
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | REYAZ HASSAN MIR | -- | 4419 | 2023-04-17 12:35:40 | | | |
| 2 | Jessie Wu | Meta information modification | 4419 | 2023-04-18 02:06:46 | | |
Video Upload Options
Homeostasis between protein synthesis and degradation is a critical biological function involving a lot of precise and intricate regulatory systems. The ubiquitin-proteasome pathway (UPP) is a large, multi-protease complex that degrades most intracellular proteins and accounts for about 80% of cellular protein degradation. The proteasome, a massive multi-catalytic proteinase complex that plays a substantial role in protein processing, has been shown to have a wide range of catalytic activity and is at the center of this eukaryotic protein breakdown mechanism. As cancer cells overexpress proteins that induce cell proliferation, while blocking cell death pathways, UPP inhibition has been used as an anticancer therapy to change the balance between protein production and degradation towards cell death. Natural products have unique chemical diversity, which results in diversity in their biological activities and drug-like properties. Physical chemistry has been able to recognize the high structural diversity of natural products. Their efficacy is related to the complexity of their well-organized three-dimensional chemical and steric properties, which offer many advantages in terms of efficiency and the selectivity of molecular targets.
1. Emodin
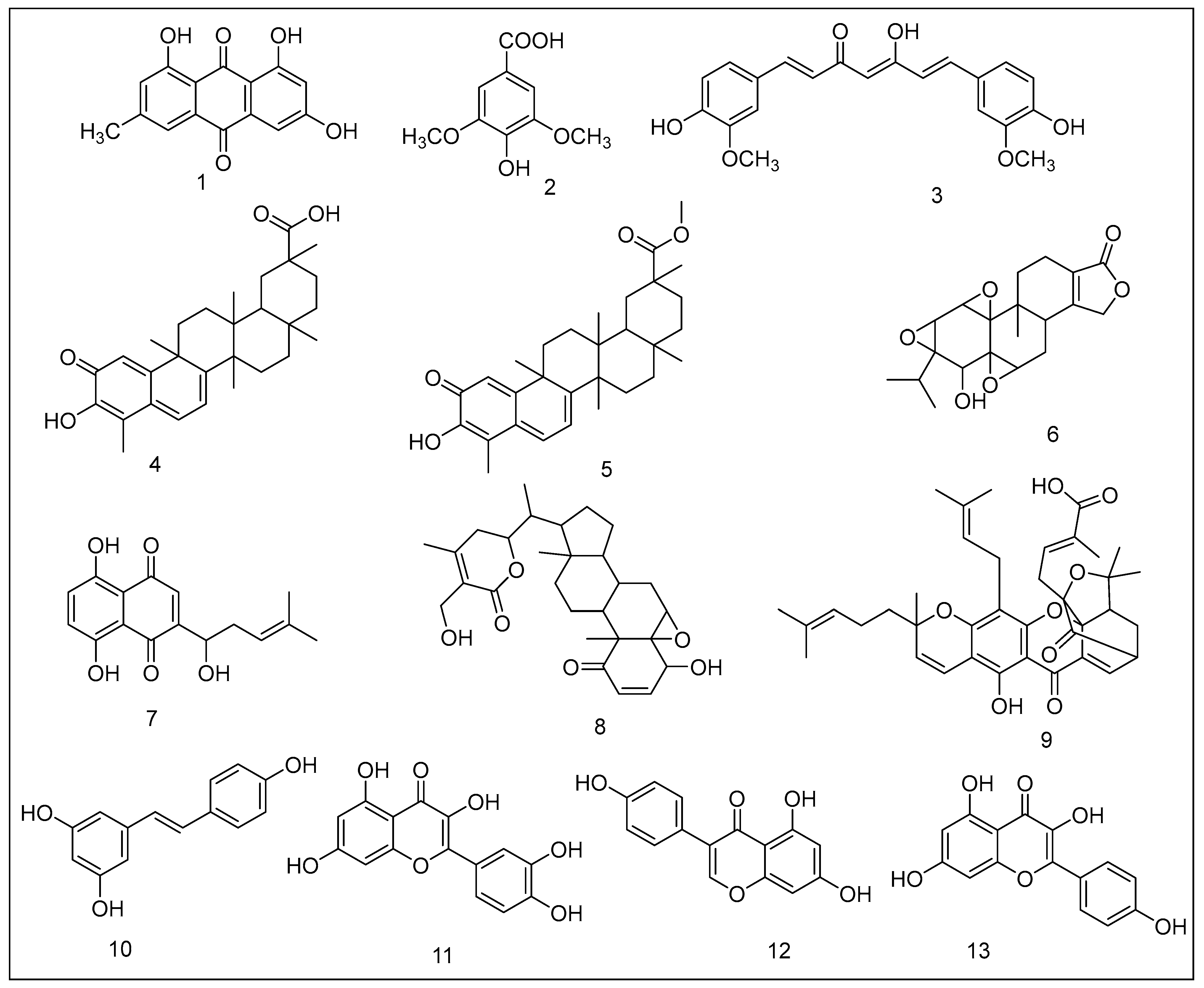
2. Syringic Acid Derivatives
3. Curcumin
4. Celastrol
5. Pristimerin
6. Triptolide
7. Shikonin
8. Withaferin A
9. Gambogic Acid
10. Resveratrol
11. Quercetin
12. Genistein
13. Kaempferol
14. Green Tea Polyphenols
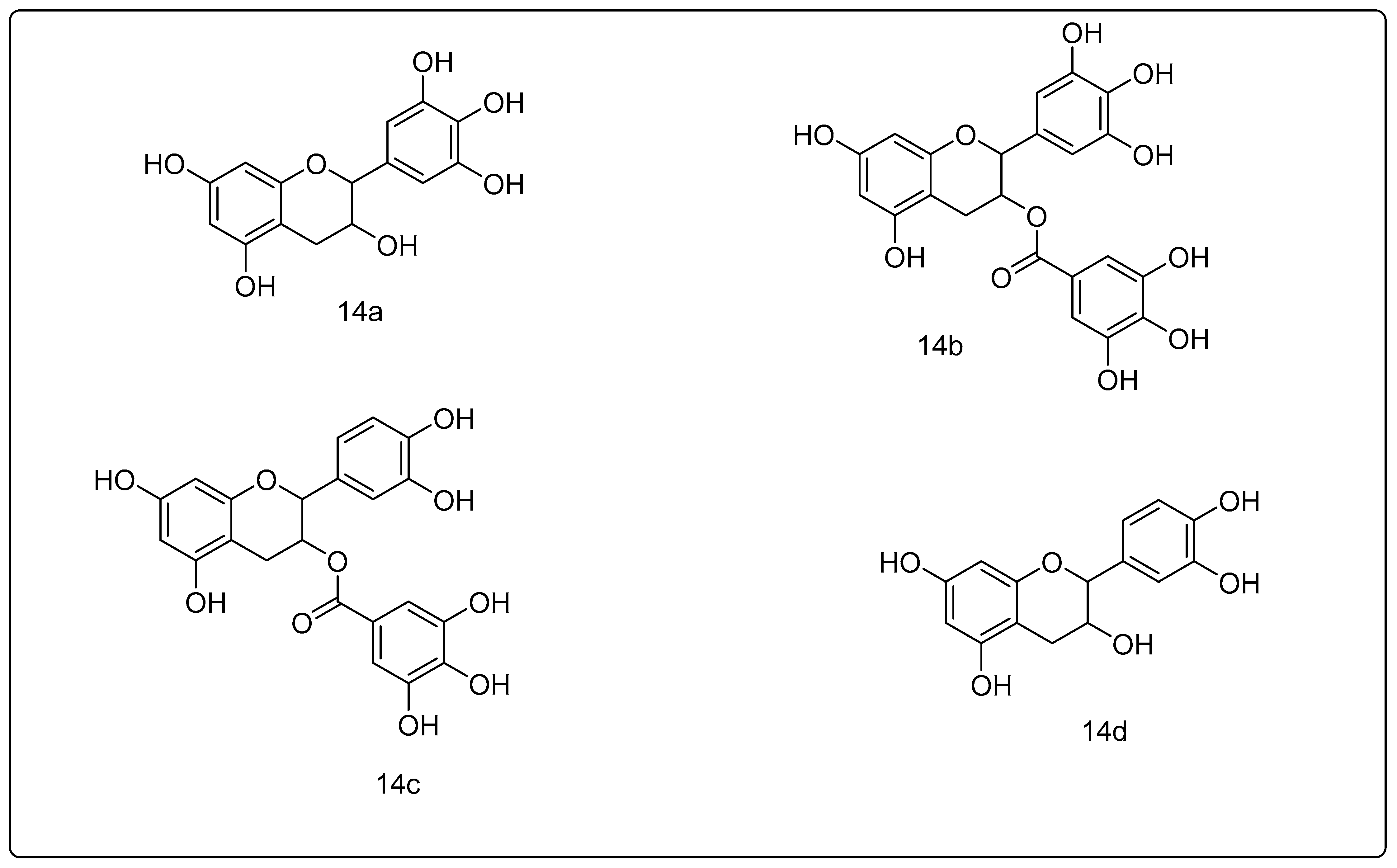
References
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Dalla Vecchia, F.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M.J.C.R. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804.
