You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | A K M Helal Morshed | -- | 1319 | 2023-04-18 07:44:06 | | | |
| 2 | Conner Chen | Meta information modification | 1319 | 2023-04-19 07:36:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Morshed, A.K.M.H.; Paul, S.; Hossain, A.; Basak, T.; Hossain, M.S.; Hasan, M.M.; Hasibuzzaman, M.A.; Rahaman, T.I.; Mia, M.A.R.; Shing, P.; et al. Baicalein. Encyclopedia. Available online: https://encyclopedia.pub/entry/43145 (accessed on 02 January 2026).
Morshed AKMH, Paul S, Hossain A, Basak T, Hossain MS, Hasan MM, et al. Baicalein. Encyclopedia. Available at: https://encyclopedia.pub/entry/43145. Accessed January 02, 2026.
Morshed, A K M Helal, Supti Paul, Arafat Hossain, Tuli Basak, Md. Sanower Hossain, Md. Mehedi Hasan, Md. Al Hasibuzzaman, Tanjim Ishraq Rahaman, Md. Abdur Rashid Mia, Pollob Shing, et al. "Baicalein" Encyclopedia, https://encyclopedia.pub/entry/43145 (accessed January 02, 2026).
Morshed, A.K.M.H., Paul, S., Hossain, A., Basak, T., Hossain, M.S., Hasan, M.M., Hasibuzzaman, M.A., Rahaman, T.I., Mia, M.A.R., Shing, P., Sohel, M., Bibi, S., Dey, D., Biswas, P., Hasan, M.N., Ming, L.C., & Tan, C.S. (2023, April 18). Baicalein. In Encyclopedia. https://encyclopedia.pub/entry/43145
Morshed, A K M Helal, et al. "Baicalein." Encyclopedia. Web. 18 April, 2023.
Copy Citation
Baicalein, a flavonoid extract (5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one) derived from the dried root of Scutellaria baicalensis Georgi, can inhibit cancer-promoting mechanisms such as metastasis, angiogenesis, and inflammation without harming healthy cells. Despite having enormous prospects for anti-cancer use, low bioavailability limits its applications.
Baicalein
ROS
apoptosis
1. Introduction
Cancer is a group of diseases defined by aberrant cell proliferation, which can invade or spread to different body parts [1]. Cancer is one of the leading causes of death worldwide and a major global health problem, and death and morbidity are escalating in both industrialized and developing regions. As a result, this public health burden needs to receive much greater attention. In wealthy countries, the survival rate of childhood cancer, in particular, has substantially increased over time, related to economic, environmental, and genetic factors. Still, it remains low in poor and middle-income countries [2]. Recent data suggest that tobacco use accounts for roughly 22% of cancer fatalities [3], and obesity, poor nutrition, lack of physical exercise, and excessive alcohol consumption account for 10% [4].
Extraordinary improvements in the detection and treatment of cancer have been made possible by developments in science and technology. An estimation showed that two out of every five people would develop cancer at some point [5]. The American Cancer Society evaluates cancer cases and related reports annually in the US, and its most recent estimation in 2021 publicized 1,898,160 cases with 608,570 mortalities [6]. According to the Global Cancer Observatory, owned by WHO, the highest cancer rate was found in Hungary at 371 cancer patients per 100,000 people. In Asia, the Republic of Korea (314 cases per 100,000 population) has the highest reported cancer incidence [7].
Most tumors remain an insurmountable challenge to eradicate for the modern medical system. Today, surgery, radiation, chemotherapy, and immunotherapy are the main therapeutic modalities for most malignancies. Due to the severe side effects of chemotherapy medications and the prevalence of numerous types of drug resistance, therapeutic effects are severely diminished despite the alternative therapies. The treatment type depends on the tumor’s location, grade, and sickness stage, as well as the patient’s general condition [8]. A variety of cancer therapies in the experimental phase are also being developed.
Recent research has identified traditional Chinese medicines as a new source of anti-cancer medications to lessen the side effects of cancer chemotherapies. Natural agents provide various benefits, including excellent price, accessibility, and lower toxicity. In today’s market, there are four types of plant-derived anti-cancer agents available: taxanes (paclitaxel and docetaxel), epipodophyllotoxins (etoposide and teniposide), camptothecin derivatives (camptothecin and irinotecan), and the vinca alkaloids (vinblastine, vincristine, and vindesine) [9]. Disease prevention and treatment have traditionally been accomplished by using herbal plants. Many individuals still use herbal nutraceuticals as their primary form of medicine. More than half of the medications used in clinical trials are made from natural products. Many researchers have looked into numerous herbal remedies and natural products in cancer treatment in recent years [10]. A lot of evidence shows the excellent efficacy of Baicalein in treating and preventing many types of cancer.
