Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aisha Umar | -- | 3668 | 2023-04-18 06:26:24 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3668 | 2023-04-18 07:28:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Umar, A.; Smółka, �.; Gancarz, M. Fungal Fuel Cells in Energy Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/43143 (accessed on 07 February 2026).
Umar A, Smółka �, Gancarz M. Fungal Fuel Cells in Energy Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/43143. Accessed February 07, 2026.
Umar, Aisha, Łukasz Smółka, Marek Gancarz. "Fungal Fuel Cells in Energy Production" Encyclopedia, https://encyclopedia.pub/entry/43143 (accessed February 07, 2026).
Umar, A., Smółka, �., & Gancarz, M. (2023, April 18). Fungal Fuel Cells in Energy Production. In Encyclopedia. https://encyclopedia.pub/entry/43143
Umar, Aisha, et al. "Fungal Fuel Cells in Energy Production." Encyclopedia. Web. 18 April, 2023.
Copy Citation
Traditional methods have certain limitations and complexities for bioenergy production, which motivates the search for new ways of sustainable bioenergy production and wastewater treatment. Biological strategies have opened new avenues to the treatment of wastewater using oxidoreductase enzymes for the degradation of pollutants. Fungal-based fuel cells (FFCs), with their catalysts, have gained considerable attention among scientists worldwide. They are a new, ecofriendly, and alternative approach to nonchemical methods due to easy handling. FFCs are efficiently used in wastewater treatment and the production of electricity for power generation.
fungi
metals
treatment
energy
1. Introduction
The rapid industrial and global population growth has polluted water and depleted the resources of fossil fuels to fulfill the excessive demand for energy production. The quality of water is deteriorating due to the continuous mixing of undesirable chemicals [1]. The need for water quality improvement and preservation is continuously growing day by day due to agricultural, civilization, and industrial activities, leading to environmental and global changes. Wastewater is defined as a combination of liquid, water with wastes from residential areas, commercial sites, institutions, and industrial establishments together with ground, surface, and storm water [2].
Nonpoint sources contaminate valuable water resources. Organic pollutants are hazardous and toxic; hence, chemical processes are most suitable to remediate and eliminate the inorganic matter, dyes, and recalcitrant matter. Various techniques (biological, physical, and chemical) are used to treat organic-compounds polluted the wastewater. Traditional methods have certain limitations for bioenergy production, e.g., large spaces, high capital cost, and complexities linked with the production process. The demands for sustainable bioenergy production have been increasing in the world as an alternative to nonchemical methods for power generation. Biological degradation involves the use of microorganisms (fungi, algae, bacteria, and enzymes), which utilize the maximum land area, exhibit very high sensitivity toward toxic agents, and require a long consumption time [3].
The exploration of novel and efficient approaches have attracted the attention of environmental scientists to cleanup and remediation of the contaminated water bodies. The fungal potential to generate bioelectricity from biodegradable wastewater reduces the cost of conversion [4]. Biotic sources exploit different species of fungi for bioenergy generation. However, very little data are available on the use of “fungal-mediated electrochemical system” for energy production. Minimal resources, higher prices of fossil fuels, and increasing global warming issues have motivated the scientists to design alternative “renewable” energy sources, e.g., fungal cell factories.
The fungal fuel cell is a device that uses fungi as catalysts to generate electricity by oxidizing the inorganic compounds of biomass [5]. A few researchers believe that this technology is not only used for the production of electricity. It also depends on the ability of the electrode associated with the fungi to degrade the toxics and waste materials [6]. Biomass/organic material is a sustainable alternative approach to address this issue. Fungal fuel cells (FFCs) provide electricity directly through the “biodegradation” of raw materials by fungal cells [7].
It is proposed that fungal species are used for energy generation, taking advantage of their potential as “novel cell factories”. Saccharomyces or Pichia fungi are used in these cells [8]. Fungal cells have nine times higher potential to generate energy accumulated in sewage sludge than conventional methods [9]. Fungal species have a strong potential to generate power using the presence of complex enzymatic systems. These species can rapidly grow on waste materials and degrade these materials within a shorter time for the production of “bioenergy”. The use of fungi in “bioremediation” is a promising technique [10].
This approach is also called an “Oxidative Biocatalyst” approach. The efficiency of this strategy can be maximized by using different fungal growth and environmental parameters with redox mediator systems [11]. .
2. Oleaginous Fungi
Oleaginous microorganisms have potential for biodiesel formulation and production. These are used as an alternative renewable energy sources. Oleaginous fungi have numerous advantages, e.g., lower land requirements, short cultivation time, and maximum production of fatty acids (oils) [12]. A few oleaginous species metabolize xylose and assist in lipid production from “lignocellulosic hydrolysates” [13]. These fungal species become more oleaginous, when different organic substrates (glucose and sucrose) are added to their growth medium. Each species has particular abilities to utilize organic substrates and enhance the lipid yield. It is noticed that in a fungal consortium, less productive species always follow a more productive species during co-metabolism. This way of combination is yielding more biomass than single cultures.
