Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dorin Ioan Cocoș | -- | 2125 | 2023-04-17 18:57:42 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2125 | 2023-04-18 03:15:42 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 2125 | 2023-04-18 03:16:48 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 2125 | 2023-04-18 03:17:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dumitriu Buzia, O.; Păduraru, A.M.; Stefan, C.S.; Dinu, M.; Cocoș, D.I.; Nwabudike, L.C.; Tatu, A.L. Strategies for Improving Transdermal Administration. Encyclopedia. Available online: https://encyclopedia.pub/entry/43132 (accessed on 07 February 2026).
Dumitriu Buzia O, Păduraru AM, Stefan CS, Dinu M, Cocoș DI, Nwabudike LC, et al. Strategies for Improving Transdermal Administration. Encyclopedia. Available at: https://encyclopedia.pub/entry/43132. Accessed February 07, 2026.
Dumitriu Buzia, Olimpia, Ana Maria Păduraru, Claudia Simona Stefan, Monica Dinu, Dorin Ioan Cocoș, Lawrence Chukwudi Nwabudike, Alin Laurențiu Tatu. "Strategies for Improving Transdermal Administration" Encyclopedia, https://encyclopedia.pub/entry/43132 (accessed February 07, 2026).
Dumitriu Buzia, O., Păduraru, A.M., Stefan, C.S., Dinu, M., Cocoș, D.I., Nwabudike, L.C., & Tatu, A.L. (2023, April 17). Strategies for Improving Transdermal Administration. In Encyclopedia. https://encyclopedia.pub/entry/43132
Dumitriu Buzia, Olimpia, et al. "Strategies for Improving Transdermal Administration." Encyclopedia. Web. 17 April, 2023.
Copy Citation
A transdermal delivery system is a painless method of drug administration through intact skin. Transdermal patches favor the controlled release of active ingredients through the skin into systemic circulation.Substances with analgesic effects can be administered on the skin in the form of topical patches made to produce local effects or transdermal patches that ensure the controlled and prolonged release of the active substance.
transdermal patches
iontophoresis
sonophoresis
1. Energy-Based Methods
At the beginning of the 20th century, the concept of ion therapy was introduced, and it was demonstrated that “ionic drugs” can penetrate the skin and exert important local and systemic effects [1].
Iontophoresis is a technique that uses low-intensity electric currents to increase the absorption of drugs through the skin [1].
The values of the intensity of the electric current used in iontophoresis are between 0.5 and 20mA (Figure 1) [1].
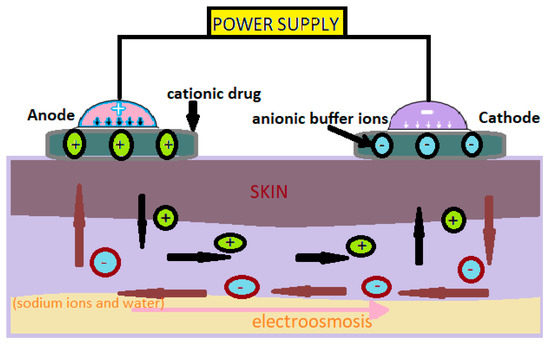
Figure 1. Administration of cationic drugs via iontophoresis. Mechanism [2].
This technology is successfully used by dermatologists to treat patients with hyperhidrosis [1].
The method uses an electrical device to inject a low-voltage current into the affected area. It is painless and relatively safe, but its effectiveness is sometimes similar to a topical antiperspirant [1].
Iontophoresis is mainly used to increase skin absorption of ionizing drugs, but neutral or weakly charged molecules can also be administered through electroosmosis [2].
Iontophoresis has been investigated with the administration of various types of drugs, including nonsteroidal anti-inflammatory drugs (ibuprofen, aspirin, and indomethacin) [1], granisetron, donepezil, and insulin [2].
In this technology, neutral or cationic products are positioned under the anode and anionic products under the cathode [3]. The low-intensity electric current used moves the ions through the skin. This method can be used to administer medicinal substances for local or systemic effects [3].
In ophthalmology, atropine, scopolamine, gentamicin and fluorescein are being tested [3]. In dentistry, they are used as local anesthesia in cases of multiple dental extractions [3][4]. The administration of systemic drugs (fentanyl [3], certain insulin formulas, antihypertensives, antirheumatics, antidiabetics, hormones, etc.) via iontophoresis is still under investigation [4].