- Monisha, B.A.; Kumar, N.; Tiku, A. Emodin and its role in chronic diseases. Anti-Inflamm. Nutraceuticals Chronic Dis. 2016, 928, 47–73.
- Hsu, S.-C.; Chung, J.-G.J.B. Anticancer potential of emodin. BioMedicine 2012, 2, 108–116.
- Liu, W.; Feng, Q.; Li, Y.; Ye, L.; Hu, M.; Liu, Z.J.T. Coupling of UDP-glucuronosyltransferases and multidrug resistance-associated proteins is responsible for the intestinal disposition and poor bioavailability of emodin. Toxicol. Appl. Pharmacol. 2012, 265, 316–324.
- Xing, J.Y.; Song, G.P.; Deng, J.P.; Jiang, L.Z.; Xiong, P.; Yang, B.J.; Liu, S.S. Antitumor Effects and Mechanism of Novel Emodin Rhamnoside Derivatives against Human Cancer Cells. PLoS ONE 2015, 10, e0144781.
- He, Y.; Huang, J.; Wang, P.; Shen, X.; Li, S.; Yang, L.; Liu, W.; Suksamrarn, A.; Zhang, G.; Wang, F.J.O. Emodin potentiates the antiproliferative effect of interferon α/β by activation of JAK/STAT pathway signaling through inhibition of the 26S proteasome. Oncotarget 2016, 7, 4664.
- Calderwood, S.K.; Gong, J. Heat shock proteins promote cancer: It’s a protection racket. Trends Biochem. Sci. 2016, 41, 311–323.
- Yan, Y.-Y.; Zheng, L.-S.; Zhang, X.; Chen, L.-K.; Singh, S.; Wang, F.; Zhang, J.-Y.; Liang, Y.-J.; Dai, C.-L.; Gu, L.-Q. Blockade of Her2/neu binding to Hsp90 by emodin azide methyl anthraquinone derivative induces proteasomal degradation of Her2/neu. Mol. Pharm. 2011, 8, 1687–1697.
- Gililland, J.M.; Anderson, L.A.; Erickson, J.; Pelt, C.E.; Peters, C.L. Mean 5-year clinical and radiographic outcomes of cementless total hip arthroplasty in patients under the age of 30. BioMed Res. Int. 2013, 2013, 1–7.
- Abaza, M.-S.; Al-Attiyah, R.A.; Bhardwaj, R.; Abbadi, G.; Koyippally, M.; Afzal, M.J.P. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol. 2013, 51, 1110–1124.
- Kampa, M.; Alexaki, V.-I.; Notas, G.; Nifli, A.-P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.J. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63–R74.
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.-M.; Chang, P.-S.; Jeong, C.-H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445.
- Carlsson, J.; Davidsson, S.; Helenius, G.; Karlsson, M.; Lubovac, Z.; Andrén, O.; Olsson, B.; Klinga-Levan, K.J. A miRNA expression signature that separates between normal and malignant prostate tissues. Cancer Cell Int. 2011, 11, 14.