The genera Mucor and Aspergillus have been recognized to store up to 80% of oils (in cells) in specific conditions [14]. Strains that have high lipid contents and metabolize TAG (triacylglycerides) usually preferred to formulate and generate biofuels efficiently. Zygomycetes are a class of excellent oleaginous fungal species, providing palmitic and oleic acids that are used for biodiesel formation. Additionally, anaerobic fungi are an arsenal of extracellular multienzyme complexes. These fungi are involved in the breakdown of various biomasses for biogas generation. Zygomycetes, such as Mortierella isabelline has reported to have a 60–70% lipid content [15].
Oleaginous yeast (Rhodotorula mucilaginosa SML) has been using for the treatment of food industry effluents. The overall yeast lipid content for the effluent treatment was 67.95 w/w% of dry cell biomass. The extracted yeast oil was used for transesterification and showed a 98% conversion of oil to methanol. The fatty acid composition was compatible with petroleum diesel, making it applicable for alternative biofuel production. Thus, this strategy proved efficient in the removal of contaminants of industrial wastes suggested as a new sustainable source for biodiesel production [16].
The biofilm of Wickerhamomyces anomalus (yeast) on the anodic electrode of a single-chamber fuel cell fed with zinc and copper electrodes and pineapple waste (substrate) is used for fuel production. Current (4.95667 ± 0.54 mA) and voltage peaks (0.99 ± 0.03 V) were generated for 16 and 20 days, respectively. The maximum power density of 513.99 ± 6.54 mW/m2 at a current density of 6.123 A/m2 was generated [17].
3. Hydrolytic and Lignolytic Fungi
Hydrolytic and ligninolytic fungi are suitable candidates for the production of biofuels or bioethanol. A few basidiomycetes have been reporting to secrete extracellular enzymes that degrade the waste materials [18]. Fungal peroxidases (manganese-dependent peroxidase and lignin peroxidase) degrade the lignin, hemicellulose, and polyaromatic phenols [19].
Fungal cells are known for the generation of bioelectricity, good-quality biofuel production, and wastewater treatment. The best-known biofuel-producing fungal species are Rhodosporidium toruloides, Cryptococcus sp., Yarrowia lipolytica, Penicillium sp., Aspergillus sp., and Trichoderma reesei. Species that have the potential to produce biodiesel or electricity generation transfer the e− via cytochrome C. These include Candida sp., Colletotrichum sp., Saccharomyces cerevisiae, Penicillium sp., Alternaria sp., Rhizopus sp., and Aspergillus sp. Cells constructed from these species are called “Fungal-based FCs” [20].
Energy-generating fungal biocatalysts increase the electron transmission rate through extensive networking of fungal hyphae and produce stable electricity, which contributes to “external electrochemical operations”. Due to this unique property, fungi, and yeasts are preferred over bacterial cells for wastewater treatment and electricity generation [21].
4. Effects of Environmental Factors on Fungal Growth and Metabolism
-
pH
A few fungal species grow in a broad pH range, while some species grow in a narrower pH range. The optimum growth of fungal species appears to correspond to a specific pH value [22]. The fungal ability to grow at a pH >7 is required during industrial production. The substrate with a pH below 7.00 inhibits the growth of contaminants (bacteria) without affecting the yield. A slight increase in pH of FFCs, inhibits fungal growth and metabolism. Fungal catalyst formation (oxidoreductase) and catalytic action are highly stable at an acidic pH (3–6). A low pH induces mobility and unfolding of the enzyme proteins.
-
Temperature
Temperature plays an important role in fungal growth, metabolism, and electricity generation using fungal fuel cells. The temperature of system facilitates the cells metabolism and their enzymatic reactions. In wood-rotting fungi, oxidoreductase is produced in an optimum temperature range (25–30 °C), which depends on mesophilic and thermophilic fungal species [23]. The enzyme system of mesophilic basidiomycetes is thermostable at elevated temperatures. Optimum temperature is also favorable for the efficient maintenance of fungal systems in fuel cells during their metabolic mechanisms. A slight decrease or increase in temperature leads to denaturation and inactivation of the cell components, which consequently stops the work of fuel cells with no power generation.
-
Ionic strength
Higher ionic conductivity also influences the work of fungal fuel cell. Ionic conductivity is directly proportional to power generation due to minimum internal resistance. High ionic conductivity increases the power output of FCs. Protons and electrons can easily move from one compartment to another for the completion of a circuit.
-
Salinity
About 90% fungal species can tolerate at 3 to 6% salt stress. Halotolerant fungal species are better adapted to the salty environment [24]. Marine fungi with a dark cell wall can tolerate higher salinity than moniliaceous fungi [25]. The habitats of marine fungal species have a strong influence on their adaptation to salt and metabolic functioning.
