
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcelo Giovanela | -- | 4553 | 2023-04-17 18:10:36 | | | |
| 2 | Catherine Yang | Meta information modification | 4553 | 2023-04-18 02:24:52 | | |
Video Upload Options
Diseases caused by infections are becoming harder to treat as the antibiotics used become less effective. A combination of strategies to develop active biomaterials that enhance antibacterial effects are desirable, especially ones that cause fewer side effects and promote healing properties. The development of new antimicrobial products is necessary to avoid the transmission of infection in healthcare environments. In this sense, metallic and metal oxide nanoparticles (NPs) have been gaining attention due to their unique size-dependent physical and chemical properties. The best known examples of this category are the NPs of elements such as silver, copper, gold, palladium, and platinum, which are used in varied areas of application (catalytic, biomedical, and electronic) as their properties are distinguished from those presented by the bulk. NPs are especially effective against Gram-negative and Gram-positive bacteria, such as Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli.
1. Silver Nanoparticles
2. Copper Nanoparticles
3. Gold Nanoparticles
4. Metal Oxide Nanoparticles
5. Synthesis of NPs
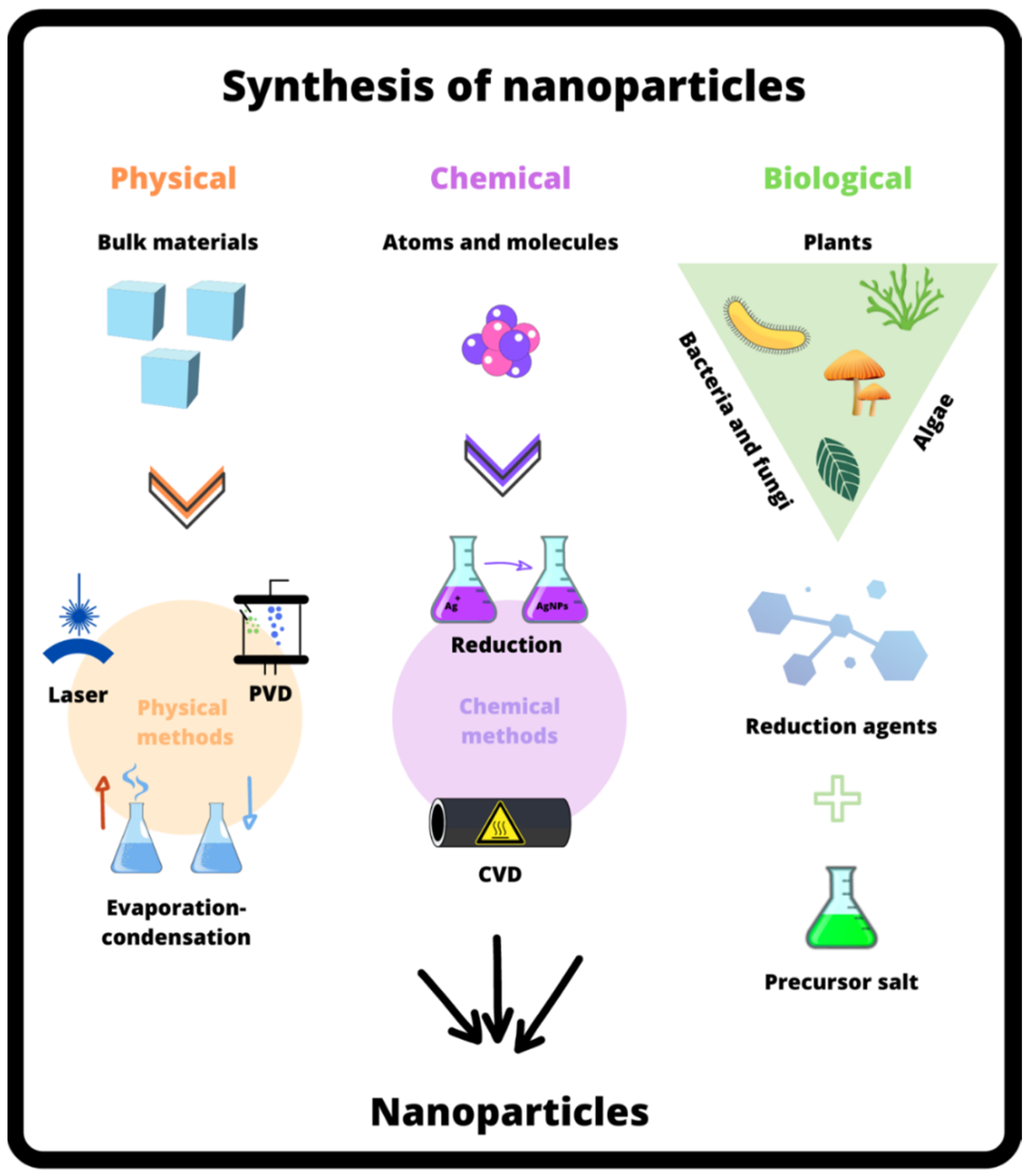
5.1. Physical Approach
5.2. Chemical Approach
5.3. Biological Approach
| Nanoparticle | Synthesis Method | Precursor Agent | Reaction Time (min) | Reaction Temperature (°C) | Average Size (nm) |
Shape | Observation | Reference |
|---|---|---|---|---|---|---|---|---|
| AgNPs | Chemical method | AgNO3 | 30 to 120 | 80 | 65 to 85 | - | - | Danna et al. [46] |
| AgNPs | Chemical method | AgNO3/NaBH4 | - | - | 10 to 20 | Sphere | NPs concentration in NRL 0.4% |
Marques et al. [47] |
| AgNPs | Physical method | AgNO3/NaBH4 | 20 | - | 4 to 10 | Sphere and aggregates | UV power light 250 W |
Bakar et al. [50] |
| AgNPs | Green synthesis | AgNO3 | 60 | 100 | 2 to 100 | Sphere and aggregates | 50 to 400 μL of NRL were tested | Guidelli et al. [49] |
| AgNPs | Chemical method | AgNO3/NaBH4 | 5 | 40 | 30 | Sphere and aggregates | - | Guidelli et al. [53] |
| Fe3O4NPs | Chemical method | FeCl3·6 H2O FeCl2·4 H2O |
60 | 90 | 12 | Sphere | - | Arsalani et al. [32] |
| Fe3O4NPs | Green synthesis | FeCl3·6 H2O FeCl2·4 H2O |
70 | 90 | 7.9 to 13 | Sphere | 100 to 800 μL of NRL were tested | Arsalani et al. [32] |
6. Mechanism of Bactericidal Activity of NPs
| Disadvantages | Consequence/Examples |
|---|---|
