Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhiwei Huang | -- | 5203 | 2023-04-18 11:15:12 | | | |
| 2 | Rita Xu | Meta information modification | 5203 | 2023-04-18 11:37:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, J.; Yang, J.; Li, X.; Liu, H.; Yao, X.; Xia, C.; Huang, Z. Biomass-Based Furan Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/43164 (accessed on 04 March 2026).
Zhang J, Yang J, Li X, Liu H, Yao X, Xia C, et al. Biomass-Based Furan Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/43164. Accessed March 04, 2026.
Zhang, Jia, Jian Yang, Xuemei Li, Hailong Liu, Xiaolan Yao, Chungu Xia, Zhiwei Huang. "Biomass-Based Furan Compounds" Encyclopedia, https://encyclopedia.pub/entry/43164 (accessed March 04, 2026).
Zhang, J., Yang, J., Li, X., Liu, H., Yao, X., Xia, C., & Huang, Z. (2023, April 18). Biomass-Based Furan Compounds. In Encyclopedia. https://encyclopedia.pub/entry/43164
Zhang, Jia, et al. "Biomass-Based Furan Compounds." Encyclopedia. Web. 18 April, 2023.
Copy Citation
Bio-based furanic oxygenates represent a well-known class of lignocellulosic biomass-derived platform molecules. In the presence of H2 and different nitrogen sources, these versatile building blocks can be transformed into valuable amine compounds via reductive amination or hydrogen-borrowing amination mechanisms, yet they still face many challenges due to the co-existence of many side-reactions, such as direct hydrogenation, polymerization and cyclization. Hence, catalysts with specific structures and functions are required to achieve satisfactory yields of target amines.
bio-based furanic oxygenate
amine
reductive amination
1. Introduction
An important challenge facing mankind in the 21st century is how to meet the growing energy demand while reducing greenhouse gas emissions [1][2]. Under the dual pressure of resources and environment, the exploitation of clean and renewable resources is greatly promoted. Biomass is the most abundant renewable carbon resource in nature, with an annual natural output of about 170 billion tons [3][4]. It is considered as a green chemical raw material due to its special advantages in terms of sustainability. For example, by 2030, the EU aims to increase the total use of bio-based chemicals and materials to 25% and to reduce greenhouse gas emissions by 40% [5]. Therefore, many governments and research institutions are paying great attention to the research on the replacement of traditional fossil resources by biomass.
Biomass-derived platform compounds have attracted great attention in reducing the dependence on fossil resources, as they are regarded as the bridges between biomass resources and the chemical industry [6][7][8]. Non-edible lignocellulose is a major component of biomass resources, which consists of polysaccharides (30–50 wt% cellulose and 20–40 wt% hemicellulose) and phenolic compounds (10–20 wt% lignin) [9][10]. Based on chemical or biological catalytic technologies, polysaccharides can be readily converted into biomass-based oxygenated platform compounds, such as furfural (FF), 5-hydroxymethylfurfural (HMF) and their derivatives [6][11] (Figure 1). The use of these low-cost and readily available furan-based oxygenates for the synthesis of high-value-added chemicals, especially various useful amines, has become a research hotspot in biorefinery in the last decade [12][13][14][15].
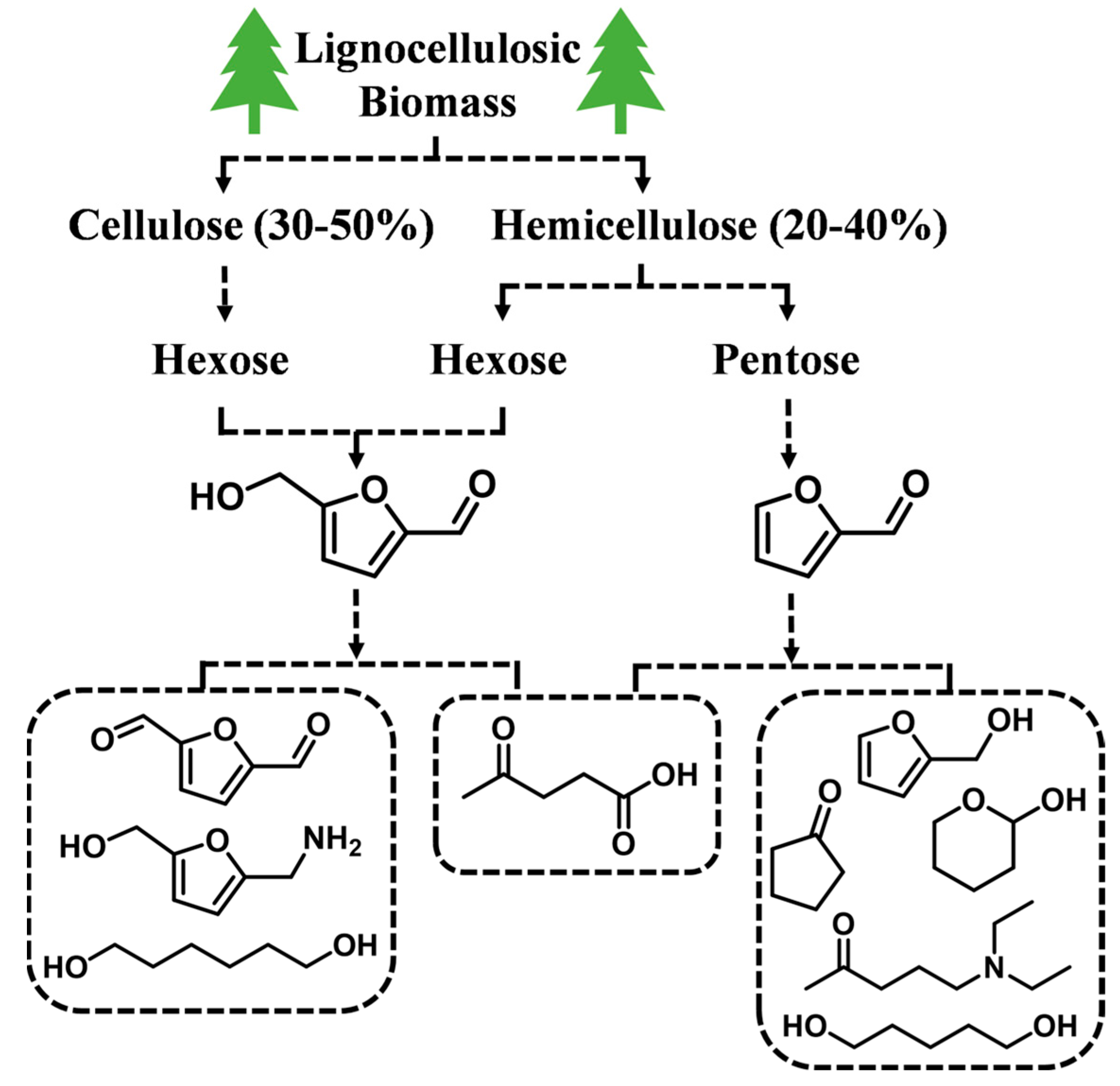
Figure 1. Schematic representation of bio-based furanic oxygenates.
Amines, an important group of stock molecules in the chemical and biological sciences, are widely employed as synthetic raw materials and essential intermediates in a variety of industries, including pharmaceuticals, insecticides, polymers, organic pigments, food additives and surfactants [14][16][17][18]. For example, in the field of drug synthesis, more than 80% of the 200 top-selling drugs in 2018 contained amines [13][19]. In the field of polymer synthesis, the annual world production of polyamide has reached a market scale of 8232 kilotons in 2018 and will reach more than 10,900 kilotons by 2025 [20]. Traditionally, amine synthesis routes are the direct amination of alkyl halides or epoxides, and the hydrogenation of nitriles or amides [20][21][22]. These processes not only depend on fossil materials, but also suffer from being high-energy intensive and/or producing heavy pollution. On the other hand, the amination of carbonyl and alcohol-based oxygenates derived from biomass undoubtedly offers a cleaner and more atom-efficient route with water as the only by-product, which complies with the 12 green chemistry principles [7][21]. In recent years, significant progress has been made in the amination of bio-based furanic oxygenates into amine products [23][24][25][26][27][28].
2. Reductive Amination of Bio-Based Furanic Aldehydes and Ketones
The reductive amination of aldehydes and ketones is an impressive approach for the synthesis of useful amines with mild reaction conditions and water as the main by-product. It is widely accepted that the reductive amination reaction involves the condensation of the carbonyl group with ammonia to form an imine intermediate, followed by the hydrogenation of the imine intermediate to yield the desired amine product (Scheme 1) [21], where the hydrogenation of imine in the second step is somewhat more difficult, likely being the rate-determining step. Achieving a highly selective synthesis of the desired amine, especially primary amine, is a difficult task due to the presence of common side-reactions, such as direct reduction of the carbonyl and overalkylation of the target amine [13][29]. Therefore, it is promising to select a catalytic system that favors the hydrogenation of imine over the hydrogenation of the carbonyl substrate and overalkylation of the target amine [30][31][32][33][34].