Hyperosmotic stress in fungi is linked with the inhibition of cell wall extension and cellular expansion, resulting a reduction in their growth [26]. Excess in everything is bad. Maximum ions can alter protein, membrane integrity, and nucleic metabolism, which may change the enzymatic activity and catalytic performance during fungal growth and functioning of fuel cells [27]. Organic osmotica (compounds) are called compatible solutes, as these solutes can store high concentrations of salts without interfering with cell metabolism. Polyols (mannitol, arabitol, and glycerol) and non-reducing saccharides (trehalose) are soluble carbohydrates found in basidiomycetes and ascomycetes. These solutes help the fungi to grow efficiently in a salt-stress environment. A maximum salt range destroys the fungal product yield as well.
5. Enzymatic Treatment by Biocatalytic Fungal Species
Catalysts are needed to accelerate the maximum biodiesel production. Biofuels are categorized into bioethanol, biohydrogen, and biodiesel (Figure 1). During the enzymatic treatment of wastewater (substrate), low-chain carbon compounds are produced, which utilized during microbial oxidation. The basic oxidoreductases, such as laccases, lignin peroxidases, and manganese peroxidases, are extracellular enzymes. In contrast, glucose oxidase and cellobiose dehydrogenase are auxiliary enzymes isolated from white-rot fungi.
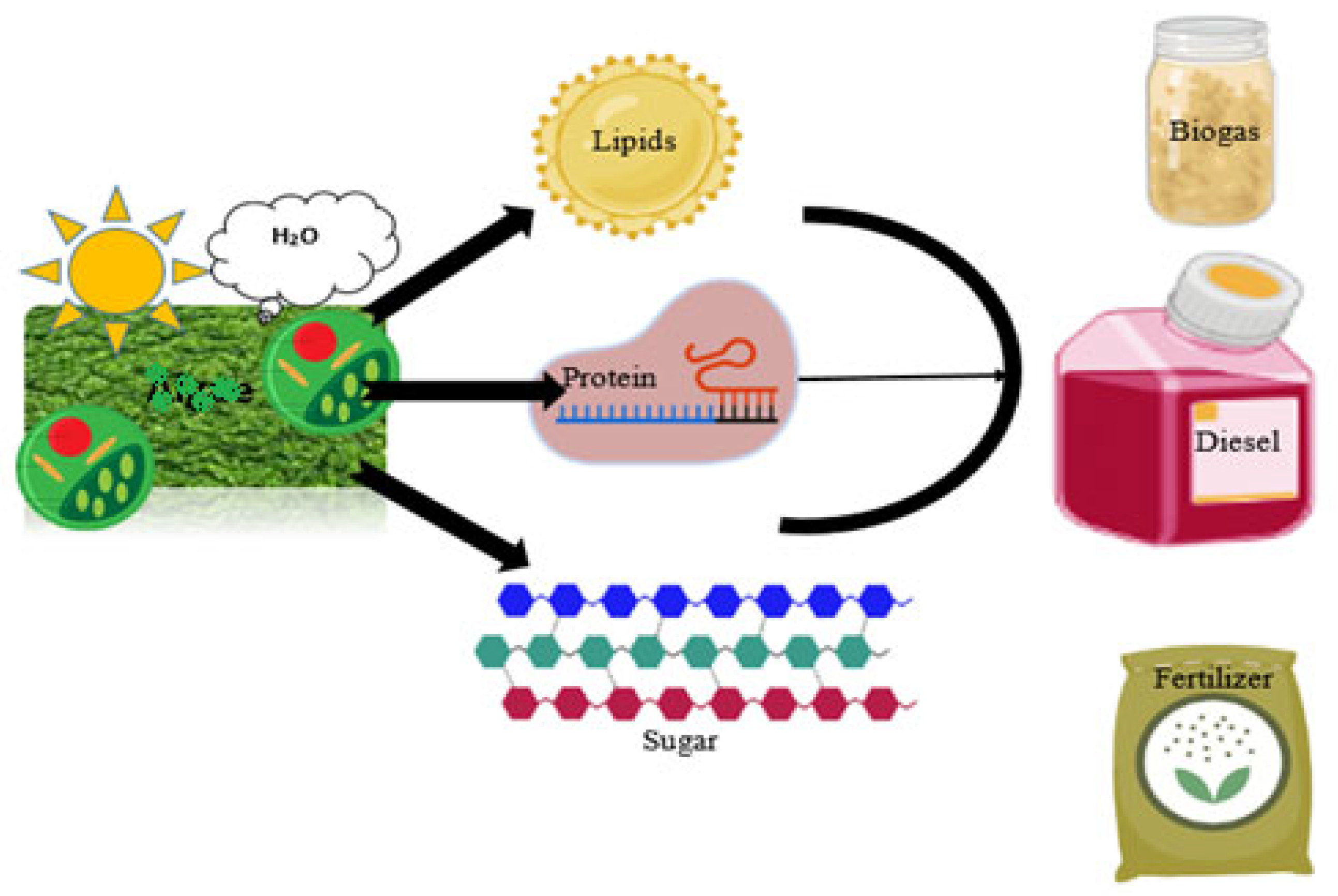
Figure 1. Fungal species utilize sunlight and water for the metabolic production of lipids, protein, and sugar used for the manufacturing of biodiesel, biogas, and biofertilizers.