| Cytotoxicity | There are concerns about the cytotoxic effect of metallic NPs, as the mechanism of interaction of NPs with cells is still not fully understood [58]. This may occur because there is a large variation in the parameters in relation to NPs, such as their size, shape, and surface charge [59][60]. Extended exposure to AgNPs through oral and inhalation can lead to Argyria or Argyrosis, i.e., chronic disorders of skin microvessels and eyes in humans. In vitro cell culture studies have indicated the toxic effects of AgNPs in immortal human skin keratinocytes, human erythrocytes, human neuroblastoma cells, human embryonic kidney cells, human liver cells, and human colon cells. In vivo animal studies have revealed the toxic effects of AgNPs in rodents by accumulating in their liver, spleen, and lung [1]. |
| Interactions for different cell lineages | Different cell lineages exhibit distinct cytotoxic responses. Vero cells (African green monkey renal epithelial cells), for example, have been shown to be more susceptible to chitosan/pectin/AuNPs hydrogel than LLCMK2 cells (Macaca mulatta renal epithelial cells) [61]. We can also mention the case of the hydrogel dressing containing AgNPs, which exhibited a different level of toxicity in relation to immortal keratinocytes and primary keratinocytes [62]. Therefore, there is a certain challenge when choosing a cell line that is more suitable for biocompatibility testing in the study of a material containing NPs [59]. |
| Migration of NPs to undesirable sites | NPs cannot only be directly absorbed by the cells of exposed organs, but they can also be translocated to other organs, causing unwanted toxicity or other adverse effects [63]. The NPs leaching depends on the hydrodynamic conditions at the implantation site [64]. The material safety assessment should be extensively conducted in adjacent tissues and organs, and not limited to the site where the artifact will be implanted [59]. |
| Resistance to NPs by bacteria | Bacteria such as Bacillus subtilis have the ability to adapt to cellular oxidative stress produced by Ag(I) [65]. Bacteria can develop a resistance to AgNPs after a repeated exposure, and resistance evolves without any genetic change. Only phenotypic change is needed to reduce the stability of NPs and thus eliminate the antibacterial activity of AgNPs [66][67]. |
| Nanoparticle aggregation process | NPs tend to aggregate or flocculate and are not stable in aqueous solutions [68]. Aggregation affects the stability of NPs and limits their use as drug carriers. Particle collisions due to Brownian motion leads to aggregation and precipitation. Therefore, it is vital to obtain NPs that are well dispersed and stable in the solution phase (mainly in phosphate-buffered saline). A possible solution is to increase the repulsion between NPs, which increases their colloidal dispersion. However, in the case of NPs used in biomedical therapies, chemical stability in the biological environment is hard to obtain. For example, acidic conditions found in cancer cells can cause the aggregation of many NPs [69]. |