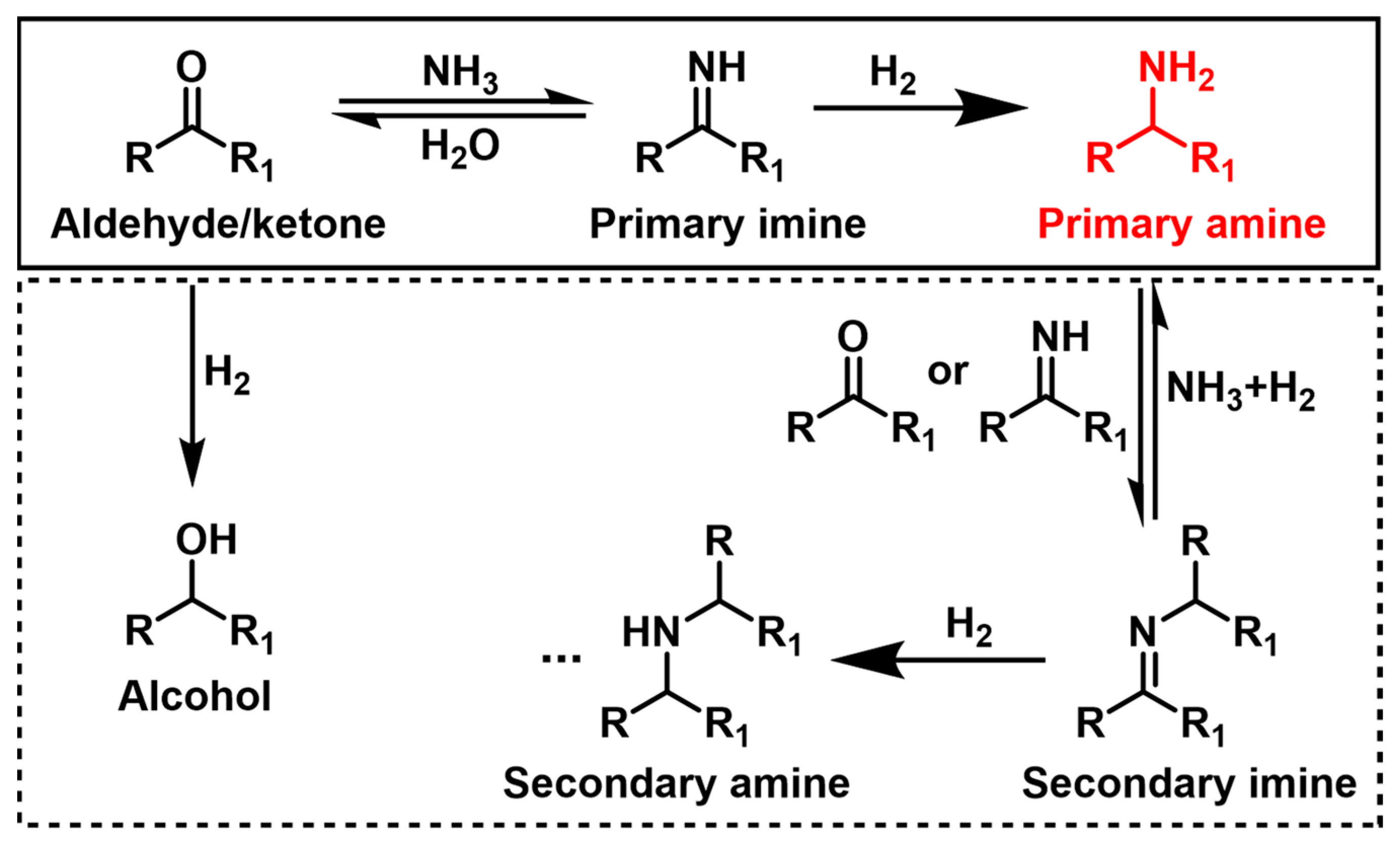
Scheme 1. Reaction pathway for the reductive amination of aldehydes and ketones.
2.1. Reductive Amination of FF
FF is one of the most prevalent industrial chemicals derived from lignocellulosic biomass, with an annual production volume of more than 200,000 tons. It can be facilely obtained by the dehydration of pentose over acidic catalysts and produced industrially on a large scale from corncobs, bagasse, wheat straw and other agroforestry wastes by hydrolytic refining [35][36]. As a promising platform compound, FF can be transformed into a wide range of value-added chemicals and biofuels [37][38]. Among the different routes for upgrading, the reductive amination of FF to furfurylamine (FAM) and its derivatives has attracted much attention in recent years due to the broad application of FAM and its derivatives in the manufacture of pharmaceuticals, pesticides and synthetic resins [39][40][41]. In this section, the reductive amination of FF will be discussed on the basis of different nitrogen sources.
2.1.1. Reductive Amination of FF with NH3
The reductive amination of FF with NH3 is a typical method for the synthesis of FAM. A plausible reaction route for the synthesis of FAM and its by-products is as follows (Scheme 2) [23][28][42]: FF (1a) is reversibly condensed with excess NH3 to form an unstable intermediate of primary imine (2a), and then 2a is hydrogenated to obtain the target FAM (3a) over an active catalyst. Meanwhile, at least three side-reactions compete with the main reaction, such as the direct hydrogenation of FF to furfuryl alcohol (5a), the trimerization and cyclization of imine (2a) to form 10a, and the condensation of 1a with 3a to form a stable intermediate of secondary imine (7a), which can be further hydrogenated to secondary amine (8a). Therefore, the selection of catalysts with special structures and functions plays an important role in achieving an efficient synthesis of FAM by inhibiting the occurrence of side-reactions.
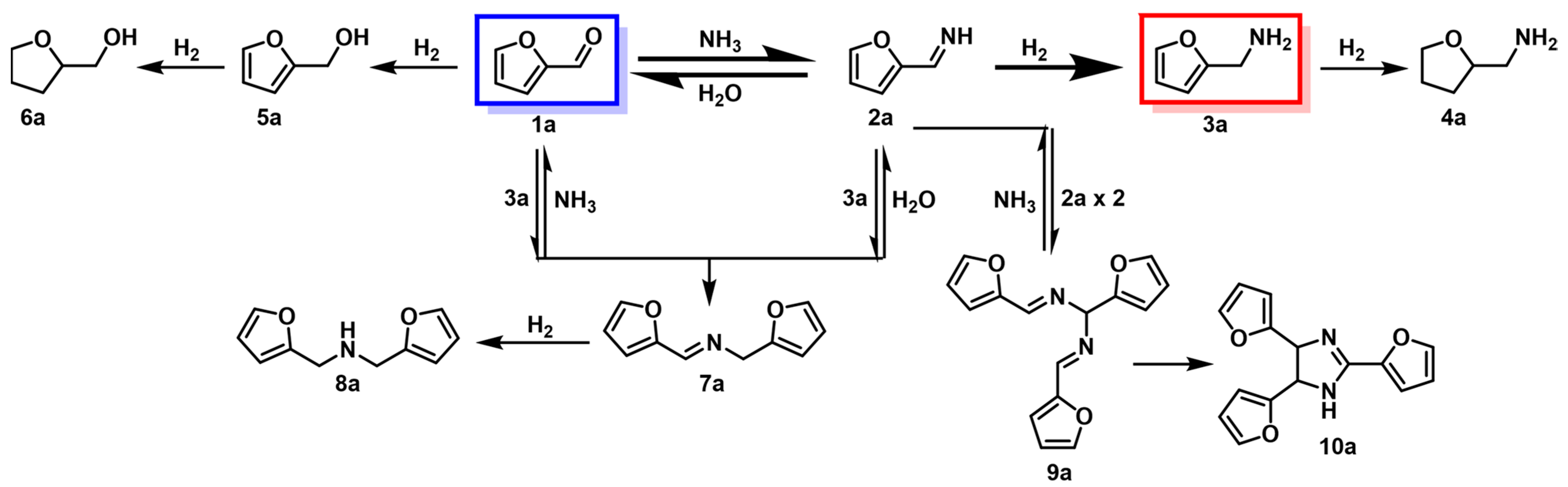
Scheme 2. Reaction pathway for the reductive amination of FF with NH3.
In searching for efficient catalysts for the reductive amination of FF to FAM, Hara and coworkers [43] investigated a wide range of metals (Ru, Rh, Pd, Pt, Ag, Ni and Cu) and oxide supports (Nb2O5, SiO2, TiO2, Al2O3, ZrO2, MgO and active carbon). Among them, Ru/Nb2O5 showed the highest FAM yield of 99% under the reaction conditions of 90 °C, 0.1 MPa NH3 and 4 MPa H2. The low electron density of Ru on the positively charged Nb2O5 surface greatly contributed to the highly selective reductive amination of FF to FAM. The effect of the type and amount of acid was also examined by systematically adjusting the reduction temperature of the Ru/Nb2O5·nH2O catalyst [44]. Ru/Nb2O5·nH2O-300 with a high density of acidic sites had the highest catalytic activity, which could facilitate the attack of Ru hydride species by activating imines, thus accelerating the hydrogenation of imines. A specific flat-shaped Ru nanoparticles (Ru-NPs) catalyst with higher activity was synthesized by the same group [45]. The conversion frequency (TOF) of the Ru-NPs catalyst was as high as 1850 h−1, which was almost six times that of the Ru/Nb2O5 catalyst (TOF = 310 h−1). The high activity of the catalyst was attributed to the exposure of a large number of electron-deficient Ru as active sites on the (111) surface of flat-shaped fcc Ru nanoparticles. The Ru-NPs catalyst showed excellent durability in long-term cycle tests.
Most current processes for producing primary amine by reductive amination are carried out with extremely excessive NH3, which raise the cost of recovery and have negative environmental effects. Xu et al. [46] designed an efficient anisotropic layered boron nitride-supported ruthenium catalyst (Ru/BN-e), which successfully achieved the quantitative conversion of FF to FAM in the presence of a relatively low amount of NH3 (2 eq) aqueous solution. The remarkable catalytic activity of Ru/BN-e was specifically related to the enhanced interface electronic effect between Ru and the edge surface of boron nitride, which consequently enhanced the hydrogenation activity.
Bimetallic catalysts can modify the surface characteristics of the catalyst through the interaction of different metals to considerably improve the catalytic performance [47][48]. Ru-based bimetallic catalysts supported on activated carbon (RuM/AC) were reported by Dai et al. [39] for the reductive amination of FF to FAM, and a 92% yield of FAM was obtained over the RuCo/AC catalyst under the reaction conditions of 80 °C and 2 MPa H2, using water as a green solvent. The good catalytic performance of RuCo/AC was ascribed to the synergistic effect between the RuCo alloy and the activated carbon, which improved its acidity and hydrogenation ability. The catalyst could be recycled five times without significant deactivation.
Single-atom catalysts (SACs), unlike conventional supported catalysts, have emerged as a new field of heterogeneous catalysis due to their well-defined active site and maximum metal atom utilization [49][50]. The group of Zhang and Wang [28] fabricated highly active, selective and robust Ru-SACs supported on N-doped carbons (Ru1/NC-900–800NH3) by pyrolyzing Ru(acac)3 and N/C precursors at 900 °C in N2 and then treating the mixture at 800 °C in NH3 (Figure 3). The Ru1/NC catalyst can afford a good yield (97%) toward FAM in the reductive amination of FF, owing to its moderate hydrogen activation capacity. The catalyst also showed outstanding stability during reuse tests and universality to a wide range of biomass-derived aldehyde/ketone. More intriguingly, Ru1/NC SAC displayed superior sulfur and CO resistance compared with traditional Ru-based nano-catalysts.