Biocatalysts are exoelectrogens that oxidize organic material and deliver electrons from anode to cathode to generate the electricity. Exoelectrogens are now investigated for the development of FFCs, which potentially convert the diverse organic substances (activated sludge of waste water) into electricity, ethanol, and H2 [28]. Sporotrichum pruinosum (white-rot fungus) efficiently degrades pollutants, organic biomass, and chemical substances with the use of an extracellular enzyme [29].
Biocatalyst is deposited onto the carbon anode as floating biomass in yeast-based FFCs [30]. Yeast-based FFCs have the following advantages: (i) degradation of very complex substrates (starch and cellulose-based substrates) into simple organic molecules; (ii) survival in an anaerobic environment [31]; and (iii) simple and easy production, rapid development, and sensitivity of the strains. Except for yeast, other fungal species are also exploited as biocatalysts for both wastewater treatment and electrochemical approaches.
Scientists are motivated toward the development of such mediator systems as fungal-based FCs [21]. Pure Saccharomyces cerevisiae (yeast) is a model organisms used as biocatalysts in FFCs. Christwardana et al. [30] indicated the significance of yeast cells in MFCs due to their unique features and sustainability. S. cerevisiae has been extensively studied and characterized as a biocatalyst in biological fuel cells [32][33][34]. This species is nonpathogenic to non-target organisms (humans), has a high growth rate, easy to culture in anaerobic environments, and grows very well at room temperature [35]; hence, it can be used for the effective treatment of wastewater [36][37]. In addition to the low cost and rapid multiplication, the species remains active and survives even in a dried environment for a longer period [38]. Carbon-neutral fuel, referred to as bioethanol, is produced from plant waste and bacterial/algal biomass [39]. It is also produced from yeast and fungi in anaerobic conditions [40], especially S. cerevisiae, which is considered to have great potential in the production of bioethanol.
Candida melibiosica, Kluyveromyces marxianus, Blastobotrys adeninivorans, Pichia anomala, P. polymorpha, and Saccharomyces cerevisiae yeasts are used as biocatalysts in FFCs with/without an external mediator. Kluyveromyces marxianus is a promising yeast species producing maximum power at higher temperatures, when grown in natural (organic) substrates. Other fungal species, e.g., Saccharomyces cerevisiae [33], Candida melibiosica [36], Blastobotrys adeninivorans [37], Hansenula polymorpha [41], and Pichia anomala [42] all are a potential source for catalysts in FFCs.
Exogenous mediators, such as methylene blue (MB) and neutral red (NR), are used to increase the transport of electrons between anodes and microbes. The yeast cell surface-displayed dehydrogenases include cellobiose dehydrogenase (CDH) and pyranose dehydrogenase (PDH) [43]. Both CDH- and PDH-based biocatalysts are used in the anodic compartment of FFCs.
6. Structure of Fungal-Mediated Fuel Cells
Protons and electrons are generated through the oxidation of organic matter in the aqueous solution of anode compartment, when fungi used as catalyst. The external circuit is used to transmit the electrons toward the cathode, while the proton exchange membrane (PEM) facilitates proton diffusion [44][45][46]. At the cathode, e− and protons are used for the reduction reaction and eventually change oxygen to water [47]. Potter [48] was the first person, who liberated electrical energy from yeast cells in 1911. Fungi can transfer electrons to the anode electrode in three possible ways: (1) direct contact; (2) pili/conductive wires; and (3) redox mediators/electron shuttle [49].
The advantages of FFCs include sustainable nonchemical character, minimum sludge generation, optimum temperature, wide range of substrates, low power consumption, and good performance [50]. This is a promising alternative technique used to explore the fungal potential in the conversion of organic substrates into electricity. The performance of FFCs depends on multiple factors, e.g., configuration of the cell, choice of the substrate, anodic material, biocatalyst, electro-catalyst (at the cathode), and environmental conditions. Prasad et al. [42] observed that fungi are more active than bacteria in MFCs.
Fungal fuel cells (FFCs) are operated in a closed-system mode on the principle of oxidation–reduction reaction through a series of electrochemical and microbial pathways. The anaerobic environment is maintained in the anodic compartment [51]. The e− and protons in the anode chamber are produced through oxidation of the substrate by fungi, and oxygen reduced by a terminal electron acceptor in the cathodic chamber.
Fungi used at the anode to transport e− via redox-active fungal protein and synthetic mediators. Anodic fungal cells oxidize the substrates and produce electrons and protons. The e− is absorbed by the electrode, and protons flow toward the cathode via a PEM. The protons are transferred through the PEM to the cathode. Subsequently, the electron and proton combination produce a molecule of water (at the cathode) for the completion of the bioelectrochemical reaction. At the cathode, the fungal enzyme catalyzes the reaction and the final electron receiver is O2 and H2O produced during this reaction. Additionally, airtight compartments, sufficient space within the chamber, an outlet, and inlets are certain prerequisites in the arrangement of the PEM and electrodes into a system (Figure 2).
