Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristiano De Azevedo | -- | 2286 | 2023-04-17 03:32:52 | | | |
| 2 | Sirius Huang | Meta information modification | 2286 | 2023-04-17 03:40:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Azevedo, C.S.; Cipreste, C.F.; Pizzutto, C.S.; Young, R.J. Effects of Enclosure Complexity on Zoo Animals. Encyclopedia. Available online: https://encyclopedia.pub/entry/43096 (accessed on 08 February 2026).
De Azevedo CS, Cipreste CF, Pizzutto CS, Young RJ. Effects of Enclosure Complexity on Zoo Animals. Encyclopedia. Available at: https://encyclopedia.pub/entry/43096. Accessed February 08, 2026.
De Azevedo, Cristiano Schetini, Cynthia Fernandes Cipreste, Cristiane Schilbach Pizzutto, Robert John Young. "Effects of Enclosure Complexity on Zoo Animals" Encyclopedia, https://encyclopedia.pub/entry/43096 (accessed February 08, 2026).
De Azevedo, C.S., Cipreste, C.F., Pizzutto, C.S., & Young, R.J. (2023, April 17). Effects of Enclosure Complexity on Zoo Animals. In Encyclopedia. https://encyclopedia.pub/entry/43096
De Azevedo, Cristiano Schetini, et al. "Effects of Enclosure Complexity on Zoo Animals." Encyclopedia. Web. 17 April, 2023.
Copy Citation
Habitat complexity is important for the maintenance of high levels of welfare for captive animals, especially at zoos. Generally, individuals who experience greater enclosure complexity express higher diversity of behaviours and show better physiological well-being. However, positive outcomes of providing habitat complexity should be species-specific, and not all species would benefit from it. Thus, it is important to provide and constantly evaluate the habitat complexity of zoo animals.
captivity
enclosure
habitat complexity
welfare
1. How Does Complexity Affect Species?
The complexity of an enclosure may vary according to the species that inhabit it. The same enclosure can have low complexity for a primate species and, at the same time, offer high complexity for a lizard species, for example (Figure 1). The environmental complexity, then, in addition to the variety in the abiotic and biotic components mentioned before, will depend on the animal species and its capacity to perceive it.