Baicalein, a flavonoid extract (5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one) derived from the dried root of Scutellaria baicalensis Georgi, can inhibit cancer-promoting mechanisms such as metastasis, angiogenesis, and inflammation without harming healthy cells [11]. Despite having enormous prospects for anti-cancer use, low bioavailability limits its applications. Baicalin is a glycoside, a compound that results from the combination of Baicalein and glucose, and is more water soluble than Baicalein with greater bioavailability. However, the anticancer and anti-angiogenesis efficacy of Baicalin is lower than that of baicalein [12]. More specifically, the advancement is that the target mechanisms of signaling pathways of baicalein’s anti-cancer potential have been well developed. Baicalein’s anti-tumor properties mostly rely on its ability to inhibit numerous complex cascades. Baicalein acts on cyclins that control the cell cycle, oxidative radical scavenging, mitogen-activated protein kinase (MAPK), protein kinase B (Akt), mammalian target of rapamycin (mTOR), MMP-2/-9 (matrix metalloproteinase-2/-9) expression, and caspase-9/-3 activation. This induces apoptosis and inhibits tumor invasion, metastasis, and progression [13]. It has been utilized as an antioxidant, anti-viral, anti-bacterial, anti-inflammatory, anti-allergic, and other applications [14]. Additionally, Baicalein has been known to have anti-cancer properties for some time [15].
Baicalein exerts its actions via several biological processes, such as inhibition of cell proliferation, metastasis, angiogenesis, and inflammation and promotion of apoptotic cancer-cell death and autophagy [16]. Since cancer treatment methods include standard resection and chemotherapy, which carry a high risk of death, there is a lot of interest in locating a natural treatment that is reasonably non-toxic and may help to reduce side effects without compromising the therapeutic efficacy. Baicalein has the potential for such a role, according to numerous studies [17]. It inhibits cancer cell growth [18], promotes apoptosis [19], and causes cell cycle arrest in hepatocellular [15], human breast [20], myeloma [21], T24 bladder cancer cells [22], and prostate cancer [23]. Baicalein has shown considerable promise as a treatment for lung cancer in many studies [24].
Additionally, Baicalein prevented the growth of various chemotherapy cancers in mouse models [25]. When used in conjunction with chemotherapy, Baicalein could be established as a novel anti-cancer medication to treat cancers [26]. It has shown considerable promise in treating and preventing cancer with few side effects like constipation, stomach pain, and vomiting [27].
2. Overview of Baicalein
As chemotherapeutic and nutritional chemopreventive agents, natural agents are gaining popularity. They have numerous benefits, including increased availability, cost-effectiveness, and reduced toxicity. Baicalein is an antioxidant sourced from the stem of Scutellaria baicalensis and Oroxylum indicum plants (generally known as Chinese Huang Qin) [10][28]. More than 50 flavonoids were isolated from the stems of S. baicalensis Georgi. Flavonoid consumption is linked to a lower risk of developing cancer, inflammatory processes, and cardiovascular disease. Baicalein has a crucial, intense characteristic feature responsible for its pharmacological activity [28][29][30][31]. Baicalein (C15H10O5), also known medically as 5,6,7- trihydroxyflavone (Figure 1), is a flavonoid substance with a polymer backbone of a two-phenyl-chromen-4-one (2-phenyl-1-benzopyran-4-one) [28]. The molecular weight of baicalein is 270.24 Da.
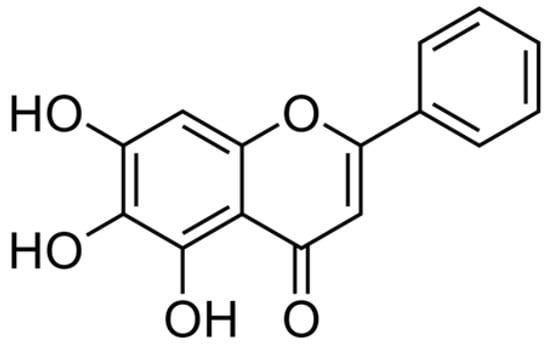
Figure 1. Chemical structure of Baicalein (Source: PubChem CID, 5281605).
Relative to the colon, the gastric region and small intestine are better at absorbing baicalein. According to Biopharmaceutic Classification System (BCS) techniques, gut permeability steadily declined from the duodenal regions to the colonic part. Baicalein absorption in various gut regions could be subjected to passive transport processes and substantial metabolism, both in vivo and in vitro [32].