- Mir, R.H.; Mir, P.A.; Shah, A.J.; Banday, N.; Sabreen, S.; Maqbool, M.; Jan, R.; Shafi, N.; Masoodi, M.H. Curcumin as a privileged scaffold molecule for various biological targets in drug development. Stud. Nat. Prod. Chem. 2022, 73, 405–434.
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020, 173, 113820.
- Huang, B.-R.; Tsai, C.-H.; Chen, C.-C.; Way, T.-D.; Kao, J.-Y.; Liu, Y.-S.; Lin, H.-Y.; Lai, S.-W.; Lu, D.-Y. Curcumin promotes connexin 43 degradation and temozolomide-induced apoptosis in glioblastoma cells. Am. J. Chin. Med. 2019, 47, 657–674.
- Chen, Q.; Tao, J.; Hei, H.; Li, F.; Wang, Y.; Peng, W.; Zhang, X.J. Up-regulatory effects of curcumin on large conductance Ca2+-activated K+ channels. PLoS ONE 2015, 10, e0144800.
- Kong, D.; Zhang, Z.; Chen, L.; Huang, W.; Zhang, F.; Wang, L.; Wang, Y.; Cao, P.; Zheng, S.J. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020, 36, 101600.
- Liu, L.; Yang, J.; Ji, W.; Wang, C.J.B. Curcumin Inhibits Proliferation of Epstein–Barr Virus-Associated Human Nasopharyngeal Carcinoma Cells by Inhibiting EBV Nuclear Antigen 1 Expression. BioMed Res. Int. 2019, 2019, 1–10.
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.-J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622.
- Chaudhary, N.; Ueno-Shuto, K.; Ono, T.; Ohira, Y.; Watanabe, K.; Nasu, A.; Fujikawa, H.; Nakashima, R.; Takahashi, N.; Suico, M.A.J.B.; et al. Curcumin down-regulates toll-like receptor-2 gene expression and function in human cystic fibrosis bronchial epithelial cells. Biol. Pharm. Bull. 2019, 42, 489–495.
- Chen, Y.; Wu, R.; Chen, W.; Liu, Y.; Liao, X.; Zeng, B.; Guo, G.; Lou, F.; Xiang, Y.; Wang, Y.J. Curcumin prevents obesity by targeting TRAF4-induced ubiquitylation in m6A-dependent manner. EMBO Rep. 2021, 22, e52146.
- Obaidi, I.; Cassidy, H.; Ibanez Gaspar, V.; McCaul, J.; Higgins, M.; Halász, M.; Reynolds, A.L.; Kennedy, B.N.; McMorrow, T.J.B. Curcumin sensitizes kidney cancer cells to TRAIL-induced apoptosis via ROS mediated activation of JNK-CHOP pathway and upregulation of DR4. Biology 2020, 9, 92.
- Buratta, S.; Chiaradia, E.; Tognoloni, A.; Gambelunghe, A.; Meschini, C.; Palmieri, L.; Muzi, G.; Urbanelli, L.; Emiliani, C.; Tancini, B.J. Effect of Curcumin on Protein Damage Induced by Rotenone in Dopaminergic PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2761.
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X.J. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160.
- Obata, K.; Kojima, T.; Masaki, T.; Okabayashi, T.; Yokota, S.; Hirakawa, S.; Nomura, K.; Takasawa, A.; Murata, M.; Tanaka, S.J. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS ONE 2013, 8, e70225.
- Khan, T.K.; You, Y.; Nelson, T.J.; Kundu, S.; Pramanik, S.K.; Das, J. Modulation of proteasome activity by curcumin and didemethylcurcumin. J. Biomol. Struct. Dyn. 2021, 40, 8332–8339.
- Liu, L.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Curcumin inhibits proteasome activity in triple-negative breast cancer cells through regulating p300/miR-142–3p/PSMB5 axis. Phytomedicine 2020, 78, 153312.