| Pollution of riverbeds | The extensive application and production of AgNPs can increase their release in aquatic environments such as rivers and lakes. For example, AgNPs can be released from antimicrobial fabrics into water during washing, thereby polluting groundwater. Once AgNPs enter the freshwater environment, they generally oxidize to Ag(I) ions that are toxic to aquatic organisms. Furthermore, ionic silver can stabilize into sparingly soluble salts. By accumulating in aquatic organisms, AgNPs can enter the human body through the food chain [1]. |
References
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449.
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14.
- Islam, A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918.
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11.
- Yaqub, A.; Malkani, N.; Shabbir, A.; Ditta, S.A.; Tanvir, F.; Ali, S.; Naz, M.; Kazmi, S.A.R.; Ullah, R. Novel Biosynthesis of Copper Nanoparticles Using Zingiber and Allium sp. with Synergic Effect of Doxycycline for Anticancer and Bactericidal Activity. Curr. Microbiol. 2020, 77, 2287–2299.
- Khademi-Azandehi, P.; Moghaddam, J. Green synthesis, characterization and physiological stability of gold nanoparticles from Stachys lavandulifolia Vahl extract. Particuology 2015, 19, 22–26.
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gopi, N.; Ekambaram, P.; Pachaiappan, R.; Velusamy, P.; Murugan, K.; Benelli, G.; Kumar, R.S.; et al. Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant Enterococcus faecalis biofilms and human lung cancer cells (A549). Microb. Pathog. 2017, 102, 173–183.
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11.
- Guerra, R.; Lima, E.; Guzmán, A. Antimicrobial supported nanoparticles: Gold versus silver for the cases of Escherichia coli and Salmonella typhi. Microporous Mesoporous Mater. 2012, 170, 62–66.
- Katas, H.; Lim, C.S.; Azlan, A.Y.H.N.; Buang, F.; Busra, M.F.M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292.
- Khan, F.U.; Chen, Y.; Ahmad, A.; Tahir, K.; Khan, Z.U.; Khan, A.U.; Khan, S.U.; Raza, M.; Wan, P. Visible light inactivation of E. coli, Cytotoxicity and ROS determination of biochemically capped gold nanoparticles. Microb. Pathog. 2017, 107, 419–424.
- Hussein, M.A.M.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. extract. Carbohydr. Polym. 2021, 256, 117498.
- Xie, Y.; Yang, J.; Zhang, J.; Zheng, W.; Jiang, X. Activating the Antibacterial Effect of 4,6-Diamino-2-pyrimidinethiol-Modified Gold Nanoparticles by Reducing their Sizes. Angew. Chem. Int. Ed. 2020, 59, 23471–23475.