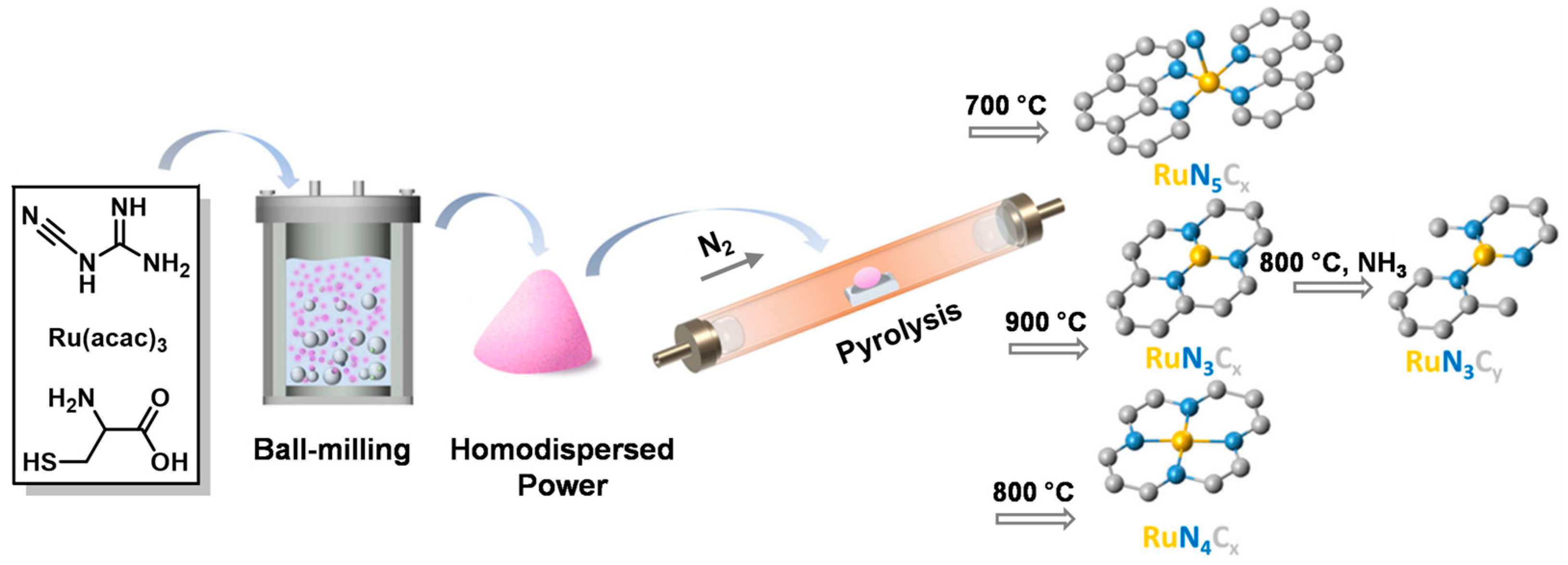
Figure 3. Schematic illustration for the synthesis procedure of Ru1/NC-T catalysts.
It is a great challenge to efficiently synthesize primary amines via ambient temperature reductive amination of carbonyl compounds. Recently, using a titanium phosphate (TiP)-supported Ru catalyst (Ru/TiP-100) reduced at 100 °C, Han and coworkers [33] carried out the reductive amination of FF and obtained a 91% yield of FAM at room temperature. The good catalytic performance of the Ru/TiP-100 catalyst originated from the relatively high acidity and the suitable electron density, provided by the combination of TiP and Ru/RuO2 with a suitable proportion of Ru0 (52%). Detailed studies showed that the suitable electron density of Ru species could balance the activation of H2 in the hydrogenation step and the desorption of intermediates (primary and secondary imines). Meanwhile, the relatively high acidity of Ru/TiP-100 could facilitate the conversion of the C=N groups in the in situ-generated primary and secondary imines to the desired primary amines.
Despite Ru-based catalysts showing good catalytic performance in the synthesis of FAM, their expensive production costs and lack of availability retard them from large-scale industrial applications. Therefore, it is important to develop cheap and easily available non-noble metal catalysts for the reductive amination of FF. Xu et al. [51] studied the reductive amination of FF on a commercially available Raney Co catalyst, achieving an FAM yield up to 99%. The excellent catalytic performance of Raney Co was attributed to its high efficiency in the ammonia-assisted hydrogenolysis of the secondary imines and low activity in the further hydrogenation of the secondary imines or the direct hydrogenation of the carbonyl and the furan ring in FF.
Recently, Ma and coworkers [52] successfully synthesized a uniform Co nanoparticles catalyst (Co@C-600-EtOH) enclosed in a multilayer graphene structure using cobalt acetate as the metal precursor. Such a catalyst achieved an 87% FAM yield under the reaction conditions of 90 °C, 7 M NH3 in MeOH and 2 MPa H2. The inserted Co species was found to function as electrophilic sites or Lewis acidic sites to promote imine formation and hydrogenation. Subsequently, Lingaiah et al. [53] prepared Co nanoparticles embedded in a N-doped carbon matrix by pyrolyzing ZIF-67 in a N2 atmosphere at different temperatures (400–700 °C). The Co/NC-700 catalyst could achieve a rather high FAM yield of 99% under the reaction conditions of 120 °C and 2 MPa H2. They found that the active center of the catalyst was the metallic Co nanoparticles anchored by coordination with the N species in the graphitic layers. Meanwhile, the large surface area, total pore volume, easy hydrogen desorption and surface defect sites together contributed to good catalytic activity, selectivity and stability of the catalyst. The direct conversion of biomass-derived xylose into FAM through a one-pot two-step process was also accomplished, with an FAM yield of 69%.
In searching for facilely synthesized non-noble Ni-based catalysts, Ding et al. [54] prepared several nano-Ni catalysts loaded on commercially available oxide supports using a simple wet impregnation method. Among them, 10Ni/Al2O3 was found to present superior activity and selectivity for FAM with a high yield of 92%. The synergistic effect of medium acidic sites and strong metal-support interaction was disclosed to be responsible for the remarkable performance of the catalyst. Recently, using a nano-Ni1Al catalyst with a Ni:Al ratio of 1:1 prepared by coprecipitation, Zhang et al. [55] attained a 91% yield of FAM in the reductive amination of FF over the Ni-Al2O3 nano-catalysts. The synergistic effect between the well-dispersed active Ni0 nanoparticles and the abundant adjacent surface Lewis acidic sites accounts for the better catalytic performance of the catalyst with a Ni/Al ratio of 1. More recently, up to a 98% yield of FAM was obtained over a Ni/SiO2 catalyst with small Ni sizes (~3 nm) prepared by deposition–precipitation in the reductive amination of FF [56]. A similar cooperative catalysis mechanism of the Lewis acidic sites and the small nickel particles of the catalyst was proposed for the efficient synthesis of FAM.
N-doped carbon materials were also applied for the fabrication of supported non-noble Ni-based catalysts. For instance, Song et al. [57] created an effective N-doped porous carbon-supported Ni catalyst (Ni/pNC) with a uniform pore structure utilizing a template-assisted pyrolysis and impregnation approach. The Ni/pNC catalyst showed a >99% yield of FAM. The formation of Ni–Nx sites and the electronic interaction between the Ni and N species were found to facilitate the reductive amination of the C=O bonds and significantly decrease the activation energy for the conversion of trimers and secondary imines to the target amines. Additionally, the Ni/pNC catalyst demonstrated good durability and broad application in the reductive amination of various carbonyl compounds with high primary amine yields.
The development of heterogeneous non-noble metal catalysts for the efficient reductive amination of FF under quite mild conditions is still challenging. The Hara group [58] used a stable Ni@DS catalyst, which was prepared by low-temperature pyrolysis of polydentate oxygen-coordinated chelating ligands and mesoporous dendritic silica supports, for the reductive amination of FF to produce FAM with a yield of 89% under rather mild conditions of 50 °C and 0.9 MPa H2. This catalyst showed high activity in both water (polar and protic) and toluene (apolar) solvents, which might be attributed to the synergistic effect between small metals and their metal oxide nanoparticles uniformly fixed on silica.
2.1.2. Reductive Amination of FF with Aniline
One example of the heterogeneous catalytic reductive amination of FF with aniline at room temperature was reported by Martínez and coworkers [59]. They applied sulfonic acid functionalized silica as a support to fabricate a bifunctional Ir/SiO2-SO3H catalyst. A 21% yield of the target secondary amine (N-(furan-2-ylmethyl)aniline) was obtained at room temperature using ethyl acetate as the solvent, which might be associated with the fact that use of ethyl acetate as a solvent inhibited the formation of a tertiary amine by-product. The synergistic effect of the metal center and acidic sites exerted a vital role in the reductive amination of FF with aniline, in which acidic sites facilitated both the formation of imine and its subsequent hydrogenation to the desired amine. However, according to the results, the Ir/SiO2-SO3H catalyst is not active enough to be used in the reductive amination of FF with aniline.
2.1.3. Reductive Amination of FF with HCOONH4
Tertiary amines, mainly produced synthetically from fossil resources using a multi-step process, are essential substances in the chemical industry. Lin et al. [60] reported the first example of the continuous reductive amination of FF with HCOONH4 to synthesize a biomass-based tertiary amine of tris(2-furanylmethyl)amine. Over an efficient Rh2P/NC catalyst, up to a 92% yield of tris(2-furanylmethyl)amine was achieved under mild reaction conditions of 60 °C and 3 MPa H2 in an ethyl acetate solvent. The good performance of the catalyst was ascribed to the efficient electron-transfer from the P atoms (on the P-terminated Rh2P) to their bonded and dissociated H atoms, which resulted in the partial filling of the antibonding orbitals of the H atoms, favoring the adsorption and activation of H2 on the surface of Rh2P. The Rh2P/NC catalyst showed good durability in six continuous cycle tests without a noticeable decrease in activity.
2.2. Reductive Amination of HMF
HMF, which is a fascinating molecule due to simultaneously containing three functional groups of aldehyde, alcohol and a furan ring, is produced by dehydration of hexoses (mainly fructose) or by hydrolysis/dehydration of cellulose in the presence of proper acidic catalysts [11]. It is known as a ‘‘sleeping giant’’ and is included together with FF as the top 10 value-added bio-based chemicals by the U.S. Department of Energy [61][62]. 5-(hydroxymethyl)-2-furfurylamine (HMFA) and its derivatives synthesized by the reductive amination of HMF are utilized in the manufacturing of bioactive compounds, including hypertension medications, diuretics and preservatives, and also as monomers in the synthesis of polymers, textiles and perfumes [63][64]. The reductive amination of HMF will be summarized and discussed on the basis of different nitrogen sources in this section.
