Förster resonance energy transfer (FRET)-based biosensors are being fabricated for specific detection of biomolecules or changes in the microenvironment. FRET is a non-radiative transfer of energy from an excited donor fluorophore molecule to a nearby acceptor fluorophore molecule. In a FRET-based biosensor, the donor and acceptor molecules are typically fluorescent proteins or fluorescent nanomaterials such as quantum dots (QDs) or small molecules that are engineered to be in close proximity to each other. When the biomolecule of interest is present, it can cause a change in the distance between the donor and acceptor, leading to a change in the efficiency of FRET and a corresponding change in the fluorescence intensity of the acceptor. This change in fluorescence can be used to detect and quantify the biomolecule of interest.
1. What Is the Importance of FRET in Biosensor?
FRET is frequently used in biosensor devising because it allows for the specific and sensitive detection of biomolecules in a highly specific manner with high sensitivity without the need for modification of the biomolecule or direct labeling. The fluorescence of the acceptor molecule is only activated when both the donor and acceptor fluorophore molecule are in close juxtaposition, so any changes in the environment that affect the distance between the two molecules will also affect the fluorescence. This allows for the detection of small changes in the environment, such as the presence of a specific biomolecule, without the need to directly label or modify that biomolecule
[1][2]. Additionally, FRET is a non-radiative process, which means that it does not produce any ionizing radiation. This makes FRET-based biosensors safer to use and handle than other types of biosensors that rely on radioactive or ionizing radiation
[3]. Furthermore, FRET biosensors are highly specific, meaning that they can detect a specific biomolecule or change in the environment without being affected by other molecules or changes that might be present. This specificity is achieved by designing the biosensor to have a high binding affinity for the target biomolecule and by engineering the donor and acceptor molecules to be close to each other
[4]. FRET biosensors are also highly sensitive and versatile, as they can detect a wide range of biomolecules and changes in the environment and can be used in a variety of applications, such as detecting protein–protein interactions, monitoring changes in pH, measuring the activity of enzymes, etc.
[5][6][7][8][9][10][11][12]. FRET is a photo-physio-chemical, quantum mechanical, distance-dependent, non-radiative transfer of energy from the photon excited donor fluorophore to a suitable electron-acceptor fluorophore in ground state when both the fluorophores are close to each other (1–10 nm). It is also called FRET in honor of the discovery of a phenomenon by German scientist Theodor Förster
[13]. It is a widely accepted accessory tool to quantify molecular dynamics for biomolecular interactions such as protein–DNA interactions, protein–protein interactions, and protein conformational changes in biochemistry and biophysics. In order to monitor the conjugate formation between two different molecules, FRET is labeled with a donor and acceptor fluorophore molecule, respectively. When these fluorophore-labeled molecules are dissociated after the mixing, the emission of donor gets detected upon the donor excitation. Additionally, emission of the acceptor is predominantly observed due to intermolecular FRET between the donor and acceptor due to their interaction when they are in proximity (1–10 nm) with each other. To monitor the conformational changes in protein, the target protein molecule is labeled with a donor as well as an acceptor molecule at two loci. FRET changes are observed upon twisting or bending of protein molecules. This occurs due to relative orientation or distance change between donor and acceptor fluorophores. If change in protein conformation or molecular interaction is associated with binding of ligand, this technique is useful for ligand detection via fluorescent indicators
[14][15][16]. Researchers can use FRET to measure the distance between the two parts and infer whether the protein is in an active or inactive state.