- Wan, S.B.; Yang, H.; Zhou, Z.; Cui, Q.C.; Chen, D.; Kanwar, J.; Mohammad, I.; Dou, O.P.; Chan, T.H. Evaluation of curcumin acetates and amino acid conjugates as proteasome inhibitors. Int. J. Mol. Med. 2010, 26, 447–455.
- Yue, X.; Zuo, Y.; Ke, H.; Luo, J.; Lou, L.; Qin, W.; Wang, Y.; Liu, Z.; Chen, D.; Sun, H.J. Identification of 4-arylidene curcumin analogues as novel proteasome inhibitors for potential anticancer agents targeting 19S regulatory particle associated deubiquitinase. Biochem. Pharmacol. 2017, 137, 29–50.
- Dai, Y.; DeSano, J.; Tang, W.; Meng, X.; Meng, Y.; Burstein, E.; Lawrence, T.S.; Xu, L. Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS ONE 2010, 5, e14153.
- Pang, X.; Yi, Z.; Zhang, J.; Lu, B.; Sung, B.; Qu, W.; Aggarwal, B.B.; Liu, M. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010, 70, 1951–1959.
- Raja, S.M.; Clubb, R.J.; Ortega-Cava, C.; Williams, S.H.; Bailey, T.A.; Duan, L.; Zhao, X.; Reddi, A.L.; Nyong, A.M.; Natarajan, A. Anticancer activity of Celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers. Cancer Biol. Ther. 2011, 11, 263–276.
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765.
- Soave, C.L.; Guerin, T.; Liu, J.; Dou, Q.P. Targeting the ubiquitin-proteasome system for cancer treatment: Discovering novel inhibitors from nature and drug repurposing. Cancer Metastasis Rev. 2017, 36, 717–736.
- Mahajan, K.; Malla, P.; Lawrence, H.R.; Chen, Z.; Kumar-Sinha, C.; Malik, R.; Shukla, S.; Kim, J.; Coppola, D.; Lawrence, N.J. ACK1/TNK2 regulates histone H4 Tyr88-phosphorylation and AR gene expression in castration-resistant prostate cancer. Cancer Cell 2017, 31, 790–803.e8.
- Chen, M.; Rose, A.E.; Doudican, N.; Osman, I.; Orlow, S.J. Celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells. Mol. Cancer Res. 2009, 7, 1946–1953.
- Yang, H.; Landis-Piwowar, K.R.; Lu, D.; Yuan, P.; Li, L.; Reddy, G.P.V.; Yuan, X.; Dou, Q.P. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J. Cell. Biochem. 2008, 103, 234–244.
- Lu, L.; Kanwar, J.; Schmitt, S.; Cui, Q.C.; Zhang, C.; Zhao, C.; Dou, Q.P. Inhibition of tumor cellular proteasome activity by triptolide extracted from the Chinese medicinal plant ‘thunder god vine’. Anticancer Res. 2011, 31, 1–10.
- Yang, H.; Zhou, P.; Huang, H.; Chen, D.; Ma, N.; Cui, Q.C.; Shen, S.; Dong, W.; Zhang, X.; Lian, W. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int. J. Cancer 2009, 124, 2450–2459.
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393.
- Yang, H.; Shi, G.; Dou, Q.P. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol. Pharmacol. 2007, 71, 426–437.
- Kashyap, D.; Mondal, R.; Tuli, H.S.; Kumar, G.; Sharma, A.K. Molecular targets of gambogic acid in cancer: Recent trends and advancements. Tumor Biol. 2016, 37, 12915–12925.
- Zhou, Z.; Wan, J. Phase I human tolerability trial of gambogic acid. Chin. J. New Drugs 2007, 16, 679–682.
- Li, X.; Liu, S.; Huang, H.; Liu, N.; Zhao, C.; Liao, S.; Yang, C.; Liu, Y.; Zhao, C.; Li, S. Gambogic acid is a tissue-specific proteasome inhibitor in vitro and in vivo. Cell Rep. 2013, 3, 211–222.
- Shi, X.; Chen, X.; Li, X.; Lan, X.; Zhao, C.; Liu, S.; Huang, H.; Liu, N.; Liao, S.; Song, W. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin. Cancer Res. 2014, 20, 151–163.