According to a laboratory study in a rat model, bile dramatically enhances the absorption of Baicalein [33]. Baicalein metabolites dominated the rats’ circulatory bloodstream following direct and parenteral bulk application [34]. After receiving oral medication, it was shown that intestinal flora was crucial in determining the distinct baicalein metabolites (Baicalin, Oroxylin A, Oroxin A, Chrysin, Baicalein-6-O-glucoside, Baicalein-6-O-glucuronide, Baicalein-6,7-di-O-glucuronide, Baicalein-sulfate, etc.) which were accessible in multiple digestive tract regions [34]. Those metabolites were found by some complex approaches that occurred in the gut mucosa, like glucuronidation, glycoxidation, methylation, and sulfation.
In addition to the gut, the liver substantially metabolizes Baicalein, which aids in pre-systemic metabolism [35]. A metabolic by-product known as 7-methoxybaicalein 6-O-glucuronide was found in a serum specimen following oral ingestion of baicalein granules in human subjects [36]. Significant glucuronides of baicalein, such as Baicalin, are produced in large quantities in the hepatocytes and intestinal microsomes of humans and mice.
Pre-systemic metabolism of Baicalein involves the enzyme UDP-glucuronosyltransferase. UDP-glucuronosyltransferase 1A9 was shown to have the maximum hepatic drug clearance and Vmax when calculated against other UDP-glucuronosyltransferases, making it the most effective in converting baicalein into Baicalin [37]. Baicalein is converted by catechol-O-methyltransferase to the methylated residue oroxylin A. Oroxylin A-7-O-β-D-glucuronide is produced by additional metabolic processing with UGT and is a component of human micturition [38].
Studies on Baicalein have indicated that it possesses multiple beneficial characteristics, such as being effective against oxidative stress, acute and chronic inflammation, malignancy, diabetes mellitus, and ulcerative colitis. Additionally, it has anti-thrombotic and anti-viral effects with cardioprotective, neuroprotective, eye-protective, and hepatoprotective features [39][40][41]. All of these functions are achieved by targeting a variety of critical signaling cascades. However, there is a paucity of information concerning these substances’ therapeutic applications and optimum dosages.
References
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612.
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183.
- Yang, M.; Li, X.; Li, H.; Zhang, X.; Liu, X.; Song, Y. Baicalein inhibits RLS3-induced ferroptosis in melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72.
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci 2018, 18, 251–279.
- Sajwani, F.H. Frondoside A is a potential anticancer agent from sea cucumbers. J. Cancer Res. Ther. 2019, 15, 953.
- Huang, S.; Zhang, Z.; Li, W.; Kong, F.; Yi, P.; Huang, J.; Mao, D.; Peng, W.; Zhang, S. Network pharmacology-based prediction and verification of the active ingredients and potential targets of zuojinwan for treating colorectal cancer. Drug Des. Dev. Ther. 2020, 14, 2725.
- Donald, G.; Hertzer, K.; Eibl, G. Baicalein-an intriguing therapeutic phytochemical in pancreatic cancer. Curr. Drug Targets 2012, 13, 1772–1776.
- Tuan, N.M.; Lee, C.H. Penfluridol as a candidate of drug repurposing for anticancer agent. Molecules 2019, 24, 3659.
- Baby, J.; Devan, A.R.; Kumar, A.R.; Gorantla, J.N.; Nair, B.; Aishwarya, T.S.; Nath, L.R. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: A review. J. Food Biochem. 2021, 45, e13761.
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11.
- Gupta, S.; Buttar, H.S.; Kaur, G.; Tuli, H.S. Baicalein: Promising therapeutic applications with special reference to published patents. Pharm. Pat. Anal. 2022, 11, 23–32.
- Liu, J.J.; Huang, T.S.; Cheng, W.F.; Lu, F.J. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer 2003, 106, 559–565.
- Chen, Y.; Zhang, J.; Zhang, M.; Song, Y.; Zhang, Y.; Fan, S.; Ren, S.; Fu, L.; Zhang, N.; Hui, H. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin. Transl. Med. 2021, 11, e577.
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291.
- Gong, W.Y.; Zhao, Z.X.; Liu, B.J.; Lu, L.W.; Dong, J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852.
- Kuo, Y.-T.; Liu, C.-H.; Wong, S.H.; Pan, Y.-C.; Lin, L.-T. Small molecules baicalein and cinnamaldehyde are potentiators of measles virus-induced breast cancer oncolysis. Phytomedicine 2021, 89, 153611.
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.S.; Wang, C.Z. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol. Rep. 2013, 30, 2411–2418.