Apart from protein interaction and protein detection, FRET is also extensively used for quantitative detection in polymerase chain reaction (PCR)
[3]. In PCR, a pair of short synthetic DNA primers are used to amplify a specific region of DNA. The primers bind to the target DNA sequence and provide a starting point for the amplification process
[4]. FRET can be used as a detection method for PCR. In this technique, one of the PCR primers is labeled with a donor fluorophore, and the other primer is labeled with an acceptor fluorophore. When the two primers bind to the target DNA sequence and are in proximity, the donor fluorophore transfers its energy to the acceptor fluorophore, resulting in a fluorescent signal
[5]. FRET can be used in real-time PCR to monitor the amplification of DNA as it occurs
[6]. As the PCR reaction progresses, the amount of amplified DNA increases, which leads to an increase in FRET signal. This allows for the quantification of the amount of DNA present in the reaction
[7]. Various fluorophores can be used as donors and acceptors in FRET-based PCR
[8]. It can also be called real time quantitative PCR, including fluorescein, rhodamine, and cyanine dyes. The specific choice of fluorophores will depend on factors such as the instrument used for detection and the specific requirements of the experiment. The article “A novel coronavirus outbreak of global health concern” published in Eurosurveillance in January 2020 describes the use of FRET in PCR for the detection of the novel coronavirus (SARS-CoV-2) responsible for the COVID-19 pandemic. The authors used a FRET-based assay targeting two regions of the SARS-CoV-2 genome, the E gene and the RdRp gene, to detect the virus in respiratory specimens from patients with suspected COVID-19. The assay consisted of two sets of primers labeled with different fluorescent dyes, and a probe that binds to a specific sequence between the primers. During PCR amplification, the probe binds to the target sequence and is cleaved by the Taq polymerase, which releases the two fluorescent dyes. The resulting increase in FRET signal can be detected in real time using a fluorescence-based PCR instrument. The authors reported that the FRET-based assay showed high sensitivity and specificity for the detection of SARS-CoV-2, with a limit of detection of 10 copies of viral RNA per reaction. They also noted that the FRET-based assay could be easily adapted for high-throughput testing in clinical laboratories. Overall, the use of FRET in PCR has become a valuable tool for the detection and monitoring of SARS-CoV-2, as well as for a wide range of other infectious agents. The high sensitivity and specificity of FRET-based assays make them ideal for the rapid and accurate detection of pathogens in clinical and research settings
[9]. In 2022, Zhang et al. developed a nucleic acid biosensor for the rapid detection of SARS-CoV-2 viral sequence. The authors used FRET concept for the detection of viral sequence. In the given experiment, they selected ssDNA as a donor motif and 2D nanomaterials as an acceptor motif
[10].
For nano–bio system also, FRET is a reliable, accurate, and highly sensitive tool to monitor nano–bio interaction at ultra-low concentration, i.e., nanomolar and picomolar level
[17]. FRET is also helpful to monitor the complex cellular events, dynamics, and interactions of biological systems in vitro as well as in vivo. The results gained in life science research are analyzed by numerous established approaches. FRET is very much crucial in terms of understanding the interactions of nano-systems with biomolecules for efficient use of nanotechnology. On the contrary, understanding the enzyme kinetics at lower concentration of enzymes is highly crucial to understand the mechanism that could be possible by the use of fluorescent QDs probing. Despite several limitations, FRET is still a highly accurate and sensitive tool to monitor such type of interactions
[18][19][20][21][22].
2. Importance over Other Conventional Techniques
It is very difficult to monitor the interaction between the biomolecule at very short distance (less than 100 nm), which makes FRET technique dominant over the other conventional technique. FRET also has an advantage over the other technique in terms of the monitoring of reactions at ultra-low concentration
[17]. This is because FRET measures energy transfer between molecules, rather than the amount of light emitted. FRET is a non-invasive technique that does not require any chemical modification of the molecules being studied. This means that the molecules can retain their natural properties, and the results obtained from FRET studies are more representative of the actual biological processes
[11]. FRET can be used to monitor dynamic changes in molecular interactions in real time. This is particularly useful for studying biological processes such as protein–protein interactions, DNA replication, and signal transduction. FRET has a high spatial resolution and can measure distances between molecules within a few nanometers
[12]. This makes it possible to study molecular interactions within specific cellular compartments or even within single cells. FRET can be used to study a wide range of biological processes and interactions, including protein–protein interactions, protein–DNA interactions, and protein–lipid interactions
[13][14]. Several techniques have their own advantages and limitations, but FRET has an extra corner on these techniques such as SPR, ELISA, electrochemical biosensor, mass spectrophotometry, colorimetric assays fluorescence microscopy, impedance biosensors, and quartz crystal microbalance.