2.2.1. Reductive Amination of HMF with NH3
The reductive amination of HMF with NH3 is mainly based on non-noble Ni and Co catalysts. A Ni/SBA-15 catalyst was used by Chen et al. [65] for the reductive amination of HMF and ~90% yield of HMFA was obtained under the reaction conditions of 100 °C and 1.5 MPa H2, which was significantly higher than that of Ru/C, Pd/C and Pt/C noble metal catalysts. They ascribed the high selectivity of the Ni/SBA-15 catalyst to its moderate hydrogenation activity. They also indicated that the presence of a small amount of water in the reaction system could preferentially promote the hydrolysis of HMFA to form ammonium ions, thus inhibiting the further condensation of HMFA with HMF to form secondary imines. In the catalyst life test, the Ni/SBA-15 catalyst showed slight deactivation after five consecutive runs, mainly caused by oxidation, aggregation, loss of nickel species and carbon deposition. Subsequently, a Ni6AlOx nano-catalyst was prepared by Yuan et al. [66] showing an outstanding HMFA yield of 99% under reaction conditions of 100 °C and 0.1 MPa H2. The partial encapsulation of the Ni and NiO nanoparticles by alumina in the Ni6AlOx catalyst played an important role in the high selectivity of the catalyst. The catalyst could be recycled four times without any apparent loss of activity.
Room-temperature reductive amination of HMF over non-noble metal catalysts was first reported by Wang et al. [64]. They prepared an Al2O3-supported carbon-doped Ni catalyst by the direct pyrolysis-reduction of the mixture of Ni3(BTC)2·12H2O and Al2O3 (Figure 4), and obtained a 96% yield of HMFA. By controlling the pyrolysis reduction temperature, the state of the Ni species and carbon-doping were finely adjusted to provide an air-stable metal Ni0 species, which served as the active site in the reductive amination of HMF. Its remarkable performance was attributed to the synergistic effect of doped carbon, acidic Al2O3 support and air-stabilized metal Ni0 species. Such a Ni@C/Al2O3-400 catalyst also demonstrated stable reusability and a wide range of substrate applicability.
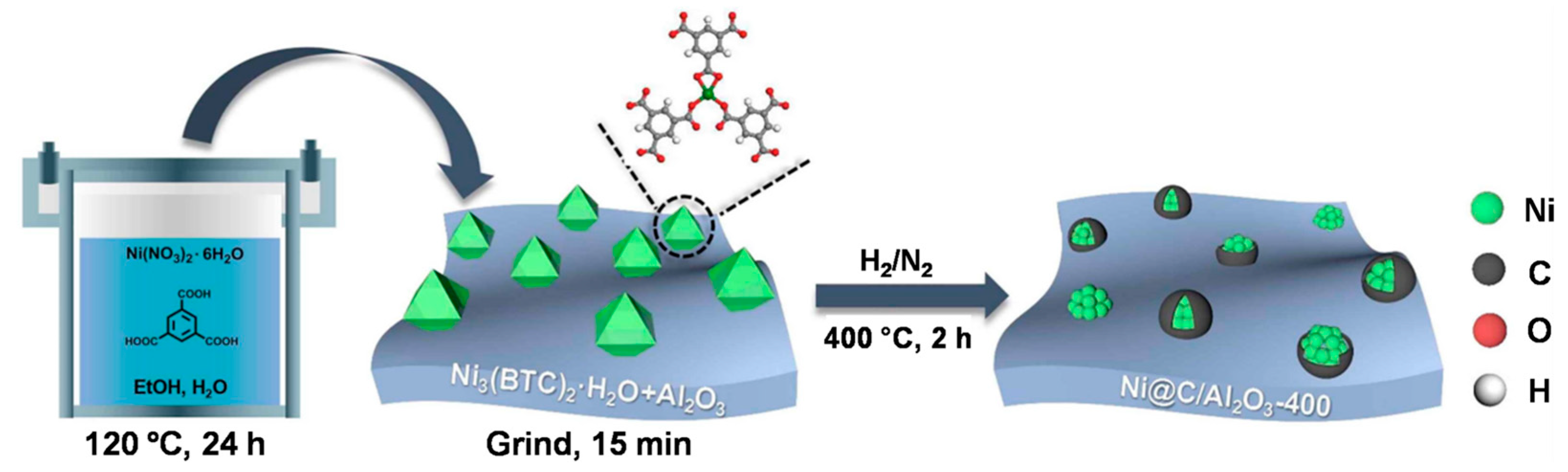
Figure 4. Schematic illustration for the synthesis procedure of a Ni@C/Al2O3-400 catalyst.
Besides nickel catalysts, cobalt-based catalysts were also used in the reductive amination of HMF with NH3. Jagadeesh et al. [63] successfully prepared silica-supported Co nanoparticles by immobilizing and pyrolyzing a cobalt-terephthalic acid-piperazine MOF template on silica. Over this Co-Co3O4@SiO2 catalyst, a 94% yield of HMFA was achieved under mild reaction conditions of 50 °C, 0.5 MPa NH3 and 1 MPa H2. However, the structure–activity relationship of the Co-Co3O4@SiO2 catalyst has not been studied in depth.
2.2.2. Reductive Amination of HMF with Aniline
N-substituted HMFAs are a significant family of chemicals due to their well-known pharmacological properties. Iborra et al. [67] investigated the reductive amination of HMF with aniline over Pd-based catalysts with a similar particle size supported on activated carbon and alumina, respectively, and found that the Pd/C catalyst quantitatively converted HMF to the target N-phenyl-HMFA, while the Pd/Al2O3 showed low selectivity for N-phenyl-HMFA due to the hydrogenation of the furan ring. In the DRIFTS spectra of CO, only the unsaturated Pd atoms were observed over the Pd/C catalyst due to carbonaceous species deposited on the terraces. However, the existence of Pd (111) crystal planes in the Pd/Al2O3 catalyst provided a suitable interaction with the furan ring, leading to the hydrogenation of the furan ring. The Pd/C catalyst also presented high activity in the reductive amination of HMF with other amines and the one-pot reaction starting from nitrobenzene.
Queen et al. [68] developed a novel and highly stable MOF/polymer composite catalyst functionalized with Pd nanoparticles, defined as UiO-67/PpPDA/Pd, giving a high yield of 95% for N-phenyl-HMFA under mild reaction conditions of 50 °C and 0.5 MPa H2. Even at room temperature, HMF could be quantitatively converted into the target amine with extended time. They ascribed the good activity and stability of the composite UiO-67/PpPDA/Pd catalyst to the synergy between the rigid structure of MOF and the high-density Lewis base of the polymer, which together inhibited the aggregation and leaching of Pd nanoparticles.
Cu-based catalysts have been used as active species for reductive amination because they favor the hydrogenation of C=N bonds over C=C bonds. A CuAlOx catalyst was used by Bukhtiyarov et al. [69] for the continuous synthesis of N-substituted HMFAs from the reductive amination of HMF in a flow reactor, and a 97% yield of N-phenyl-HMFA was obtained under reaction conditions of 100 °C and 1 MPa H2, with methanol as a solvent to facilitate the formation of imine intermediates. Over this low-cost Cu-based catalyst, other N-substituted HMFAs could also be produced with good yields.
3. Hydrogen-Borrowing Amination of Bio-Based Furanic Alcohols
The plentiful biomass-based alcohols derived from non-edible lignocellulose are ideal candidates for the sustainable production of amine chemicals. The amination of alcohols to amine can be realized by the so-called hydrogen-borrowing mechanism, also known as the automated hydrogen transfer method, which includes three consecutive steps of dehydrogenation, amination and hydrogenation (Scheme 3) [70]. Specifically, these steps are (i) the dehydrogenation of alcohols to highly reactive aldehydes (or ketones), (ii) the condensation of carbonyl compounds with ammonia to form imines and (iii) the hydrogenation of the imines to form amines over the active sites of the catalyst. Theoretically, no additional H2 is consumed and water is the only by-product, which highlights the advantages of this route with high atomic economy and environmental protection [71][72]. However, high reaction temperatures (150~250 °C) are usually required in the amination of alcohols to activate the C–O bond of alcohols, which is thought of as a rate-determining step. High temperatures will lead to an increase in side-reactions, such as cyclization, overalkylation and polymerization [73]. Therefore, it is important to find an appropriate balance between activity (i.e., the conversion of C–OH bonds) and selectivity (i.e., the avoidance of unwanted side-reactions) by designing specific heterogeneous catalysts.