Sonophoresis is a method of increasing the absorption of drugs through the skin, which uses ultrasounds with frequencies varying between 20 kHz and 16 MHz to modify the structure of the lipid layer of the stratum corneum (Figure 2) [3].
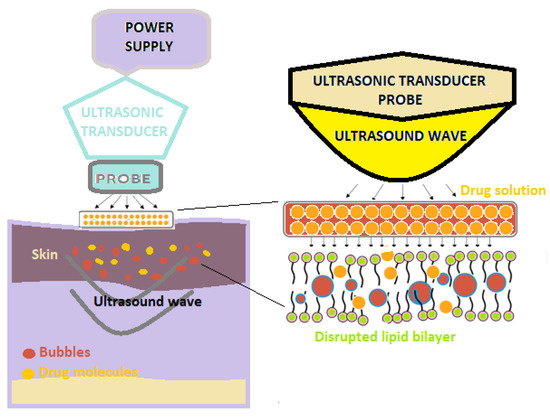
Figure 2. A schematic illustration of sonophoresis-assisted transdermal drug delivery [2].
The use of ultrasound results in an increase in skin temperature and, implicitly, the better absorption of the medicine at this level [2].
In this technique, a predetermined frequency of ultrasound is used on the drug solution that is positioned under the probe, as well as on the skin [3][4].
Various studies have shown that ultrasound-mediated transdermal delivery had good results in the case of the administration of some proteins (erythropoietin, interferon y and insulin), ketoprofen [5] and vancomycin, which demonstrates that a wide range of hydrophilic and hydrophobic products, as well as macromolecules and small molecules, can be administered through sonophoresis [2][3][4].
The result of the sequential ultrasound procedure is so-called sonophoresis. Sonophoresis is a process of the penetration and migration (spreading) of dermo-cosmetic substances in the skin under the action of these sequential ultrasounds [2].
Electroporation is a method used to facilitate the transdermal delivery of drugs; it is based on the penetration of the skin and the creation of micropores by using high voltages (between 10 and 1000 V) for a duration of less than a few hundred milliseconds (Figure 3) [2][3].
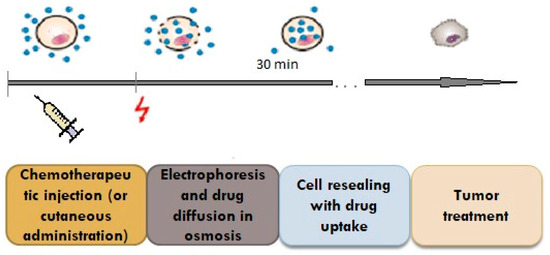
Figure 3. Penetration of the drug through the pores created by the impulse.
With the help of pulse waves, aqueous pores are created in the double lipid layer of the stratum corneum, the solution with the active substance on the skin penetrates deeply, and thus, superior absorption is achieved (Figure 4) [2].
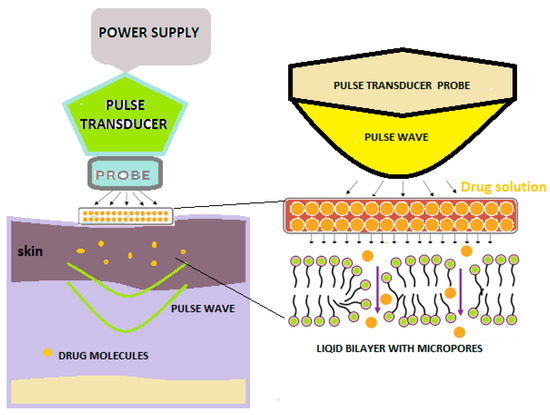
Figure 4. Schematic diagram showing pulse wave transdermal drug delivery [2].
By using this method, good results have been reported regarding the in vitro absorption of insulin, tetracaine, thymol and fentanyl [3].
Blagus et al. performed in vivo research using this method on rats, following the degree of absorption of fentanyl, dextrin and doxorubicin [4].
Galvanization achieves superior absorption in the skin [2].
Microns (MNs) are defined as needles with heights between 25 μm and 2000 μm, and the materials used in their preparation determine their applications [2][3]. The principle of the microneedle release system consists of the creation of microns that penetrate the stratum corneum, which limits absorption, to create micropipes through which the medicine is deposited in the dermis. (Figure 5) [2].