3. Importance of FRET in Biosensor
Biosensing of drug molecules, biomolecules, and chemical compounds at ultra-low concentration is very much crucial in biochemical research. FRET-based sensors and biosensors based on fluorescent material enables us to deal with such types of challenges, especially quantum dots probe-based biosensors. Nowadays, FRET has become the most powerful spectroscopic technique to detect such molecules including heavy metal ions and transition elements with very high sensitivity at picomolar and nanomolar concentration
[23]. FRET is an important technique in biosensors because it allows for the specific and sensitive trace of biomolecules without the need for direct labeling or modification of the biomolecule. This allows for the detection of small changes in the environment, such as the presence of a specific biomolecule, without the need to directly label or modify that biomolecule. FRET biosensors are also highly specific, meaning that they can detect a specific biomolecule or change in the environment without being affected by other molecules or changes that might be present. This specificity is achieved by designing the biosensor to have a high binding affinity for the target biomolecule, and by engineering the donor and acceptor molecules to be in adjoined proximity to each other. Sensitivity of FRET biosensors is also very high, often in the picomolar range, making them able to detect very low amounts of biomolecules, which is especially important for early detection of diseases or for detecting low levels of environmental pollutants. FRET biosensors are also highly versatile, as they can detect a wide range of biomolecules and changes in the environment, and can be used in a variety of applications, such as detecting protein–protein interactions, monitoring changes in pH, or measuring the activity of enzymes. This versatility makes FRET-based biosensors a valuable tool for research, diagnosis, and monitoring in various fields, including biochemistry, cell biology, and drug discovery. In recent days, the FRET-based biosensor has touched almost every aspect of research from physiology to pathology, from diagnosis up to treatment, and from health to environment
[24].
4. Detection of Conformational Changes Using FRET
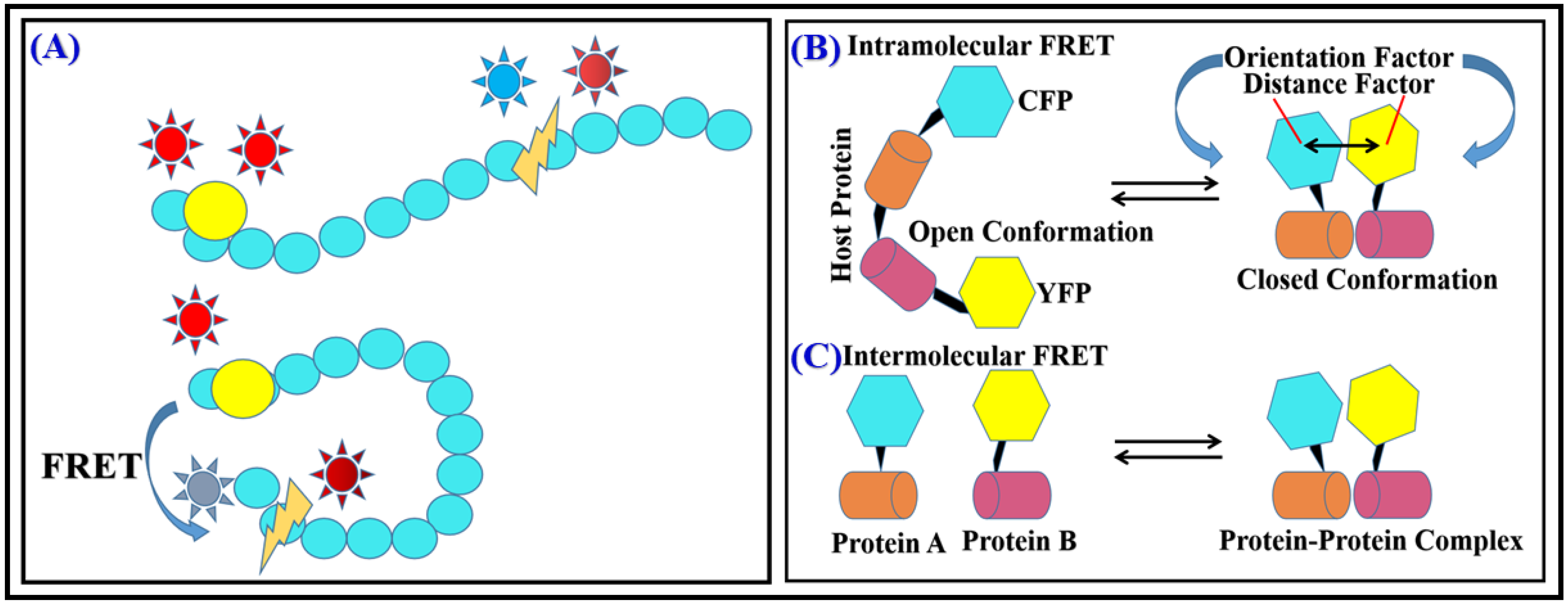
FRET is a hihgly powerful tool for detecting conformational changes in biomolecules because it is sensitive to distance-dependent changes between the FRET donor–acceptor pair. When a conformational change occurs in a biomolecule, the distance amid the FRET donor–acceptor pair can change, which can affect the efficiency of energy transfer between them. By measuring the FRET efficiency, researchers can infer the conformation of the biomolecule. FRET is widely used in structural biology, molecular biology, and biochemistry to study the structural dynamics of biomolecules and their interactions with other molecules in real time and in a non-invasive way. For example, by attaching a FRET donor and acceptor molecule to different parts of a protein of interest, researchers can use FRET to measure the distance between the two parts and infer whether or not the protein is in an active or inactive state. Similarly, by using FRET, researchers can detect conformational changes in nucleic acids, lipids, and other biomolecules (
Figure 1A)
[15][16].