- Andreani, C.; Bartolacci, C.; Wijnant, K.; Crinelli, R.; Bianchi, M.; Magnani, M.; Hysi, A.; Iezzi, M.; Amici, A.; Marchini, C. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging 2017, 9, 508.
- Mir, R.H.; Banday, N.; Sabreen, S.; Shah, A.J.; Jan, R.; Wani, T.U.; Farooq, S.; Bhat, Z.A. Resveratrol: A potential drug candidate with multispectrum therapeutic application. Stud. Nat. Prod. Chem. 2022, 73, 99–137.
- Kwon, K.J.; Kim, J.N.; Kim, M.K.; Lee, J.; Ignarro, L.J.; Kim, H.J.; Shin, C.Y.; Han, S.H. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: A possible role in neuroprotection. J. Pineal Res. 2011, 50, 110–123.
- Golonko, A.; Pienkowski, T.; Swislocka, R.; Lazny, R.; Roszko, M.; Lewandowski, W. Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system. Eur. J. Med. Chem. 2019, 167, 291–311.
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2008, 214, 149–160.
- Silswal, N.; Reddy, N.; Qureshi, N. Resveratrol Modulates Cytokine Expression in LPS-induced Human Monocytes: Role of Proteasome Subunits. FASEB J. 2016, 30, 597.6.
- Sato, A.; Okada, M.; Shibuya, K.; Watanabe, E.; Seino, S.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; Kitanaka, C. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res. 2013, 11, 601–610.
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828.
- Dosenko, V.; Nagibin, V.; Tumanovskaya, L.; Zagorii, V.Y.; Moibenko, A. Effect of quercetin on the activity of purified 20S and 26S proteasomes and proteasomal activity in isolated cardiomyocytes. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2007, 1, 40–44.
- Klappan, A.K.; Hones, S.; Mylonas, I.; Brüning, A. Proteasome inhibition by quercetin triggers macroautophagy and blocks mTOR activity. Histochem. Cell Biol. 2012, 137, 25–36.
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Dou, Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432.
- Chen, D.; Chen, M.S.; Cui, Q.C.; Yang, H.; Dou, Q.P. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front Biosci. 2007, 12, 1935–1945.
- Zhu, J.; Zhang, C.; Qing, Y.; Cheng, Y.; Jiang, X.; Li, M.; Yang, Z.; Wang, D. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radic. Biol. Med. 2015, 86, 209–218.
- Zhang, Z.; Wang, C.-Z.; Du, G.-J.; Qi, L.-W.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296.
- Wu, T.-C.; Lin, Y.-C.; Chen, H.-L.; Huang, P.-R.; Liu, S.-Y.; Yeh, S.-L. The enhancing effect of genistein on apoptosis induced by trichostatin A in lung cancer cells with wild type p53 genes is associated with upregulation of histone acetyltransferase. Toxicol. Appl. Pharmacol. 2016, 292, 94–102.
- Kazi, A.; Daniel, K.G.; Smith, D.M.; Kumar, N.B.; Dou, Q.P. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem. Pharmacol. 2003, 66, 965–976.
- Zhou, N.; Yan, Y.; Li, W.; Wang, Y.; Zheng, L.; Han, S.; Yan, Y.; Li, Y. Genistein inhibition of topoisomerase IIα expression participated by Sp1 and Sp3 in HeLa cell. Int. J. Mol. Sci. 2009, 10, 3255–3268.
- Azarova, A.M.; Lin, R.-K.; Tsai, Y.-C.; Liu, L.F.; Lin, C.-P.; Lyu, Y.L. Genistein induces topoisomerase IIbeta-and proteasome-mediated DNA sequence rearrangements: Implications in infant leukemia. Biochem. Biophys. Res. Commun. 2010, 399, 66–71.
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350.
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553.
- Shields, M. Pharmacognosy: Fundamentals, Applications and Strategies; Elsevier: Atlanta, GA, USA, 2017.
- Kim, S.-H.; Choi, K.-C. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013, 29, 229–234.