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745.
- Krishnan, B.; Mahalingam, S. Improved surface morphology of silver/copper oxide/bentonite nanocomposite using aliphatic ammonium based ionic liquid for enhanced biological activities. J. Mol. Liq. 2017, 241, 1044–1058.
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27.
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808.
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978.
- Kovalishyn, V.; Abramenko, N.; Kopernyk, I.; Charochkina, L.; Metelytsia, L.; Tetko, I.V.; Peijnenburg, W.; Kustov, L. Modelling the toxicity of a large set of metal and metal oxide nanoparticles using the OCHEM platform. Food Chem. Toxicol. 2018, 112, 507–517.
- Winkler, D.A. Role of Artificial Intelligence and Machine Learning in Nanosafety. Small 2020, 16, 2001883–2001889.
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758.
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481.
- Sahoo, S.; Maiti, M.; Ganguly, A.; George, J.J.; Bhowmick, A.K. Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 2007, 105, 2407–2415.
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Albar, N.H.M.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990.
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562.
- Çeşmeli, S.; Avci, C.B. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766.
- Ilyas, M.; Waris, A.; Khan, A.U.; Zamel, D.; Yar, L.; Baset, A.; Muhaymin, A.; Khan, S.; Ali, A.; Ahmad, A. Biological synthesis of titanium dioxide nanoparticles from plants and microorganisms and their potential biomedical applications. Inorg. Chem. Commun. 2021, 133, 108968.
- Naseri, N.; Janfaza, S.; Irani, R. Visible light switchable bR/TiO 2 nanostructured photoanodes for bio-inspired solar energy conversion. RSC Adv. 2015, 5, 18642–18646.
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222.
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470.
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324.
- Arsalani, S.; Guidelli, E.J.; Araujo, J.; Bruno, A.C.; Baffa, O. Green Synthesis and Surface Modification of Iron Oxide Nanoparticles with Enhanced Magnetization Using Natural Rubber Latex. ACS Sustain. Chem. Eng. 2018, 6, 13756–13765.
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378.
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469.
- Giovanela, C.S.C.G.; Crespo, M.J.S.; Roesch-Ely, M.; Henriques, J.A.P.; Aguzzoli, C.; Maddalozzo, A.E.D. Filmes, Processos de Obtenção dos Filmes e Uso dos Filmes. Patent BR1020190107154 2019.
- Garcia, C.S.C.; Maddalozzo, A.E.D.; Garcia, P.M.C.; Fontoura, C.P.; Rodrigues, M.M.; Giovanela, M.; Henriques, J.A.P.; Aguzzoli, C.; Crespo, J.D.S.; Roesch-Ely, M. Natural Rubber Films Incorporated with Red Propolis and Silver Nanoparticles Aimed for Occlusive Dressing Application. Mater. Res. 2021, 24, 1–16.
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.B.H.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756.
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220.
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325.
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174.
- Gunnarsson, R.; Helmersson, U.; Pilch, I. Synthesis of titanium-oxide nanoparticles with size and stoichiometry control. J. Nanopart. Res. 2015, 17, 353–358.
- Dreesen, L.; Colomer, J.-F.; Limage, H.; Giguère, A.; Lucas, S. Synthesis of titanium dioxide nanoparticles by reactive DC magnetron sputtering. Thin Solid Films 2009, 518, 112–115.
- Kwoka, M.; Lyson-Sypien, B.; Kulis, A.; Maslyk, M.; Borysiewicz, M.A.; Kaminska, E.; Szuber, J. Surface Properties of Nanostructured, Porous ZnO Thin Films Prepared by Direct Current Reactive Magnetron Sputtering. Materials 2018, 11, 131.
- Shi, L.; Buhler, E.; Boué, F.; Carn, F. How does the size of gold nanoparticles depend on citrate to gold ratio in Turkevich synthesis? Final answer to a debated question. J. Colloid Interface Sci. 2017, 492, 191–198.
