Chang, A.T.; Liu, Y.; Ayyanathan, K.; Benner, C.; Jiang, Y.; Prokop, J.W.; Paz, H.; Wang, D.; Li, H.-R.; Fu, X.-D.; et al. An evolutionarily conserved DNA architecture determines target specificity of the TWIST family bHLH transcription factors. Genes Dev. 2015, 29, 603–616.
- Deribe, Y.L.; Pawson, T.; Dikic, I. Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 2010, 17, 666–672.
- Pejaver, V.; Hsu, W.-L.; Xin, F.; Dunker, A.K.; Uversky, V.N.; Radivojac, P. The structural and functional signatures of proteins that undergo multiple events of post-translational modification. Protein Sci. 2014, 23, 1077–1093.
- Llorens, M.C.; Lorenzatti, G.; Cavallo, N.L.; Vaglienti, M.V.; Perrone, A.P.; Carenbauer, A.L.; Darling, D.S.; Cabanillas, A.M. Phosphorylation Regulates Functions of ZEB1 Transcription Factor. J. Cell. Physiol. 2016, 231, 2205–2217.
- Zhang, W.; Sun, H.; Shi, X.; Wang, H.; Cui, C.; Xiao, F.; Wu, C.; Guo, X.; Wang, L. SENP1 regulates hepatocyte growth factor-induced migration and epithelial-mesenchymal transition of hepatocellular carcinoma. Tumor Biol. 2016, 37, 7741–7748.
- Chen, A.; Wong, C.S.; Liu, M.C.; House, C.M.; Sceneay, J.; Bowtell, D.D.; Thompson, E.W.; Möller, A. The ubiquitin ligase Siah is a novel regulator of Zeb1 in breast cancer. Oncotarget 2015, 6, 862–873.
- Zhou, Z.; Zhang, P.; Hu, X.; Kim, J.; Yao, F.; Xiao, Z.; Zeng, L.; Chang, L.; Sun, Y.; Ma, L. USP51 promotes deubiquitination and stabilization of ZEB1. Am. J. Cancer Res. 2017, 7, 2020–2031.
- Zhang, S.; Hong, Z.; Chai, Y.; Liu, Z.; Du, Y.; Li, Q.; Liu, Q. CSN5 promotes renal cell carcinoma metastasis and EMT by inhibiting ZEB1 degradation. Biochem. Biophys. Res. Commun. 2017, 488, 101–108.
- Song, C.; Peng, J.; Wei, Y.; Shao, J.; Chen, X.; Zhang, X.; Xu, J. USP18 promotes tumor metastasis in esophageal squamous cell carcinomas via deubiquitinating ZEB1. Exp. Cell Res. 2021, 409, 112884.
- Hlubek, F.; Löhberg, C.; Meiler, J.; Jung, A.; Kirchner, T.; Brabletz, T. Tip60 Is a Cell-Type-Specific Transcriptional Regulator. J. Biochem. 2001, 129, 635–641.
- Aghdassi, A.; Sendler, M.; Guenther, A.; Mayerle, J.; Behn, C.-O.; Heidecke, C.-D.; Friess, H.; Büchler, M.; Evert, M.; Lerch, M.M.; et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012, 61, 439–448.
- Schneider, G.; Krämer, O.H.; Saur, D. A ZEB1-HDAC pathway enters the epithelial to mesenchymal transition world in pancreatic cancer. Gut 2012, 61, 329–330.
- Li, N.; Babaei-Jadidi, R.; Lorenzi, F.; Spencer-Dene, B.; Clarke, P.; Domingo, E.; Tulchinsky, E.; Vries, R.G.J.; Kerr, D.; Pan, Y.; et al. An FBXW7-ZEB2 axis links EMT and tumour microenvironment to promote colorectal cancer stem cells and chemoresistance. Oncogenesis 2019, 8, 13.
- Xu, M.; Zhu, C.; Zhao, X.; Chen, C.; Zhang, H.; Yuan, H.; Deng, R.; Dou, J.; Wang, Y.; Huang, J.; et al. Atypical ubiquitin E3 ligase complex Skp1-Pam-Fbxo45 controls the core epithelial-to-mesenchymal transition-inducing transcription factors. Oncotarget 2015, 6, 979–994.
- Lander, R.; Nordin, K.; LaBonne, C. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. J. Cell Biol. 2011, 194, 17–25.