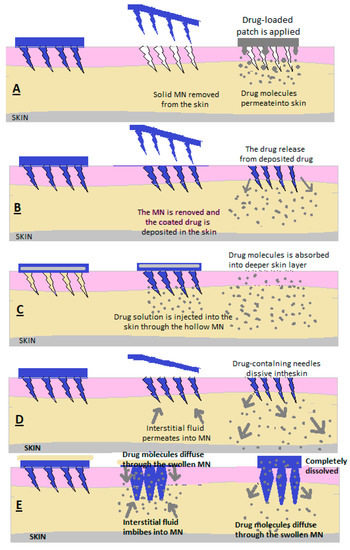
Figure 5. Drug release through (A) solid MN, (B) covered MN, (C) empty MN, (D) dissolving MN and (E) MN that forms a hydrogel [2].
This new technology presents multiple advantages from many points of view:
- -
- -
-
It does not require specialized personnel for administration.
- -
- -
-
The patient’s skin can regenerate very quickly after the removal of MNs; thus, there is only a very low probability of infection and irritation at the application site, as well as possible occurrences of isomorphic lesions due to the Koebner phenomenon, such as lichen planus and psoriasis [6][7][8].
2. Solid MNs
MNs are used to make micropipes with an absorption role in the patient’s skin; after removing the needles, a transdermal patch is applied with a medicinal substance that is released through passive diffusion in the skin [7][8].
2.3. Covered MNs (Figure 5)
Covered MNs are obtained through different methods to ensure uniform coverage [7][8]. A coating is used consisting of the medicinal substance combined with surfactants and thickening agents, which ensure adhesion to the needle ends [7][8]. The needle tips are coated with a formulation in the form of a solution or dispersion [6][7][8]. To make the MNs, polymers or metals are used [6][7][8].
After the MNs have penetrated the skin, at the moment of contact between the interstitial liquid and the coating, it is dissolved and absorbed [6][7][8].
2.4. Empty MNs
Empty MNs are formulated to release a drug continuously into the skin through needle holes [2][3][4][5]. The solution or dispersion containing the medicinal substance is loaded inside the MNs and then administered transdermally [2][3][4][5]. (Figure 5c) Empty MNs and solid MNs can be made of glass, polymers, silicon and metals [2][3][4][5].
As an advantage, compared to solid MNs and coated MNs, MNgol has a greater capacity to fill with medicine [2][6].
2.5. MN of Dissolution
Following application to the skin, the needles also dissolve from the MN matrix, and the active substance is released slowly (Figure 5d) [2].
In the case of the manufacture of these forms of transdermal administration methods, mixtures of biocompatible and soluble polymers with medicinal substances are used [2].
Polymers can be vinyl alcohol, polycarboxymethylcellulose, vinylpyrrolidone, hyaluronic acid and copolymers of methyl vinyl ether and maleic acid [2].
2.6. MN Hydrogel Former
A reservoir containing the active substance is embedded in the empty MN upon application, and after penetration through the skin, the MN swells due to the absorption of interstitial liquid, the reservoir dissolves and the medicine is absorbed through the already-dilated ducts [2][5]. A hydrogel mass is formed to control the release of the drug depending on the crosslinking power of the hydrogel network. This allows the drug to be released slowly over a long period (Figure 5e) [2][5].
In dentistry, special needle-based devices could be very useful and beneficial, because oral carcinoma is often oral squamous cell cancer and is very difficult to detect in the early stages, being asymptomatic [2][5]. These special devices can detect elevated levels of biomarkers, such as Cyfra 21-1, TPA, CA-125 antigen, MMP-9 and TNF-α [9][10].
Microneedles can be used in dental periodontal surgery as an adjuvant because they can accelerate the healing process and locally increase platelet-derived growth factor (PDGF), transforming beta and alpha (TGF-β and TGF-α), connective growth factor and fibroblasts (FGF) [10][11]. They can potentiate neocollagenases and neovascularization through intercellular matrix layering and fibroblast proliferation [11].
Antimicrobial patches that hold gingival-grip microneedles could help speed up the healing of periodontal lesions [11][12][13]. These microneedles are loaded with antimicrobial agents, possibly with green tea and nano-silver, and these patches have been used in experiments designed to suppress the microbial load at the periodontal wound site [12][13].
Following a study, rat experiments demonstrated that analgesic microneedle (AMN) patches have been developed using pain-free soluble content microneedles with the transdermal transfer of the selective antagonist peptide CGRP to treat localized neuropathic pain [14].