- Han, B.; Yu, Y.-Q.; Yang, Q.-L.; Shen, C.-Y.; Wang, X.-J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget 2017, 8, 86227.
- Xu, H.; Zhou, Y.; Coughlan, K.A.; Ding, Y.; Wang, S.; Wu, Y.; Song, P.; Zou, M.-H. AMPKα1 deficiency promotes cellular proliferation and DNA damage via p21 reduction in mouse embryonic fibroblasts. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 65–73.
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; Von Deimling, A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. 2008, 7, 3566–3574.
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol modulates DNA methylation and downregulates DNMT3B in bladder cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335.
- Seely, D.; Mills, E.J.; Wu, P.; Verma, S.; Guyatt, G. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: A systematic review and meta-analysis. Integr. Cancer Ther. 2005, 4, 144–155.
- Arab, L.; Il’yasova, D.J. The epidemiology of tea consumption and colorectal cancer incidence. J. Nutr. 2003, 133, 3310S–3318S.
- Imai, K.; Suga, K.; Nakachi, K.J. Cancer-preventive effects of drinking green tea among a Japanese population. Prev. Med. 1997, 26, 769–775.
- Mir, R.H.; Masoodi, M.H. Anti-inflammatory plant polyphenolics and cellular action mechanisms. Curr. Bioact. Compd. 2020, 16, 809–817.
- Dou, Q.; Landis-Piwowar, K.; Chen, D.; Huo, C.; Wan, S.; Chan, T.J. Green tea polyphenols as a natural tumour cell proteasome inhibitor. Inflammopharmacology 2008, 16, 208–212.
- Nam, S.; Smith, D.M.; Dou, Q.P. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 2001, 276, 13322–13330.
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241.
- Smith, D.M.; Wang, Z.; Kazi, A.; Li, L.-H.; Chan, T.-H.; Dou, Q.P. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol. Med. 2002, 8, 382–392.
- Kazi, A.; Wang, Z.; Kumar, N.; Falsetti, S.C.; Chan, T.-H.; Dou, Q.P. Structure-activity relationships of synthetic analogs of (-)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004, 24, 943–954.
- Chen, Z.P.; Schell, J.B.; Ho, C.-T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179.
- Kuhn, D.; Lam, W.H.; Kazi, A.; Daniel, K.G.; Song, S.; Chow, L.; Chan, T.H.; Dou, Q.P. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front. Biosci. Landmark 2005, 10, 1010–1023.
- Quan, Y.; Li, L.; Dong, L.; Wang, S.; Jiang, X.; Zhang, T.; Jin, P.; Fan, J.; Mao, S.; Fan, X.; et al. Epigallocatechin-3-gallate (EGCG) inhibits aggregation of pulmonary fibrosis associated mutant surfactant protein A2 via a proteasomal degradation pathway. Int. J. Biochem. Cell Biol. 2019, 116, 105612.
- Jordan, J.D.; He, J.C.; Eungdamrong, N.J.; Gomes, I.; Ali, W.; Nguyen, T.; Bivona, T.G.; Philips, M.R.; Devi, L.A.; Iyengar, R.J. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through Gαo/i-triggered proteasomal degradation of Rap1GAPII. J. Biol. Chem. 2005, 280, 11413–11421.
- Nam, B.; Rho, J.K.; Shin, D.-M.; Son, J.J. Gallic acid induces apoptosis in EGFR-mutant non-small cell lung cancers by accelerating EGFR turnover. Bioorg. Med. Chem. Lett. 2016, 26, 4571–4575.
- Zhang, L.; Wei, Y.; Zhang, J.J. Novel mechanisms of anticancer activities of green tea component epigallocatechin-3-gallate. Anti-Cancer Agents Med. Chem. 2014, 14, 779–786.
- Xiang, L.-P.; Wang, A.; Ye, J.-H.; Zheng, X.-Q.; Polito, C.A.; Lu, J.-L.; Li, Q.-S.; Liang, Y.-R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458.
- Ju, J.; Lu, G.; Lambert, J.D.; Yang, C.S. Inhibition of carcinogenesis by tea constituents. Semin. Cancer Biol. 2007, 17, 395–402.