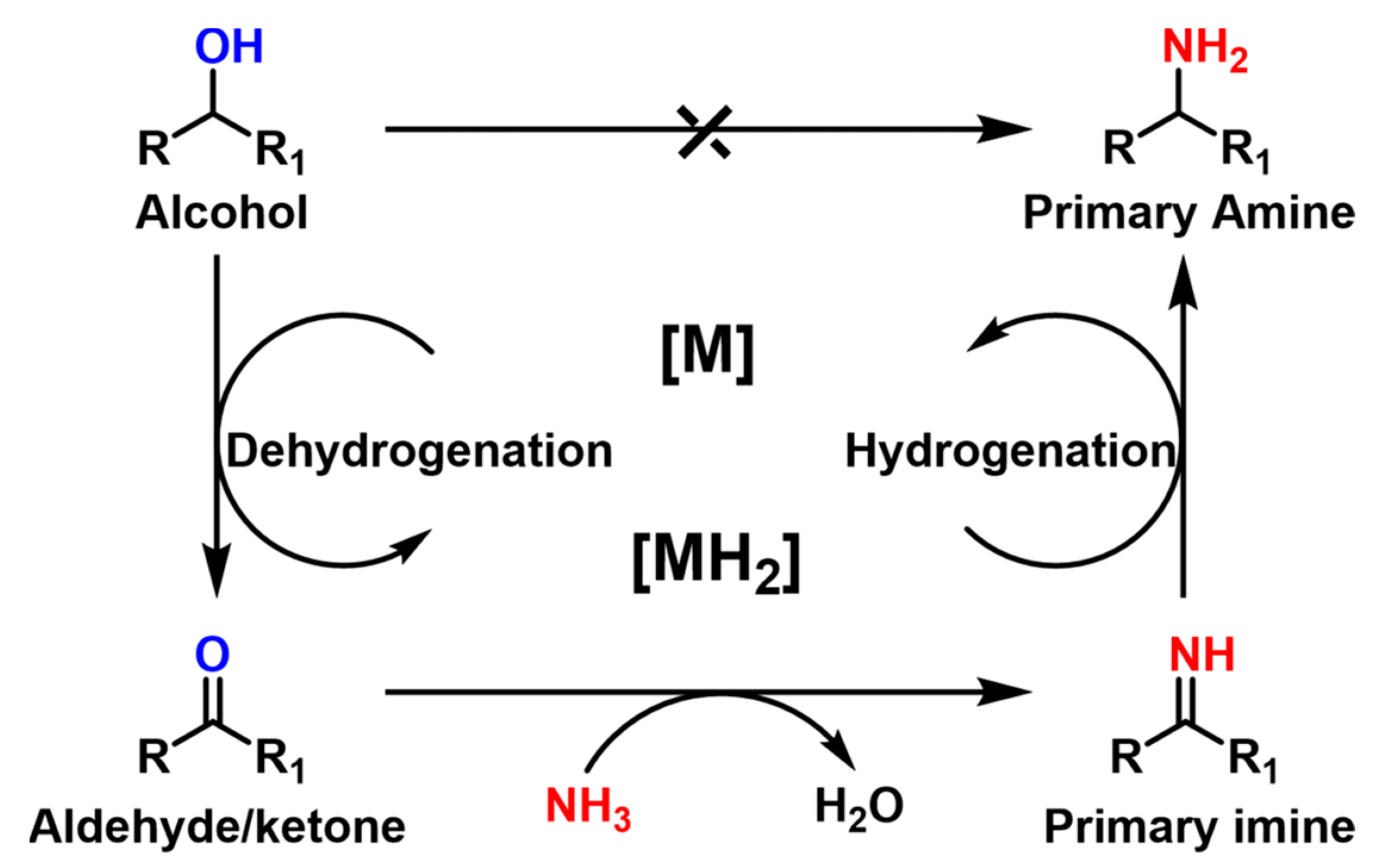
Scheme 3. Amination of alcohols with NH3 by the hydrogen-borrowing mechanism.
3.1. Hydrogen-Borrowing Amination of Furfuryl Alcohol (FA)
FA is one of the most prevalent products of FF hydrogenation and ~62% of the FF produced globally each year is estimated to be converted into FA [74]. In addition to the reductive amination of FF, the amination of FA also represents one of the potential pathways for the synthesis of FAM through a hydrogen-borrowing amination mechanism. Some heterogeneous catalysts containing Ni, Co or Ru are currently available for the amination of FA to FAM.
Hara et al. [75] used a Ru-20MgO/TiO2 catalyst for the amination of FA with a 94% yield of FAM in the absence of H2. They proposed a reaction mechanism that the alcohol was adsorbed on the Ru surface to form deprotonated Ru alkoxide, followed by β-hydride elimination to generate aldehydes. After that, the reversible condensation of the resulting aldehydes with NH3 formed the imines, which were hydrogenated on the concomitant Ru-H species to produce the desired primary amines (Scheme 4). The addition of MgO to the catalyst not only provided electrons for metal Ru, but also promoted the high dispersion of Ru nanoparticles.
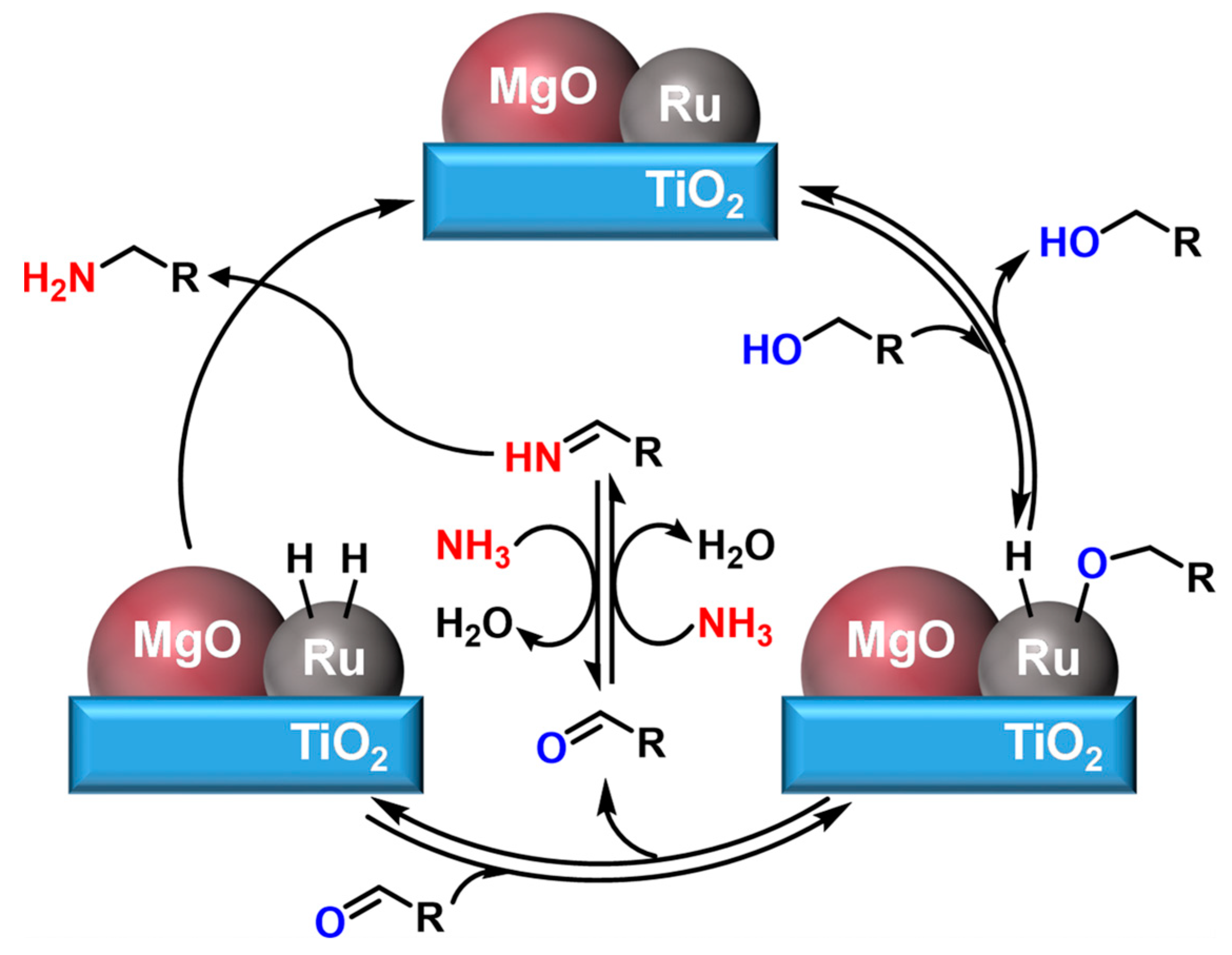
Scheme 4. Proposed reaction mechanism for the amination of alcohols over Ru-20MgO/TiO2.
Besides noble metal Ru, non-noble metal Ni-based catalysts are also active in the amination of FA. Wei et al. [71] used commercial Raney Ni for the amination of FA, obtaining a 77% yield of FAM in the absence of H2. They ascribed the formation of FAM to the proper adsorption capacity of Raney Ni for NH3, H2 and FA molecules. However, the formation of a new Ni3N phase led to the deactivation of the catalyst. It should be noted that Raney Ni could be recycled at least five times without noticeable deactivation in the presence of 1 MPa H2.
Subsequently, using the NixAl catalyst with different Ni/Al mole ratios prepared from a hydrotalcite precursor, Xu et al. [76] carried out the amination of both FA and HMFA to the corresponding FAM and BAMF products. Over a Ni2Al-600 catalyst reduced at 600 °C, an 84% yield of FAM and a 71% yield of BAMF were achieved, respectively. The cooperative effect of its abundant Ni0 sites and appropriate acid–base site density is suggested as an important parameter for the catalyst with good catalytic activity (Scheme 5). However, the formation of Ni3N during the recycling tests led to the gradual deactivation of the catalyst.
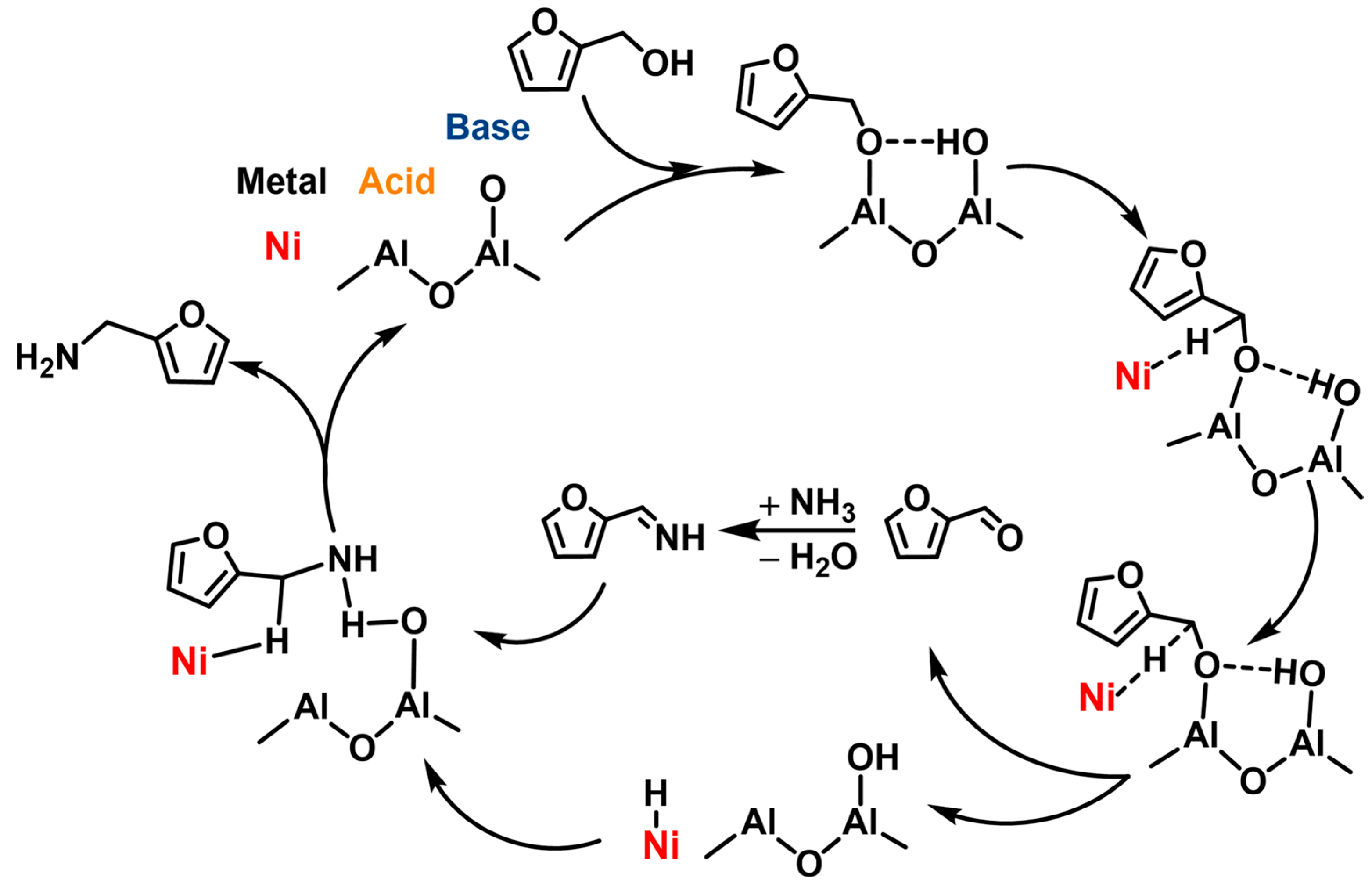
Scheme 5. A possible mechanism of synergistic catalysis of Ni0 and acid-base sites for the amination of FA.