Compared to conventional therapies, AMN patches produced a satisfactory analgesic effect without influencing the motor function of laboratory animals, indicating the high specificity of the peptides released against CGRP receptors [14].
2.7. Hybrid Approaches
Recently, the use of a combination of a stainless steel nanoemulsion and microns was studied to improve the transdermal delivery of aceclofenac [15].
2.8. Transdermal System with Amorphous Content of Flurbiprofen and Lidocaine
Researchers created an amorphous molecular complex by combining different anti-inflammatory substances (flurbiprofen, etodolac, naproxen, piroxicam and indomethacin) and lidocaine and examined the physicochemical properties of the complex [16][17][18][19].
The focus was on intermolecular flurbiprofen–lidocaine interaction. They found that the solubility of flurbiprofen was 100 times higher than the product alone, and the solubility of lidocaine was 2 times higher [18]. The conclusion was that it was possible to develop a transdermal preparation with anti-inflammatory and analgesic effects useful for treating the pain of various diseases [18][19].
2.9. Transdermal System with Flurbiprofen and Lidocaine—Ionic Liquids
Ionic liquids (ILs) are ionic salts in a liquid state over a wide temperature range (between ambient temperature and below 100 °C), with high polarity and very high ionic conductivity, high thermal stability, negligible vapor pressure, low volatility and viscosity, non-flammability and a very good recycling capacity [19][20][21].
Third-generation ionic liquids comprise an innovative class of functional materials with improved properties, characterized by excellent biological activity, due to their physical–chemical and biological properties, low toxicity and especially their biotechnological and pharmaceutical potential [22][23][24][25]. These ILs are distinguished by surprising antimicrobial and antibacterial, antibiofilm (bacterial resistance) and antitumor pharmaceutical activity [22][23][24][25].
The role of third-generation biodegradable ILs has been highlighted in the case of transdermal drug delivery systems [26]. Within these systems, ILs can play various roles: as active pharmaceutical ingredients, in drug modification or as transdermal permeation enhancers, as individual agents or in combination with other agents or as chemical permeation enhancers [26].
The incorporation of active pharmaceutical ingredients (APIs) brings about efficient API-IL drug delivery systems, which currently represent an innovative solution for improving the pharmacokinetic and pharmacodynamic properties of many less water-soluble drugs by increasing their solubility, bioavailability, permeability and stability, as well as by eliminating polymorphisms [19][27].
IL molecules are able to act as ideal solvents (green solvents) for drug substances, allowing topical and transdermal administration [28]. Incorporating ILs into water, oils or hydroalcoholic solutions bring about topical IL-based drug administration systems, which have very good therapeutic efficiency at the level of the hydrolipidic film of the skin barrier, due to the increase in drug solubility and the increase in the local permeability of the skin [19][28].
Cholinium-amino-acid-based ionic liquids display high efficiency in the transdermal delivery of ferulic acid and puerarin by improving the solubility and permeability of these two APIs [28].
The skin release capacity of hyaluronic acid has been improved using different choline-based ionic liquids, the strongest being obtained for an IL consisting of choline and citric acid [29].
1-butyl-3-methylimidazolium hexafluorophosphate and 1-hexyl-3-methylimidazolium chloride showed 5% higher antimicrobial activity and higher skin penetration levels [30].
In vitro studies on the production of insulin biopolymer films based on biocompatible ILs have also been performed to improve the transdermal permeability of insulin [31].
A study showed that ionic fluids are safe, contribute to the increase in skin permeability and are promising applications in the field of transdermal systems used in pain treatment [32].
The design of non-irritating drug-IL systems, with high efficiency for permeability through the skin, is based on the effects of the structure of counterions: polarizability, their molecular weight and their polar surface area [33].
The therapeutic potential of the topical delivery of siRNA (small interfering RNA) using ionic liquids (ILs) for the treatment of genetic skin diseases is enhanced by the use of choline-geranic acid ionic liquid [34].
2.10. Capsaicin–Diclofenac Transdermal System
The exact mechanism of this anti-inflammatory combination with low-dose capsaicin for the treatment of osteoarthritis pain is still under investigation [35].
2.11. The Potential of Bee Venom in the Treatment of Pain via Topical or Transdermal Administration
The efficacy of transdermal administration for biologics is limited due to the barrier function of the stratum corneum and possible adverse reactions [36].
Bee venom has been studied since the early 19th century and has been shown to have a complex composition that confers numerous potential therapeutic uses [37][38][39][40].