- Piszczek, P.; Lewandowska, A.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274.
- Danna, C.S.; Cavalcante, D.G.S.M.; Gomes, A.S.; Kerche-Silva, L.E.; Yoshihara, E.; Osorio-Román, I.O.; Salmazo, L.O.; Rodríguez-Pérez, M.A.; Aroca, R.F.; Job, A.E. Silver Nanoparticles Embedded in Natural Rubber Films: Synthesis, Characterization, and Evaluation of In Vitro Toxicity. J. Nanomater. 2016, 2016, 2368630.
- Marques, L.; Martinez, G.; Guidelli, J.; Tamashiro, J.; Segato, R.; Payão, S.L.M.; Baffa, O.; Kinoshita, A. Performance on Bone Regeneration of a Silver Nanoparticle Delivery System Based on Natural Rubber Membrane NRL-AgNP. Coatings 2020, 10, 323.
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84–89.
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Baffa, O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 140–145.
- Bakar, N.H.H.; Ismail, J.; Bakar, M. Synthesis and characterization of silver nanoparticles in natural rubber. Mater. Chem. Phys. 2007, 104, 276–283.
- Rathnayake, I.; Ismail, H.; Azahari, B.; De Silva, C.; Darsanasiri, N. Imparting antimicrobial properties to natural rubber latex foam via green synthesized silver nanoparticles. J. Appl. Polym. Sci. 2013, 131, 1–10.
- Phinyocheep, P. In-situ green synthesis of silver nanoparticles in natural rubber latex for fabricating rubber composite with antimicrobial property. Int. J. Sci. Innov. Technol. 2021, 4, 11–20.
- Guidelli, É.J.; Kinoshita, A.; Ramos, A.P.; Baffa, O. Silver nanoparticles delivery system based on natural rubber latex membranes. J. Nanopart. Res. 2013, 15, 1536.
- Fanoro, O.T.; Oluwafemi, O.S. Bactericidal Antibacterial Mechanism of Plant Synthesized Silver, Gold and Bimetallic Nanoparticles. Pharmaceutics 2020, 12, 1044.
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024.
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769.
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562.
- Souza, T.A.; Franchi, L.P.; Rosa, L.R.; Veiga, M.; Takahashi, C.S. Cytotoxicity and genotoxicity of silver nanoparticles of different sizes in CHO-K1 and CHO-XRS5 cell lines. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 795, 70–83.
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration. Bioengineering 2019, 6, 17.
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702.
- Tentor, F.R.; de Oliveira, J.H.; Scariot, D.B.; Lazarin-Bidóia, D.; Bonafé, E.G.; Nakamura, C.V.; Venter, S.A.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Scaffolds based on chitosan/pectin thermosensitive hydrogels containing gold nanoparticles. Int. J. Biol. Macromol. 2017, 102, 1186–1194.
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns 2014, 40, 1562–1569.
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and Gold Nanoparticles Exposure to In Vitro Cultured Retina—Studies on Nanoparticle Internalization, Apoptosis, Oxidative Stress, Glial- and Microglial Activity. PLoS ONE 2014, 9, e105359.
- Kostić, D.D.; Malagurski, I.S.; Obradović, B.M. Transport of silver nanoparticles from nanocomposite Ag/alginate hydrogels under conditions mimicking tissue implantation. Chem. Ind. 2017, 71, 383–394.
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Induced Adaptation of Bacillus sp. to Antimicrobial Nanosilver. Small 2013, 9, 3554–3560.
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71.
- Guo, Z.; Chen, Y.; Wang, Y.-H.; Jiang, H.; Wang, X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J. Mater. Chem. B 2020, 8, 4764–4777.
- Avirdi, E.; Hooshmand, S.E.; Sepahvand, H.; Vishwanathan, V.; Bahadur, I.; Katata-Seru, L.M.; Varma, R.S. Ionic liquids-assisted greener preparation of silver nanoparticles. Curr. Opin. Green Sustain. Chem. 2021, 33, 100581–100593.
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279.