3.2. Hydrogen-Borrowing Amination of Bio-Based Furanic Alkanediols
Alkanediols with five and six carbon atoms generated from FF and HMF are versatile intermediate platform molecules [77][78]. The amination of diols represents an efficient method for biomass valorization to value-added chemicals due to the importance of amines, including aminoalcohols, diamines and N-heterocyclic amines, in the polymer industry [18][20]. More efforts have been made to convert biomass-derived diols into amines, particularly straight-chain diamines, in light of the rising demand for biopolymers.
3.2.1. Hydrogen-Borrowing Amination of 1,5-Pentanediol (PDO)
Research on the amination of PDO over heterogeneous catalysts is quite limited. Kulkarni et al. [79] used a Co-modified ZSM-5 catalyst for the amination of PDO with 87% conversion and a 43% yield toward 5-AP (Figure 5). Moreover, its stability under reaction conditions needs to improve, and the structure–activity relationship of the catalyst needs to be further elucidated.
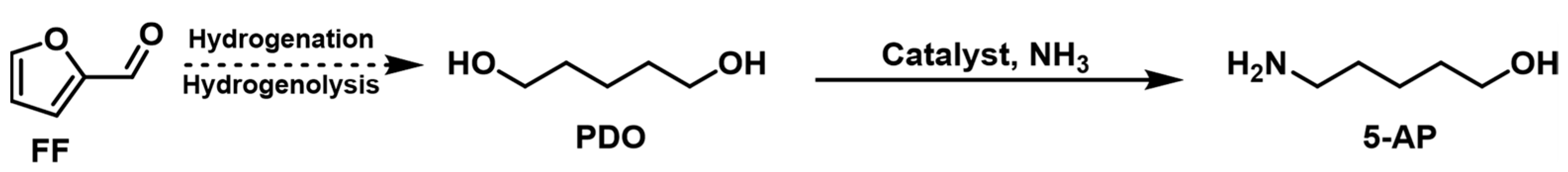
Figure 5. Hydrogen-borrowing amination of PDO and NH3 to synthesize 5-AP.
The heterogeneous catalysts currently used for the di-amination of PDO give a cyclic piperidine product instead of 1,5-pentanediamine because the direct cyclization of the intermediate 5-AP is thermodynamically more favorable than further hydrogen-borrowing amination (Figure 6). For example, Rose et al. [80] studied the amination of PDO with NH3 into piperidine (86% yield) as the main product over a solid Ru/C catalyst in an aqueous solution.
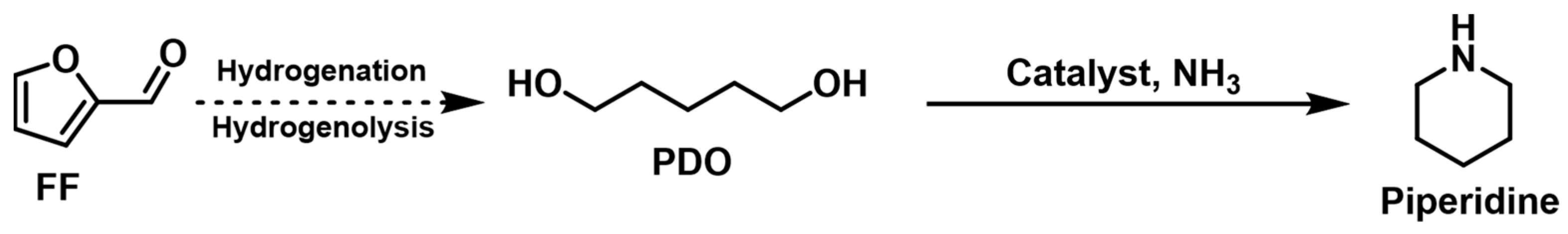
Figure 6. Hydrogen-borrowing amination of PDO and NH3 to synthesize piperidine.
3.2.2. Hydrogen-Borrowing Amination of 1,6-Hexanediol (HDO)
1,6-Hexanediamine (HDA), a key monomer in the synthesis of nylon-66, has traditionally been obtained by the hydrogenation of adiponitrile produced with butadiene and toxic HCN [81]. Many efforts have been made to achieve a green and sustainable synthesis of HDA by the amination of renewable HDO derived from biomass.
Zhao and coworkers [82] performed the amination of HDO over a Ru/Al2O3 catalyst with a 38% yield of HDA in the presence of supercritical NH3. The high dispersion of Ru and medium acid–basicity in the catalyst are crucial factors for its good activity and selectivity. The NH3 with 15 MPa at a supercritical state inhibited side-reactions, such as cyclization, dimerization and oligomerization, thus improving the selectivity of the target product.
The amination of HDO is usually carried out at high temperatures and NH3 pressures, which significantly hinder the actual large-scale amination of HDO. Recently, Rose et al. [83] used a Ru/C catalyst for the amination of HDO to aminoalcohols or diamines under the reaction conditions of 190 °C, 2.5 MPa H2 and NH3 aqueous solution. In the presence of Cs2CO3, a 26% yield of 6-amino-1-hexanol was achieved. On the other hand, a 34% yield of HDA was obtained in the presence of Ba(OH)2. They concluded that the presence of the base facilitated the dehydrogenation of alcohols and the condensation of carbonyl intermediates with NH3.
Tetramethyl diamines, an industrially important class of fine chemicals, are often produced from alkyl halides with large salt-containing waste. The amination of diols with dimethylamine represents one of the potential pathways for producing tetramethyl diamines. Yan et al. [84] developed a Cu/ZnO/γ-Al2O3 catalyst for the amination of HDO with dimethylamine to produce N,N,N′,N′-tetramethyl-1,6-hexanediamine (TMHDA) in a fixed-bed reactor. Over this Cu/ZnO/γ-Al2O3 catalyst, a 93% yield of TMHDA was achieved under reaction conditions of 180 °C and 3 MPa H2. The doped ZnO could effectively improve the dispersion of active Cu and reduce the particle size of Cu, owing to the strong interaction between Cu and ZnO, which help the catalyst expose more active sites and thus promote the catalytic activity.
N-alkyl amines are extensively used in the production of various materials, pharmaceuticals and pesticides. Shi and coworkers [85] used a non-noble CuNiAlOx catalyst synthesized by the coprecipitation method for the amination of C4-C6 linear diols to produce aminoalcohol or diamine products. At low catalyst concentrations, aminoalcohol was obtained as the main product. At high catalyst concentrations, the conversion of diol to dialdehyde was enhanced, followed by dehydration to form an imine and hydrogenation to form a cyclization by-product. Additionally, the presence of abundant amine could quickly react with the in-situ generated dialdehyde, which prohibited the cyclization side-reaction and promoted diamine production.
3.3. Reductive Amination and Hydrogen-Borrowing Amination of HMF to Synthesize BAMF
As mentioned in the section on the reductive amination of DFF, BAMF is an emerging monomer for various bio-based polymers, such as polyurethane, polyamine and polyurea [86][87][88]. Except for the reduced amination of DFF, the direct amination of HMF provided an alternative pathway for the synthesis of BAMF. As shown in Figure 7, many multi-step routes for the synthesis of BAMF from HMF have been developed [27][43][89][90][91]. The direct amination of HMF to BAMF is possibly preferable to multi-step procedures.
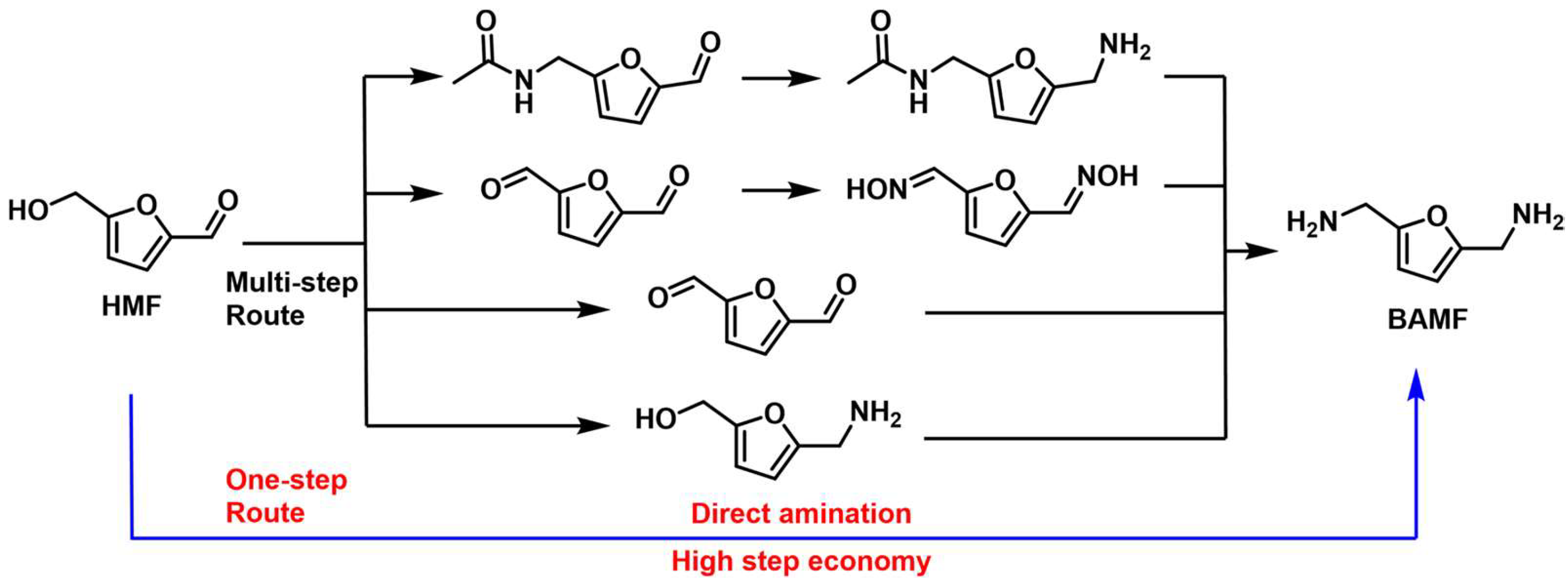
Figure 7. Catalytic synthesis of BAMF from HMF.