Many studies are conducted specifically to test the anti-inflammatory effect of bee venom and its mechanism of action [41][42][43][44][45].
Efforts are being made to discover safe practices combined with modern delivery systems to minimize adverse reactions [46].
References
- Zuo, J.; Du, L.; Li, M.; Liu, B.; Zhu, W.; Jin, Y. Transdermal enhancement effect and mechanism of iontophoresis for non-steroidal anti-inflammatory drugs. Int. J. Pharm. 2014, 466, 76–82.
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791.
- Vanbever, R.; Langers, G.; Montmayeur, S.; Préat, V. Transdermal delivery of fentanyl: Rapid onset of analgesia using skin electroporation. J. Control. Release 1998, 50, 225–235.
- Blagus, T.; Markelc, B.; Cemazar, M.; Kosjek, T.; Preat, V.; Miklavcic, D.; Sersa, G. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J. Control. Release 2013, 172, 862–871.
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012, 423, 289–296.
- Brănișteanu, D.E.; Pintilie, A.; Dimitriu, A.; Cerbu, A.; Ciobanu, D.; Oanţă, A.; Tatu, A.L. Clinical, laboratory and therapeutic profile of lichen planus. Rev. Med. Chir. Soc. Med. Nat. 2017, 121, 25–32.
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424.
- Nwabudike, L.C.; Tatu, A.L.; Happle, R. Koebner’s sheep in Wolf’s clothing: Does the isotopic response exists as a distinct phenomenon? J. Eur. Acad. Dermatol. Venereol. 2018, 32, e336–e337.
- Nagler, R.; Bahar, G.; Shpitzer, T.; Feinmesser, R. Concomitant Analysis of Salivary Tumor Markers—A New Diagnostic Tool for Oral Cancer. Clin. Cancer Res. 2006, 12, 3979–3984.
- Shpitzer, T.; Hamzany, Y.; Bahar, G.; Feinmesser, R.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R.M. Salivary analysis of oral cancer biomarkers. Br. J. Cancer 2009, 101, 1194–1198.
- Aashim, S.; Savita, Y. Microneedling: Advances and widening horizons. Indian Dermatol. Online J. 2016, 7, 244–254.
- Young, P.S.; Uk, L.H.; Young-Chul, L.; Hwa, K.G.; Changkyun, P.E.; Hyun, H.S.; Gyu, L.J.; Saehae, C.; Su, H.N.; Lak, K.D.; et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 757–762.
- González García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2019, 55, 171–174.
- Xi, X.; Conrado, P.; Christopher, L.; Seajin, O.; Ji, W.; Bende, Z.; Julian, X.; Zhaohui, L.; James, X.; David, C.Y.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano. 2017, 11, 395–406.
- Ilić, T.; Savić, S.; Batinić, B.; Marković, B.; Schmidberger, M.; Lunter, D.; Savic, M.; Savić, S. Combined use of biocompatible nanoemulsions and solid microneedles to improve transport of a model NSAID across the skin: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2018, 125, 110–119.
- Alshaikh, R.A.; Essa, E.A.; El Maghraby, G.M. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: Caffeine as a melting point modulator. Int. J. Pharm. 2019, 563, 395–405.
- Fiandaca, M.; Dalwadi, G.; Wigent, R.; Gupta, P. Ionic liquid formation with deep eutectic forces at an atypical ratio (2:1) of naproxen to lidocaine in the solid-state, thermal characterization and FTIR investigation. Int. J. Pharm. 2020, 575, 118946.
- Xu, Q.; Furuishi, T.; Fukuzawa, K.; Yonemochi, E. Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form. Pharmaceutics 2023, 15, 318.
- Navti, P.D.; Pandey, A.; Nikam, A.N.; Padya, B.S.; Kalthur, G.; Koteshwara, K.B.; Mutalik, S. Ionic Liquids Assisted Topical Drug Delivery for Permeation Enhancement: Formulation Strategies, Biomedical Applications, and Toxicological Perspective. AAPS PharmSciTech 2022, 23, 161.
- Greer, A.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207.
- Zhuang, W.; Hachem, K.; Bokov, D.; Ansari, M.J.; Nakhjiri, A.T. Ionic liquids in pharmaceutical industry: A systematic review on applications and future perspectives. J. Mol. Liq. 2022, 349, 118145.
- Pedro, S.; Freire, C.R.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298.
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546.
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic Liquids as Active Pharmaceutical Ingredients. Chem. Med. Chem. 2011, 6, 975–985.