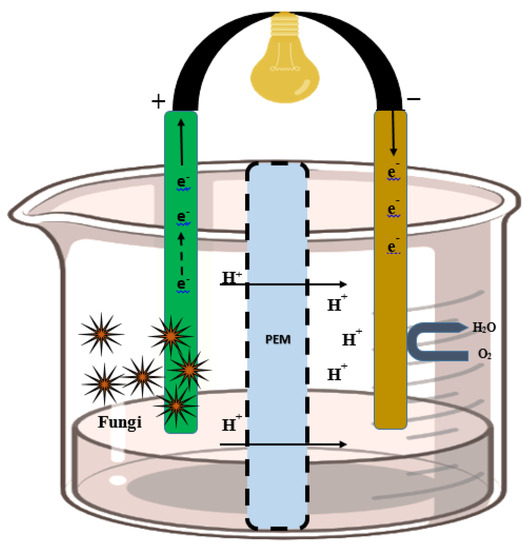
Figure 2. Structure of the fungal fuel cell. The left side of the cell is the fungal catalyst electrode (anodic chamber), and protons are transferred from the proton exchange membrane to the cathodic chamber (where catalytic oxidation takes place).
A membrane separator called a proton exchange membrane (PEM), divides the cell into two distinct cathodic and anodic chambers. An extracellular microorganism with the ability to transfer electrons is called an exoelectrogen (Biocatalyst) [52]. Membrane separators (PEM, salt bridge, anion and cation exchange membrane, microfiltration membrane, glass fiber) have such features as low permeability and high conductivity for optimum FFC performance [53].
The membrane (Nafion), cathode (Platinum), and anode (carbon cloth and carbon paper) materials are expensive and fragile. Fungal FCs with a low-cost electrode, high power output, and membrane materials with good scalability should develop to treat different effluents (de-sizing, bleaching, dyeing, and printing effluents).
Potent microbes improve the electron transfer and facilitate the degradation of biological substrates, e.g., Shewanella oneidensis or the hyphal networks of T. versicolor facilitate electron transfer onto the anode efficiently. Nearly 30 days are required for the formation of homogeneous bacterial and fungal biofilms on the electrode [54].
7. Electron Transfer (ET) Mechanism
There are two types of e− transfer mechanisms. Fungal consortia demonstrated a better ET mechanism than single species.
-
Direct ET: two types via outer cytochrome and nanowire.
-
Indirect ET: (mediated/mediator electron transfer) reactive diffusible redox mediators (RMs) enhance the reaction rate and increase the range of degraded substrates.
8. Types of Electrodes
The electrode material influences the performance of FFC, which has a direct impact on the kinetics of electrodes [55].
Anode: A carbon-based anode is cost-effective and noncorrosive (modifier carbon paper, carbon felt, carbon cloth, carbon nanotubes, graphene, stainless steel, titanium, and gold) [56]. These materials improve the characteristics of anodic surface material and provide an appropriate platform for fungal biofilm formation with an active catalyst. The anode quality enhances the high surface area, chemical/electrical stability, and biocompatibility [57]. The anodic electrode increases the efficiency of FFCs. This serves as a driving feature for power generation. Thus, the anodic material seems to be a suitable strategy to enhance performance.
Reaction in the anodic chamber:
C6H12O6 + 6H2O → 6CO2 + 24H+ + 24e−
Iron and iron oxide nanoparticles, graphite, carbon cloth, and carbon felt are effective anode catalysts improving the efficiency of a fungal fuel cell (FFC) for industrial wastewater treatment. Wastewater is used nowadays as an energy source. This is a promising approach to meet the increasing energy needs in place of fossil fuels [58]. Biocatalysts provide clean, sustainable, and renewable energy sources by utilizing exoelectrogenic organisms [59].
There are several advantages of FFCs; however, their utilization is still limited due to the high cost of their components and their low power output [60]. This limitation can overcome by using an appropriate anode surface morphology (large surface area, superhydrophilicity, high electrical conductivity, excellent chemical stability, high porosity, biocompatibility, and chemistry) with improved electron transfer process [6][61]. The hydrophobic nature of anode, negatively affects the microbial adhesion and enhances the interfacial resistance for e− transfer due to insufficient adhesion on anode surface. This minimize the power and current density [62], which can be overcome with the use of a carbonaceous anode and modification methods (chemical function group treatment, physical treatment, acid heat treatment, and transition metal coating techniques) [63].
Cathode and Cathodic compartment: Types of substrate act as a cathode in FFCs (biodegradable waste such as brewery and sewage wastewater, rich in organics such as glucose, sucrose, lignocellulose, acetate, biomass materials, etc). The reduction of oxygen takes place in this compartment. This is a key interaction in energy conversion and biological respiration [64]. Electrons from the anode are received by cathode through an external circuit and protons are transported via the PEM. This is essential in the reduction reaction between electrons and protons resulting in H2O formation. The cathode affects the total cell voltage output and has a high redox potential.