- Park, Y.G.; Choi, J.; Jung, H.K.; Kim, B.; Kim, C.; Park, S.Y.; Seol, J.W. Baicalein inhibits tumor progression by inhibiting tumor cell growth and tumor angiogenesis. Oncol. Rep. 2017, 38, 3011–3018.
- Huang, X.; Mao, W.; Zhang, T.; Wang, M.; Wang, X.; Li, Y.; Zhang, L.; Yao, D.; Cai, X.; Wang, L. Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complement. Altern. Med. 2018, 18, 330.
- Zhou, Q.M.; Wang, S.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Motoo, Y.; Su, S.B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658.
- Kumagai, T.; Müller, C.I.; Desmond, J.C.; Imai, Y.; Heber, D.; Koeffler, H.P. Scutellaria baicalensis, a herbal medicine: Anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk. Res. 2007, 31, 523–530.
- Lin, C.; Tsai, S.C.; Tseng, M.T.; Peng, S.F.; Kuo, S.C.; Lin, M.W.; Hsu, Y.M.; Lee, M.R.; Amagaya, S.; Huang, W.W.; et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int. J. Oncol. 2013, 42, 993–1000.
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228.
- Xu, Z.; Mei, J.; Tan, Y. Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 2017, 50, 93–100.
- Chandrashekar, N.; Pandi, A. Baicalein: A review on its anti-cancer effects and mechanisms in lung carcinoma. J. Food Biochem. 2022, 46, e14230.
- Li, S.; Wang, L.; Li, N.; Liu, Y.; Su, H. Combination lung cancer chemotherapy: Design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017, 95, 548–555.
- Andouard, D.; Gueye, R.; Hantz, S.; Fagnère, C.; Liagre, B.; Bernardaud, L.; Pouget, C.; Duroux, J.; Alain, S. Impact of new cyclooxygenase 2 inhibitors on human cytomegalovirus replication in vitro. Antivir. Ther. 2021, 26, 117–125.
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523.
- Yu, X.; Tang, W.; Yang, Y.; Tang, L.; Dai, R.; Pu, B.; Feng, C.; Xia, J. Long noncoding RNA NKILA enhances the anti-cancer effects of baicalein in hepatocellular carcinoma via the regulation of NF-κB signaling. Chem. Biol. Interact. 2018, 285, 48–58.
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681.
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The biological activities and therapeutic potentials of baicalein extracted from oroxylum indicum: A systematic review. Molecules 2020, 25, 5677.
- Liu, Y.; Sun, J.; Zhong, L.; Li, Y.; Er, A.N.; Li, T.; Yang, L.; Dong, L. Combination of a biopharmaceutic classification system and physiologically based pharmacokinetic models to predict absorption properties of baicalein in vitro and in vivo. J. Tradit. Chin. Med. Sci. 2021, 8, 238–247.
- Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z.; Dong, R. Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin. Transl. Sci. 2021, 14, 2017–2024.
- Zhang, B.; Dong, Y.; Yu, N.; Sun, Y.; Xing, Y.; Yang, F.; Yu, X.; Sun, W.; Sun, J.; Li, X.; et al. Intestinal metabolism of baicalein after oral administration in mice: Pharmacokinetics and mechanisms. J. Funct. Foods 2019, 54, 53–63.
- Zhang, L.; Li, C.; Lin, G.; Krajcsi, P.; Zuo, Z. Hepatic metabolism and disposition of baicalein via the coupling of conjugation enzymes and transporters-in vitro and in vivo evidences. AAPS J. 2011, 13, 378–389.
- Guo, X.Y.; Yang, L.; Chen, Y.; Wang, Q.F.; Sun, Q.S.; Che, Y.X.; Che, Q.M. Identification of the metabolites of baicalein in human plasma. J. Asian Nat. Prod. Res. 2011, 13, 861–868.
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89.
- Zhang, R.; Cui, Y.; Wang, Y.; Tian, X.; Zheng, L.; Cong, H.; Wu, B.; Huo, X.; Wang, C.; Zhang, B.; et al. Catechol-O-Methyltransferase and UDP-Glucuronosyltransferases in the Metabolism of Baicalein in Different Species. Eur. J. Drug Metab. Pharm. 2017, 42, 981–992.
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother. Res. 2021, 35, 3558–3574.
- Liu, H.T.; Lin, Y.N.; Tsai, M.C.; Wu, Y.C.; Lee, M.C. Baicalein Exerts Therapeutic Effects against Endotoxin-Induced Depression-like Behavior in Mice by Decreasing Inflammatory Cytokines and Increasing Brain-Derived Neurotrophic Factor Levels. Antioxidants 2022, 11, 947.
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell Longev. 2021, 2021, 8377362.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