Based on the high dehydrogenation performance of copper and the good hydrogenation performance of nickel, Shi et al. [92] developed a simple and efficient bifunctional non-noble CuNiAlOx catalyst for the conversion of HMF to BAMF in a one-pot process. Owing to bulk Cu and highly dispersed Ni species, BAMF with a yield up to 86% was attained over Cu4Ni1Al4Ox in the presence of a Na2CO3 co-catalyst for promoting the dehydrogenation reaction.
Wei et al. [93] investigated different commercial catalysts for the conversion of HMF to BAMF and obtained a 61% BAMF yield over Raney Ni under the conditions of 160 °C, 0.35 MPa NH3 and 1.0 MPa H2. The DFT calculation reveals that the difference in the adsorption energies of metal Ni to NH3 and H2 is much lower than that of other metals, which reduces ammonia coverage on the catalyst surface, resulting in more vacancy active centers to adsorb and activate hydrogen. Later, they [94] achieved up to an 86% yield of BAMF over γ-Al2O3-supported Ni catalysts with around 10 wt% Ni loadings under the same conditions as Raney Ni. The incorporation of a proper amount of Mn enhanced the reaction stability of the catalyst.
References
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402.
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421.
- Feng, Y.; Long, S.; Tang, X.; Sun, Y.; Luque, R.; Zeng, X.; Lin, L. Earth-abundant 3d-transition-metal catalysts for lignocellulosic biomass conversion. Chem. Soc. Rev. 2021, 50, 6042–6093.
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624.
- Gerardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347.
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117.
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963.
- Mura, M.G.; Luca, L.D.; Giacomelli, G.; Porcheddu, A. Formic acid: A promising bio-renewable feedstock for fine chemicals. Adv. Synth. Catal. 2012, 354, 3180–3186.
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613.
- Bhaumik, P.; Dhepe, P.L. Solid acid catalyzed synthesis of furans from carbohydrates. Catal. Rev. 2016, 58, 36–112.
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306.
- Jiang, S.; Ramdani, W.; Muller, E.; Ma, C.; Pera-Titus, M.; Jerome, F.; De Oliveira Vigiera, K. Direct catalytic conversion of furfural to furan-derived amines in the presence of Ru-based catalyst. ChemSusChem 2020, 13, 1699–1704.
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328.
- He, J.; Chen, L.; Liu, S.; Song, K.; Yang, S.; Riisager, A. Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds. Green Chem. 2020, 22, 6714–6747.
- Chen, X.; Liu, Y.; Wang, J. Lignocellulosic biomass upgrading into valuable nitrogen-containing compounds by heterogeneous catalysts. Ind. Eng. Chem. Res. 2020, 59, 17008–17025.
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332.
- Chandrashekhar, V.G.; Baumann, W.; Beller, M.; Jagadeesh, R.V. Nickel-catalyzed hydrogenative coupling of nitriles and amines for general amine synthesis. Science 2022, 376, 1433–1441.
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224.
- Chen, X.; Song, S.; Li, H.; Gözaydın, G.; Yan, N. Expanding the boundary of biorefinery: Organonitrogen chemicals from biomass. Acc. Chem. Res. 2021, 54, 1711–1722.
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward renewable amines: Recent advances in the catalytic amination of biomass-derived oxygenates. ACS Catal. 2022, 12, 10400–10440.
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331.
- Pera-Titus, M.; Shi, F. Catalytic amination of biomass-based alcohols. ChemSusChem 2014, 7, 720–722.
- Saini, M.K.; Kumar, S.; Li, H.; Babu, S.A.; Saravanamurugan, S. Advances in the catalytic reductive amination of furfural to furfural amine: The momentous role of active metal sites. ChemSusChem 2022, 15, e202200107.
- Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Reductive amination of levulinic acid or its derivatives to pyrrolidones over heterogeneous catalysts in the batch and continuous flow reactors: A review. Renew. Sustain. Energy Rev. 2021, 143, 110876.
- Truong, C.C.; Mishra, D.K.; Suh, Y.W. Recent catalytic advances on the sustainable production of primary furanic amines from the one-pot reductive amination of 5-hydroxymethylfurfural. ChemSusChem 2023, 16, e202201846.
- Xie, C.; Song, J.L.; Wu, H.R.; Hu, Y.; Liu, H.Z.; Zhang, Z.R.; Zhang, P.; Chen, B.F.; Han, B.X. Ambient reductive amination of levulinic acid to pyrrolidones over Pt nanocatalysts on porous TiO2 nanosheets. J. Am. Chem. Soc. 2019, 141, 4002–4009.
- Qi, H.; Liu, F.; Zhang, L.; Li, L.; Su, Y.; Yang, J.; Hao, R.; Wang, A.; Zhang, T. Modulating trans-imination and hydrogenation towards the highly selective production of primary diamines from dialdehydes. Green Chem. 2020, 22, 6897–6901.
- Qi, H.; Yang, J.; Liu, F.; Zhang, L.; Yang, J.; Liu, X.; Li, L.; Su, Y.; Liu, Y.; Hao, R.; et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones. Nat. Commun. 2021, 12, 3295.
- Irrgang, T.; Kempe, R. Transition-metal-catalyzed reductive amination employing hydrogen. Chem. Rev. 2020, 120, 9583–9674.
- Wang, Y.; Furukawa, S.; Fu, X.; Yan, N. Organonitrogen chemicals from oxygen-containing feedstock over heterogeneous catalysts. ACS Catal. 2019, 10, 311–335.
- Luo, D.; He, Y.; Yu, X.; Wang, F.; Zhao, J.; Zheng, W.; Jiao, H.; Yang, Y.; Li, Y.; Wen, X. Intrinsic mechanism of active metal dependent primary amine selectivity in the reductive amination of carbonyl compounds. J. Catal. 2021, 395, 293–301.
- Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of primary amines by reductive amination of biomass-derived aldehydes/ketones. Angew. Chem. Int. Ed. 2017, 56, 3050–3054.
- Xie, C.; Song, J.; Hua, M.; Hu, Y.; Huang, X.; Wu, H.; Yang, G.; Han, B. Ambient-temperature synthesis of primary amines via reductive amination of carbonyl compounds. ACS Catal. 2020, 10, 7763–7772.
- Ho, C.R.; Defalque, V.; Zheng, S.; Bell, A.T. Propanol amination over supported nickel catalysts: Reaction mechanism and role of the support. ACS Catal. 2019, 9, 2931–2939.
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206.
- Dutta, S.; De, S.; Saha, B.; Alam, M.I. Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels. Catal. Sci. Technol. 2012, 2, 2025–2036.
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640.
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189.
- Le, G.; Xie, L.; Wang, Y.; Dai, L. Efficient conversion of furfural to furfural amine over 4Ru1Co/AC catalyst. Appl. Catal. A Gen. 2022, 647, 118902.
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis. RSC Adv. 2021, 11, 27042–27058.
- Caetano, J.A.T.; Fernandes, A.C. One-pot synthesis of amines from biomass resources catalyzed by HReO4. Green Chem. 2018, 20, 2494–2498.
- Liu, J.; Song, Y.; Ma, L. Earth-abundant metal-catalyzed reductive amination: Recent advances and prospect for future catalysis. Chem. Asian J. 2021, 16, 2371–2391.
- Komanoya, T.; Kinemura, T.; Kita, Y.; Kamata, K.; Hara, M. Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds. J. Am. Chem. Soc. 2017, 139, 11493–11499.
- Deng, D.; Kita, Y.; Kamata, K.; Hara, M. Low-temperature reductive amination of carbonyl compounds over Ru deposited on Nb2O5·nH2O. ACS Sustain. Chem. Eng. 2018, 7, 4692–4698.
- Chandra, D.; Inoue, Y.; Sasase, M.; Kitano, M.; Bhaumik, A.; Kamata, K.; Hosono, H.; Hara, M. A high performance catalyst of shape-specific ruthenium nanoparticles for production of primary amines by reductive amination of carbonyl compounds. Chem. Sci. 2018, 9, 5949–5956.
- Gao, M.; Jia, X.; Ma, J.; Fan, X.; Gao, J.; Xu, J. Self-regulated catalysis for the selective synthesis of primary amines from carbonyl compounds. Green Chem. 2021, 23, 7115–7121.
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098.
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139.
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81.
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733.
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective synthesis of furfurylamine by reductive amination of furfural over Raney cobalt. ChemCatChem 2019, 11, 5562–5569.
- Zhuang, X.; Liu, J.; Zhong, S.; Ma, L. Selective catalysis for the reductive amination of furfural toward furfurylamine by graphene-co-shelled cobalt nanoparticles. Green Chem. 2022, 24, 271–284.
- Yogita; Rao, K.T.V.; Kumar, P.M.; Lingaiah, N. Cobalt nanoparticles embedded in a nitrogen-doped carbon matrix for reductive amination of biomass-derived furfural to furfurylamine. Sustain. Energy Fuels 2022, 6, 4692–4705.