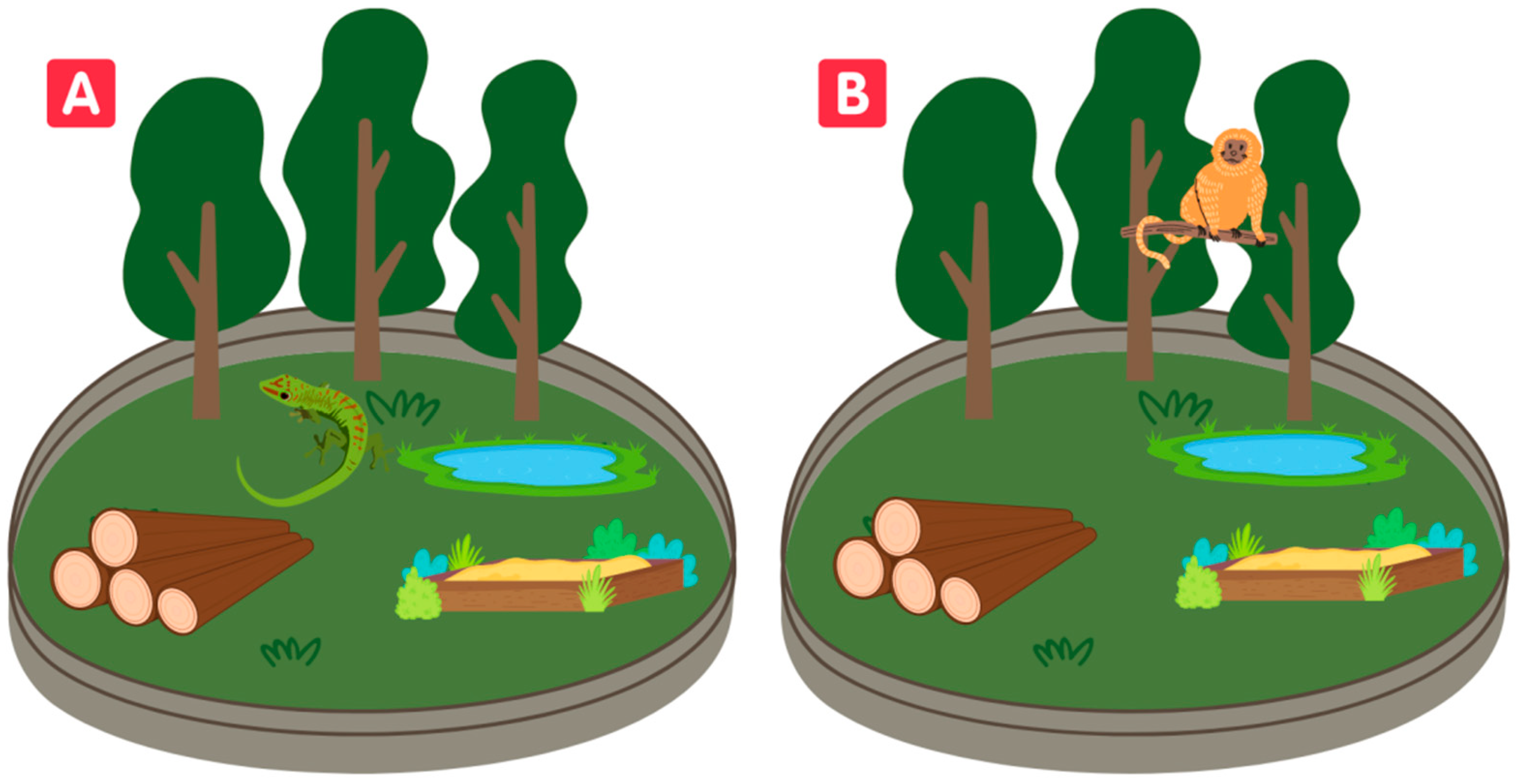
Figure 1. Habitat complexity can be perceived differently depending on the species living in the enclosure. Habitat complexity can be high for a lizard (A) and low for a monkey (B), even when evaluating the same enclosure.
Animal species have a set of sensory receptors that allow them to capture, process, and respond to different environmental stimuli [1]. These sensory receptors can be simple or complex, shared or not by different animal groups [2]. Animal species can vary in their sensory capabilities, with some using more visual cues (birds and primates [3][4]), others more auditory cues (amphibians, birds, and bats [5][6][7]), and others more tactile cues (fish and cave living animals [8][9]) from their environment, for example. The combination of using different senses is what allows animals to perceive the complexity of their environment [10]. After the stimuli perceived by sensory organs are processed in the brain, the animals respond appropriately to them [11]. However, brain morphology also varies between species, with the brain being modified in areas intended for processing stimuli captured by sensory organs [12][13]. For example, birds have olfactory bulbs (i.e., site for processing olfactory stimuli) varying from little to highly developed, with more basal birds (Ratites, Anseriformes, Columbiformes, etc.) having less developed bulbs than more derived birds (parrots and songbirds [14]). Reptiles have a well-developed olfactory bulb [15]. Reptiles probably would be benefited more by olfactory complexity in their enclosures than birds, depending on the species. Therefore, the brain capacity to process environmental stimuli is another important characteristic that must be considered when evaluating how animals respond to environmental complexity [16].