Reaction in the cathodic chamber
6O2 + 24H+ + 24e− → 12H2O
The biocathode is an alternative low-cost, sustainable, stable, and nonchemical option used currently in FFCs. Due to certain biological components in the cathode, the term ’biocathode’ is used. The fungus is embedded in an oxygenated cathodic chamber and establishes a mutual configuration of a dual compartment-based yeast fuel cell.
9. Reactor Configuration
The reactor configuration influences the performance of biological fuel cells. There are two construction designs: (1) single [65] and (2) dual-chambers [66] to evaluate the in vitro performance. According to the mode of aeration, FFCs are classified into different configurations (Figure 3):
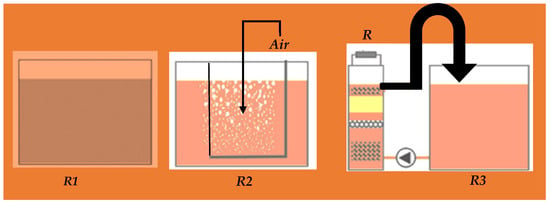
Figure 3. Reactors for the measurement of wastewater parameters; without-air reactor (R1)—the reactor 15 L and the air is interface in wastewater sewage; with-air reactor (R2)—15 L with constantly aerated wastewater; and membrane less FFC (R3)—reduces COD to 90%.
(1) Aqueous cathode: in this cell, water is bubbled with air for the supply of dissolved O2 to the electrode [67]; (2) air cathode: to minimize the cost and maximize the energy output, the air cathode is designed. Carbon electrodes generate energy in the absence of a PEM. The power density (494 mW/m2) is much better in this type of cell than in the aqueous cathode [68]; (3) downflow: this membrane less fuel cell is constructed with downflow feeding to generate electricity from wastewater. Water is fed directly onto the cathode, which is horizontally installed in the upper part of the FFC. Oxygen is utilized readily from the air and concentration of dissolved oxygen in the wastewater has little effect on the power generation. The maximum power density of 37.4 mW/m2 is generated by this type of cell and mostly used in brewery wastewater treatment [69]; (4) upflow: upflow reactors have advantages in retaining the maximum cell density and mass transfer efficiency. In this type of cell, the recirculation rate can improve the upflow rate. At a recirculation rate of 4.8 RV/h, a power density of 356 ± 24 mW/m2 is produced from this cell [70]; (5) miniature: a low-cost mini tubular fuel cell is developed for the treatment of groundwater contaminated with benzene and for the monitoring of wells. An increase in the length and density and a decrease in size of char particles at the anode effectively reduce the internal resistance. This type of cell removes 95% of benzene and generates a power density of 38 mW/m2 [71]; (6) stacked: this easy-to-operate FFC in septic tanks comprises a common base and multiple pluggable units, which are connected in series or parallel for electricity generation during waste treatment. Three parallel-connected units produce a power density of 142 ± 6.71 mW/m2 [72]; (7) large scale: in this cell, multiple operational conditions can be tested (different flow rates, application of external resistors, and poised anodic potentials). This results in the highest COD removal efficiency (94.6 ± 1.0%) at an applied resistance of 10 Ω across each circuit. Results of eight stages of operation (325 days total) indicate that this fuel cell can sustain treatment rates over a long-term period and are robust enough to sustain performance even after system perturbations [73]; (8) tubular: two ceramic stacks, mullite (m-stack) and terracotta (t-stack) are developed to produce energy. Each stack contains 12 identical fuel cells, which are arranged in cascades and tested under different electrical configurations. The m-stack and the t-stack are found to produce a maximum power of 800 μW and 520 μW, respectively [74]; and (9) salt bridge: a salt bridge is used instead of membrane system. The low power output (2.2 mW/m2) is directly attributed to the higher internal resistance of the salt bridge (19920 ± 50 Ω) compared to the membrane system (1286 ± 1 Ω). Oxygen diffusion from the cathode to the anode chamber is a factor in power generation [75].
References
- Lvovich, M.I. Water Resources and Their Future; Litho Crafters Inc.: Eastpointe, MI, USA, 1979.
- Adeel, S.; Abrar, S.; Kiran, S.; Farooq, T.; Gulzar, T.; Jamal, M. Sustainable Application of Natural Dyes in Cosmetic Industry. Handbook of Renewable Materials for Coloration and Finishing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 189–211.
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today Proc. 2022, 31, A158–A165.
- Narita, J.; Okano, K.; Tateno, T.; Tanino, T.; Sewaki, T.; Sung, M.H.; Fukuda, H.; Kondo, A. Display of active enzymes on the cell surface of Escherichia coli using PgsA anchor protein and their application to bioconversion. Appl. Microbiol. Biotechnol. 2006, 70, 564–572.
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332.