- Dong, C.; Wu, Y.; Wang, H.; Peng, J.; Li, Y.; Samart, C.; Ding, M. Facile and efficient synthesis of primary amines via reductive amination over a Ni/Al2O3 catalyst. ACS Sustain. Chem. Eng. 2021, 9, 7318–7327.
- Zhang, J.; Yang, J.; Li, X.; Liu, H.; Wang, A.; Xia, C.; Chen, J.; Huang, Z. Efficient synthesis of pharmaceutical intermediates from biomass-derived aldehydes and ketones over robust NixAl nanocatalysts. ACS Sustain. Chem. Eng. 2022, 10, 5526–5537.
- Yang, Y.Z.; Zhou, L.L.; Wang, X.C.; Zhang, L.Y.; Cheng, H.Y.; Zhao, F.Y. Catalytic reductive amination of furfural to furfurylamine on robust ultra-small Ni nanoparticles. Nano Res. 2022.
- Song, W.; Wan, Y.; Li, Y.; Luo, X.; Fang, W.; Zheng, Q.; Ma, P.; Zhang, J.; Lai, W. Electronic Ni–N interaction enhanced reductive amination on an N-doped porous carbon supported Ni catalyst. Catal. Sci. Technol. 2022, 12, 7208–7218.
- Bhunia, M.K.; Chandra, D.; Abe, H.; Niwa, Y.; Hara, M. Synergistic effects of earth-abundant metal-metal oxide enable reductive amination of carbonyls at 50 °C. ACS Appl. Mater. Interfaces 2022, 14, 4144–4154.
- Martínez, J.J.; Nope, E.; Rojas, H.; Brijaldo, M.H.; Passos, F.; Romanelli, G. Reductive amination of furfural over Me/SiO2–SO3H (Me: Pt, Ir, Au) catalysts. J. Mol. Catal. A Chem. 2014, 392, 235–240.
- Lin, C.; Zhou, J.; Zheng, Z.; Chen, J. An efficient approach to biomass-based tertiary amines by direct and consecutive reductive amination of furfural. J. Catal. 2022, 410, 164–179.
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026.
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554.
- Chandrashekhar, V.G.; Natte, K.; Alenad, A.M.; Alshammari, A.S.; Kreyenschulte, C.; Jagadeesh, R.V. Reductive amination, hydrogenation and hydrodeoxygenation of 5-hydroxymethylfurfural using silica-supported cobalt-nanoparticles. ChemCatChem 2021, 14, e202101234.
- Hu, Q.; Jiang, S.; Wu, Y.; Xu, H.; Li, G.; Zhou, Y.; Wang, J. Ambient-temperature reductive amination of 5-hydroxymethylfurfural over Al2O3-supported carbon-doped nickel catalyst. ChemSusChem 2022, 15, e202200192.
- Chen, W.; Sun, Y.; Du, J.; Si, Z.; Tang, X.; Zeng, X.; Lin, L.; Liu, S.; Lei, T. Preparation of 5-(aminomethyl)-2-furanmethanol by direct reductive amination of 5-hydroxymethylfurfural with aqueous ammonia over the Ni/SBA-15 catalyst. J. Appl. Chem. Biotechnol. 2018, 93, 3028–3034.
- Yuan, H.; Li, J.-P.; Su, F.; Yan, Z.; Kusema, B.T.; Streiff, S.; Huang, Y.; Pera-Titus, M.; Shi, F. Reductive amination of furanic aldehydes in aqueous solution over versatile NiyAlOx catalysts. ACS Omega 2019, 4, 2510–2516.
- García-Ortiz, A.; Vidal, J.D.; Climent, M.J.; Concepción, P.; Corma, A.; Iborra, S. Chemicals from biomass: Selective synthesis of N-substituted furfuryl amines by the one-pot direct reductive amination of furanic aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 6243–6250.
- Karve, V.V.; Sun, D.T.; Trukhina, O.; Yang, S.; Oveisi, E.; Luterbacher, J.; Queen, W.L. Efficient reductive amination of HMF with well dispersed Pd nanoparticles immobilized in a porous MOF/polymer composite. Green Chem. 2020, 22, 368–378.
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarov, V.I. Two-step one-pot reductive amination of furanic aldehydes using CuAlOx catalyst in a flow reactor. Molecules 2020, 25, 4771.
- Niu, F.; Bahri, M.; Ersen, O.; Yan, Z.; Kusema, B.T.; Khodakov, A.Y.; Ordomsky, V.V. A multifaceted role of a mobile bismuth promoter in alcohol amination over cobalt catalysts. Green Chem. 2020, 22, 4270–4278.
- Liu, Y.; Zhou, K.; Shu, H.; Liu, H.; Lou, J.; Guo, D.; Wei, Z.; Li, X. Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel. Catal. Sci. Technol. 2017, 7, 4129–4135.
- Niu, F.; Xie, S.; Yan, Z.; Kusema, B.T.; Ordomsky, V.V.; Khodakov, A.Y. Alcohol amination over titania-supported ruthenium nanoparticles. Catal. Sci. Technol. 2020, 10, 4396–4404.
- Liang, G.; Zhou, Y.; Zhao, J.; Khodakov, A.Y.; Ordomsky, V.V. Structure-sensitive and insensitive reactions in alcohol amination over nonsupported Ru nanoparticles. ACS Catal. 2018, 8, 11226–11234.
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676.
- Kita, Y.; Kuwabara, M.; Yamadera, S.; Kamata, K.; Hara, M. Effects of ruthenium hydride species on primary amine synthesis by direct amination of alcohols over a heterogeneous Ru catalyst. Chem. Sci. 2020, 11, 9884–9890.
- Zhou, K.; Xie, R.; Xiao, M.; Guo, D.; Cai, Z.; Kang, S.; Xu, Y.; Wei, J. Direct amination of biomass-based furfuryl alcohol and 5-(aminomethyl)-2-furanmethanol with NH3 over hydrotalcite-derived nickel catalysts via the hydrogen-borrowing strategy. ChemCatChem 2021, 13, 2074–2085.
- Enjamuri, N.; Darbha, S. Solid catalysts for conversion of furfural and its derivatives to alkanediols. Catal. Rev. 2020, 62, 566–606.
- He, J.; Huang, K.; Barnett, K.J.; Krishna, S.H.; Alonso, D.M.; Brentzel, Z.J.; Burt, S.P.; Walker, T.; Banholzer, W.F.; Maravelias, C.T.; et al. New catalytic strategies for alpha,omega-diols production from lignocellulosic biomass. Faraday Discuss. 2017, 202, 247–267.
- Rani, V.R.; Srinivas, N.; Kulkarni, S.J.; Raghavan, K.V. Amino cyclization of terminal (alpha,omega)-diols over modified ZSM-5 catalysts. J. Mol. Catal. A Chem. 2002, 187, 237–246.
- Niemeier, J.; Engel, R.V.; Rose, M. Is water a suitable solvent for the catalytic amination of alcohols? Green Chem. 2017, 19, 2839–2845.
- Dros, A.B.; Larue, O.; Reimond, A.; De Campo, F.; Pera-Titus, M. Hexamethylenediamine (HMDA) from fossil- vs. bio-based routes: An economic and life cycle assessment comparative study. Green Chem. 2015, 17, 4760–4772.
- Li, Y.; Cheng, H.Y.; Zhang, C.; Zhang, B.; Liu, T.; Wu, Q.F.; Su, X.L.N.; Lin, W.W.; Zhao, F.Y. Reductive amination of 1,6-hexanediol with Ru/Al2O3 catalyst in supercritical ammonia. Sci. China Chem. 2017, 60, 920–926.
- Gupta, N.K.; Palenicek, P.; Nortmeyer, L.; Meyer, G.M.; Schäfer, T.; Hellmann, T.; Hofmann, J.P.; Rose, M. Modulating catalytic selectivity by base addition in aqueous reductive amination of 1,6-hexanediol using Ru/C. ACS Sustain. Chem. Eng. 2022, 10, 14560–14567.
- Cai, X.; Ke, Y.; Wang, B.; Zeng, Y.; Chen, L.; Li, Y.; Bai, G.; Yan, X. Efficient catalytic amination of diols to diamines over Cu/ZnO/γ-Al2O3. Mol. Catal. 2021, 508, 111608.
- Wu, Y.; Yuan, H.; Shi, F. Sustainable catalytic amination of diols: From cycloamination to monoamination. ACS Sustain. Chem. Eng. 2017, 6, 1061–1067.
- Lichtenthaler, F.W. Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737.
- Kreye, O.; Mutlu, H.; Meier, M.A.R. Sustainable routes to polyurethane precursors. Green Chem. 2013, 15, 1431–1455.
- Gandini, A.; Belgacem, M.N. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379.
- Le, N.-T.; Byun, A.; Han, Y.; Lee, K.-I.; Kim, H. Preparation of 2,5-bis(aminomethyl)furan by direct reductive amination of 2,5-diformylfuran over nickel-Raney catalysts. Green Sustain. Chem. 2015, 5, 115–127.
- Wang, X.; Chen, W.; Li, Z.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Synthesis of bis(amino)furans from biomass based 5-hydroxymethylfurfural. J. Energy Chem. 2018, 27, 209–214.
- Xu, Y.; Jia, X.; Ma, J.; Gao, J.; Xia, F.; Li, X.; Xu, J. Selective synthesis of 2,5-bis(aminomethyl)furan via enhancing the catalytic dehydration–hydrogenation of 2,5-diformylfuran dioxime. Green Chem. 2018, 20, 2697–2701.
- Yuan, H.; Kusema, B.T.; Yan, Z.; Streiff, S.; Shi, F. Highly selective synthesis of 2,5-bis(aminomethyl)furan via catalytic amination of 5-(hydroxymethyl)furfural with NH3 over a bifunctional catalyst. RSC Adv. 2019, 9, 38877–38881.
- Zhou, K.; Liu, H.; Shu, H.; Xiao, S.; Guo, D.; Liu, Y.; Wei, Z.; Li, X. A comprehensive study on the reductive amination of 5-hydroxymethylfurfural into 2,5-bisaminomethylfuran over Raney Ni through DFT calculations. ChemCatChem 2019, 11, 2649–2656.
- Wei, Z.; Cheng, Y.; Huang, H.; Ma, Z.; Zhou, K.; Liu, Y. Reductive amination of 5-hydroxymethylfurfural to 2,5-bis(aminomethyl)furan over alumina-supported Ni-based catalytic systems. ChemSusChem 2022, 15, e202200233.
More
Information
Subjects:
Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
18 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