- Benítez-Mateos, A.I.; Roura Padrosa, D.; Paradisi, F. Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses. Nat. Chem. 2022, 14, 489–499.
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96.
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic liquids as a useful tool for tailoring active pharmaceutical ingredients. J. Control. Release 2021, 338, 268–283.
- Yuan, J.; Zhou, N.; Wu, J.; Yin, T.; Jia, Y. Ionic liquids as effective additives to enhance the solubility and permeation for puerarin and ferulic acid. RSC Adv. 2022, 12, 3416–3422.
- Wu, X.; Zhang, H.; He, S.; Yu, Q.; Lu, Y.; Wu, W.; Ding, N.; Zhu, Q.; Chen, Z.; Ma, Y.; et al. Improving dermal delivery of hyaluronic acid by ionic liquids for attenuating skin dehydration. Int. J. Biol. Macromol. 2020, 150, 528–535.
- Caparica, R.; Júlio, A.; Rosado, C.; de Almeida, T.S. Applicability of Ionic Liquids in Topical Drug Delivery Systems: A Mini Review. J. Pharmacol. Clin. Res. 2018, 4, 555649–555655.
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M.J.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharmacol. 2020, 11, 243.
- Yang, D.; Chen, X.; Li, Z.; Yang, C. Mechanistic Study of Release Characteristics of Two Active Ingredients in Transdermal Patch Containing Lidocaine−Flurbiprofen Ionic Liquid. Pharmaceutics 2022, 14, 2158.
- Yang, D.; Liu, C.; Ding, D.; Quan, P.; Fang, L. The molecular design of drug-ionic liquids for transdermal drug delivery: Mechanistic study of counterions structure on complex formation and skin permeation. Int. J. Pharm. 2021, 602, 120560.
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.V.L.B.; Mitragotri, S. Topical delivery of siRNA into skin using ionic liquids. J. Control. Release 2020, 323, 475–482.
- Persson, M.; Stocks, J.; Walsh, D.; Doherty, M.; Zhang, W. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: A network meta-analysis of randomised controlled trials. Osteoarthr. Cartil. 2018, 26, 1575–1582.
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360.
- Baek, H.; Park, S.-Y.; Ku, S.J.; Ryu, K.; Kim, Y.; Bae, H.; Lee, Y.-S. Bee Venom Phospholipase A2 Induces Regulatory T Cell Populations by Suppressing Apoptotic Signaling Pathway. Toxins 2020, 12, 198.
- Cheng, B.; Xu, P. Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin. Toxins 2020, 12, 582.
- Kim, W. Bee Venom and Its Sub-Components: Characterization, Pharmacology, and Therapeutics. Toxins 2021, 13, 191.
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997.
- Tekeoğlu, I.; Akdoğan, M.; Çelik, I. Investigation of anti-inflammatory effects of bee venom in experimentally induced adjuvant arthritis. Reumatologia 2020, 58, 265–271.
- Bae, S.; Gu, H.; Gwon, M.-G.; An, H.-J.; Han, S.-M.; Lee, S.-J.; Leem, J.; Park, K.-K. Therapeutic Effect of Bee Venom and Melittin on Skin Infection Caused by Streptococcus pyogenes. Toxins 2022, 14, 663.
- Kurek-Górecka, A.; Komosinska-Vassev, K.; Rzepecka-Stojko, A.; Olczyk, P. Bee Venom in Wound Healing. Molecules 2020, 26, 148.
- El-Tedawy, D.M.; Abd-Alhaseeb, M.; Helmy, M.W.; Ghoneim, A.I. Systemic bee venom exerts anti-arthritic and anti-inflammatory properties in a rat model of arthritis. Biomed. Rep. 2020, 13, 20.
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective. Front. Pharmacol. 2022, 13, 1001553.
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78.
- Jung, H.; Kim, Y.S.; Jung, D.-M.; Lee, K.-S.; Lee, J.-M.; Kim, K.K. Melittin-derived peptides exhibit variations in cytotoxicity and antioxidant, anti-inflammatory and allergenic activities. Anim. Cells Syst. 2022, 26, 158–165.
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090.
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2234–2241.
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17.
- Yu, X.; Chen, L.; Liu, J.; Dai, B.; Xu, G.; Shen, G.; Luo, Q.; Zhang, Z. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 2019, 10, 574.
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
4 times
(View History)
Update Date:
18 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