Figure 1. (A) FRET during protein conformational change. (B) Intramolecular (C) Intermolecular FRET.
5. Determination of Inter and Intramolecular Interactions
One of the most common applications of FRET for detecting intermolecular interactions is to use it to measure the distance between two or more biomolecules. Intermolecular interactions involve the binding of one molecule to another, and they are crucial for many biological processes such as signal transduction, gene regulation, and protein–protein interactions. By attaching a FRET donor and acceptor molecule to different parts of the biomolecules of interest, researchers can use FRET to measure the distance between them. If the biomolecules are not interacting, the distance between the FRET donor and acceptor will be large, leading to low FRET efficiency. However, if the biomolecules bind to each other, the distance decreases between the donor–acceptor pair, leading to a higher FRET efficiency. FRET can also be used to detect intramolecular interactions within a single biomolecule. Intramolecular interactions involve the binding of different parts of the same molecule, and they are crucial for many biological processes such as enzyme catalysis, protein folding, and regulation of protein activity. For example, by attaching a FRET donor and acceptor molecule to different domains of a protein, researchers can use FRET to measure the distance between the domains. If the domains are not interacting, the distance between the FRET donor–acceptor pair will be large, leading to low FRET efficiency. However, if the domains bind to each other, the distance between both will decrease, resulting in higher FRET efficiency. Another way to detect intramolecular interactions by FRET is to use it to monitor changes in the conformation of a biomolecule caused by the interaction of different domains within the same molecule. For example, by attaching a FRET donor and acceptor molecule to different parts of a protein, researchers can use FRET to monitor changes in the conformation of the protein caused by the interaction of different domains. This constitutes FRET as a primal tool for screening inter- as well as intramolecular interactions along with conformational changes that help to understand the mechanisms of reactions. FRET is used to study numerous bimolecular interactions such as protein–DNA interaction and interaction between proteins, enzyme kinetic reaction, changes in reaction kinetics, bonding conformation, and configurationally changes. Reaction rate can also be detected by FRET as transfer efficiency is very much distance dependent, with charge-transfer property and dipolar orientations of biomolecules (
Figure 1B,C)
[17][18][19].
6. Enzyme Kinetic Studies through FRET
FRET can also be used to study enzyme kinetics, i.e., rate at which enzymes catalyze reactions. Enzyme kinetics provides important information on how enzymes work and how they can be regulated, which is crucial for understanding many biological processes. This is possible beacause of spectral overlapping J(λ) of emissin of donor fluorophore and absorption of acceptor fluorophore. This varies as interaction-dependent transfer of energy at all levels of the reaction kinetics. One way to use FRET for enzyme kinetic studies is to attach a FRET donor and acceptor molecule to different parts of an enzyme, such as the active site or a regulatory domain. By measuring the FRET efficiency, researchers can infer the conformation of the enzyme and how it changes during the course of the reaction. This can provide insights into the mechanism of enzyme catalysis and how the enzyme interacts with substrates and inhibitors. FRET-based methods are very useful in enzyme kinetic studies because they allow researchers to monitor the dynamics of enzyme–substrate and enzyme–inhibitor interactions in real time and in a non-invasive way. This can provide important insights into enzyme mechanisms, regulations, and drug discovery. Additionally, FRET can monitor the rate of product formation proteases and nucleases by using suitable substrates as donor
[20].