- Franks, A.E.; Nevin, K.P. Microbial fuel cells. A currentreview. Energies 2010, 3, 899–919.
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298.
- Mitra, P.; Hill, G.A. Continuous microbial fuel cell using a photoautotrophic cathode and a fermentative anode. Can. J. Chem. Eng. 2012, 90, 1006–1010.
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390.
- Wang, Y.; Liu, P.; Zhang, G.; Yang, Q.; Lu, J.; Xia, T.; Peng, L.; Wang, Y. Cascading of engineered bioenergy plants and fungi sustainable for low-cost bioethanol and high-value biomaterials under green-like biomass processing. Renew. Sustain. Energy Rev. 2021, 137, 110586.
- Vernet, G.; Hobisch, M.; Kara, S. Process intensification in oxidative biocatalysis. Curr. Opin. Green Sustain. Chem. 2022, 38, 100692.
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815.
- Kurosawa, K.; Wewetze, S.J.; Sinskey, A.J. Engineering xylose metabolism in triacylglycerol producing Rhodococcus opacus for lignocellulosic fuel production. Biotechnol. Biofuels 2013, 6, 134–147.
- Dhanasekaran, D.; Sundaresan, M.; Suresh, A.; Thajuddin, N.; Thangaraj, R.; Vinothini, G. Oleaginous microorganisms for biofuel development. Environ. Sci. Eng. 2017, 12, 243–263.
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Bio. Bioenergy 2009, 33, 573–580.
- Sundaramahalingam, M.A.; Sivashanmugam, P. Concomitant strategy of wastewater treatment and biodiesel production using innate yeast cell (Rhodotorula mucilaginosa) from food industry sewerage and its energy system analysis. Renew Energ. 2023, 208, 52–62.
- Rojas-Flores, S.; Nazario-Naveda, R.; Benites, S.M.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Use of Pineapple Waste as Fuel in Microbial Fuel Cell for the Generation of Bioelectricity. Molecules 2022, 27, 7389.
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? Ol. Corps Gras. Lipides 2012, 19, 22–28.
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzym. Microb. Technol. Recent Adv. Lignin Biodegrad. 2002, 30, 454–466.
- Sekrecka-Belniak, A.; Toczyłowska-Mamińska, R. Fungi-based microbial fuel cells. Energies 2018, 19, 2827.
- Sayed, T.; Abdelkareem, M.A. Yeast as a Biocatalyst in Microbial Fuel Cell. Old Yeasts-New Quest. 2017, 317, 41–65.
- Hallsworth, J.E.; Magan, N. Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl. Environ. Microbiol. 1996, 62, 2435–2442.
- Deska, M.; Kończak, B. Immobilized fungal laccase as” green catalyst” for the decolourization process–State of the art. Process Biochem. 2019, 84, 112–123.
- Huang, J.; Lu, C.; Qian, X.; Huang, Y.; Zheng, Z.; Shen, Y. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol. Sin. 2011, 30, 118.
- Cantrell, S.A.; Casillas-Martínez, L.; Molina, M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol. Res. 2006, 110, 962–970.
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499.
- Mansour, M.M.F.; Salama, K.H. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122.
- Patil, S.A.; Hagerhall, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bio electrochemical systems. Bioanal. Rev. 2012, 4, 159–192.
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896.
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11.
- Mao, L.; Verwoerd, W.S. Selection of organisms for systems biology study of microbial electricity generation: A review. Int. J. Energy Environ. Eng. 2013, 4, 17.
- Ganguli, R.; Dunn, B.S. Kinetics of anode reactions for a yeast-catalysed microbial fuel cell. Fuel Cells 2009, 9, 44–52.
- Gunawardena, A.; Fernando, S.; To, F. Performance of a yeast-mediated biological fuel cell. Int. J. Mol. Sci. 2008, 9, 1893–1907.
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 2011, 102, 2751–2757.
- Schaetzle, O.; Barriere, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from micro-organisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620.
- Hubenova, Y.; Mitov, M. Extracellular electron transfer in yeast-based biofuel cells: A review. Bioelectrochemistry 2015, 106, 177–185.
- Haslett, N.D.; Rawson, F.J.; Barriere, F.; Kunze, G.; Pasco, N.; Gooneratne, R.; Baronian, K.H.R. Characterisation of yeast microbial fuel cell with the yeast Arxula adeninivorans as the biocatalyst. Biosens. Bioelectron. 2011, 26, 3742–3747.
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403.
- Hanaki, K.; Portugal-Pereira, J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability. Science for Sustainable Societies; Takeuchi, K., Shiroyama, H., Saito, O., Matsuura, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 53–71.
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotech. 2018, 56, 174.
- Shkil, H.; Schulte, A.; Guschin, D.A.; Schuhmann, W. Electron transfer between genetically modified hansenula polymorpha yeast cells and electrode surfaces via complex modified redox polymers. Chem. Phys. Chem. 2011, 12, 806–813.
- Prasad, D.; Arun, S.; Murugesan, M.; Padmanaban, S.; Satyanarayanan, R.S.; Berchmans, S.; Yegnaraman, V. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell. Biosens. Bioelectron. 2007, 22, 2604–2610.
- Gal, I.; Schlesinger, O.; Amir, L.; Alfonta, L. Yeast surface display of dehydrogenases in microbial fuel-cells. Bioelectrochemistry. 2016, 112, 53–60.
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494.
- Miskan, M.; Ismail, M.; Ghasemi, M.; Md Jahim, J.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrog. Energy 2016, 41, 543–552.
- Darvishi, Y.; Hassan-Beygi, S.R.; Zarafshan, P.; Hooshyari, K.; Malaga-Toboła, U.; Gancarz, M. Numerical Modeling and Evaluation of PEM Used for Fuel Cell Vehicles. Materials 2021, 14, 7907.
- Woo, S.; Lee, S.; Taning, A.Z.; Yang, T.H.; Park, S.H.; Yim, S.D. Current understanding of catalyst/ionomer interfacial structure and phenomena affecting the oxygen reduction reaction in cathode catalyst layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2020, 21, 289–296.
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276.
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336.
- Sayed, E.T.; Tsujiguchi, T.; Nakagawa, N. Catalytic activity of baker’s yeast in a mediatorless microbial fuel cell. Bioelectrochemistry 2012, 86, 97–101.
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81.
- Ho, C.H.; Yi, J.; Wang, X. Biocatalytic continuous manufacturing of diabetes drug: Plantwide process modeling, optimization, and environmental and economic analysis. ACS Sustain. Chem. Engineer. 2018, 7, 1038–1051.
- Kim, I.S.; Chae, K.J.; Choi, M.J.; Verstraete, W.; Kim, I.S.; Chae, K.J.; Choi, M.J.; Verstraete, W. Microbial fuel cells: Recent advances, bacterial communities and application beyond electricity generation. Environ. Eng. Res. 2008, 13, 51–65.
- Nimje, V.R.; Chen, C.Y.; Chen, H.R.; Chen, C.C.; Huang, Y.M.; Tseng, M.J.; Cheng, K.C.; Chang, Y.F. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour. Technol. 2012, 104, 315–323.
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 1–11.
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity generation by geobacter sulfurreducens attached to gold electrodes. Langmuir 2008, 24, 4376–4379.
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536.
- Wu, D.; Yi, X.; Tang, R.; Feng, C.; Wei, C. Single microbial fuel cell reactor for coking wastewater treatment: Simultaneous carbon and nitrogen removal with zero alkaline consumption. Sci. Total Environ. 2018, 621, 497–506.
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525.
- Zhang, P.; Liu, J.; Qu, Y.; Feng, Y. Enhanced Shewanella oneidensis MR-1 anode performance by adding fumarate in microbial fuel cell. Chem. Engineer. J. 2017, 328, 697–702.
- Santoro, C.; Babanova, S.; Artyushkova, K.; Cornejo, J.A.; Ista, L.; Bretschger, O.; Marsili, E.; Atanassov, P.; Schuler, A.J. Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 2015, 106, 141–149.
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Engineer. J. 2018, 335, 539–547.
- Mohanakrishna, G.; Abu-Reesh, I.M.; Kondaveeti, S.; Al-Raoush, R.I.; He, Z. Enhanced treatment of petroleum refinery wastewater by short-term applied voltage in single chamber microbial fuel cell. Bioresour. Technol. 2018, 253, 16–21.
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Simultaneous organic matter removal and disinfection of wastewater with enhanced power generation in microbial fuel cell. Bioresour. Technol. 2014, 163, 328–334.
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329.
- Erable, B.; Bergel, A. First air-tolerant effective stainless steel microbial anode obtained from a natural marine biofilm. Bioresour. Technol. 2009, 100, 3302–3307.
- Utomo, H.D.; Yu, L.S.; Yi, D.C.Z.; Jun, O.J. Recycling solid waste and bioenergy generation in MFC dual-chamber model. Energy Procedia 2017, 143, 424429.
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 40404046.
- Zhu, F.; Wang, W.; Zhang, X.; Tao, G. Electricity generation in a membrane-less microbial fuel cell with down-flow feeding onto the cathode. Bioresour. Technol. 2011, 102, 73247328.
- Lay, C.H.; Kokko, M.E.; Puhakka, J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 2015, 91, 235241.
- Chang, S.H.; Wu, C.H.; Wang, R.C.; Lin, C.W. Electricity production and benzene removal from groundwater using low-cost mini tubular microbial fuel cells in a monitoring well. J. Environ. Manag. 2017, 193, 551557.
- Yazdi, H.; Alzate-Gaviria, L.; Ren, Z.J. Pluggable microbial fuel cell stacks for septic wastewater treatment and electricity production. Bioresour. Technol. 2015, 180, 258263.
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274287.
- Tremouli, A.; Greenman, J.; Ieropoulos, I. Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 2018, 123, 1925.
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 16751686.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
18 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