Another important consideration when assessing environmental complexity is the animal’s ability to perceive time scales. Small animals with a high metabolic rate perceive more information from the environment per unit of time compared to large animals with a low metabolic rate [17]. For example, flies can perceive more information per unit of time than turtles, perceiving the time scale more slowly [17]. Do animals that perceive time scales more slowly need more complexity in their environment? The temporal scale is involved in the habitat selection of species, since the disproportionate use of certain areas of the environment is linked to the presence of important resources for the animal, and these resources vary in space and time [18]. Resources are environmental conditions that influence biological fitness, such as resting, foraging, breeding, shelter from predators, etc. [19][20]. Therefore, both animals that perceive time more slowly and those that perceive it more quickly will select the habitat according to their needs at that moment, and the variation in possibilities of choice of resources helps the animals to supply their demands, consummating the actions for the which they were motivated, maintaining their homeostasis, and improving their welfare [20]. Thus, environmental complexity must be offered to all animals, regardless of their ability to perceive time scales. Changes in habitat complexity, however, are expected to be perceived differently, with slow time perceivers experiencing variation in habitat complexity less rapidly than fast time perceivers. Logistically, for human caregivers, it is easier to provide complexity to animals that are slow time perceivers due to the fact that humans are slow time perceivers. A recommendation for caregivers would be to evaluate the annual rhythm of each species to try to provide different but important stimuli in each life phase (such as migration, reproduction, hibernation, etc.) [21][22].
Environmental complexity offered to the animals is influenced by human perception of complexity, and the human sensory ability is often limited compared to that of other animals. As examples, humans cannot perceive ultraviolet light [23] and have lower olfactory and auditory capabilities compared to many animal species, depending on their sensitivity to the odorants [7][24][25]. In this way, the environmental complexity offered to animals kept in human care is often based only on human perception and not on the animals’ sensory capacity [26][27][28]. For example, many bird species can detect ultraviolet light and the ability can influence foraging, reproduction, and welfare of the birds [29]. Pekin ducks (Anas platyrhynchos) reared under UV lights showed decreased physiological responses of stress [30]. In another study, some individual starlings (Sturnus vulgaris) showed preferences for UV light exposition, but with no behavioural changes [31]. Environmental characteristics other than light provided by caregivers can also influence the welfare and behaviour of animals. For example, flamingos are birds that build tall, cone-shaped mud nests [32]. If in a flamingo enclosure a muddy area is not present (no flooring complexity), reproduction will be compromised [33]. This muddy area may be perceived by humans as a dirty area of the enclosure and not be offered to the animals. Consequently, human perception of the complexity of the environment that is different from the animal’s perception can harm the maintenance of the species in human care and decrease its welfare [33]. Therefore, offering adequate environmental complexity to animals stimulates natural behaviours and an adequate physiological functioning of their organisms [34]. Even within species there can be differences in perceptive abilities; for example, some human females are tetrachromats and can perceive millions of more colours than trichromats [35][36].
2. Exhibit Renovations, Environmental Enrichment, and Concepts Aiming at Habitat Complexity
The enclosure’s design should stimulate species-specific behaviours. By renovating the animals’ enclosures, managers and caretakers can promote habitat complexity that will offer to the animals different stimuli and the opportunity of choice and control of the environment, which ultimately will enhance their welfare [37][38]. The enclosures must provide necessary features and structures so that animals will display a range of welfare-related behaviours and should also provide positive husbandry practices [39].
However, institutions that do not have funds for a complete or radical renovation of their exhibits must use environmental enrichment. Environmental enrichment is a technique that offers environmental stimulation to animals from the insertion of physical, sensory, food, and cognitive structures in the enclosures, or the insertion of other individuals of the same species or of different species in the enclosures (social stimulation) [40][41]. Artificial enrichment, for example, may provide the necessary environment and it can be considerably more durable, sturdy, and easier to clean, requiring far less maintenance and lower monetary costs [39]. Of course, the use of environmental enrichment is important in its own right because it increases variation inside the exhibits, and should be offered not only in poorly designed exhibits but also in renovated exhibits.
The use of environmental enrichment has already been shown to be efficient in increasing the environmental complexity of enclosures, positively influencing animal welfare. The modification of the enclosure design associated with physical structures that stimulated crawling behaviour instead of jetting swim behaviour decreased the mantle injuries of Eledone cirrhosa octopuses, improving their behaviours and physiology [42][43]. Captive Malayan sun bears (Helarctos malayanus) housed in enriched enclosures presented better welfare than those housed in barren enclosures, based on behavioural measures [44]. Offering enclosures large enough to allow rectilinear behaviours for snakes can increase their welfare [45]. Chimpanzees used more areas of the enclosure, showing behaviours more similar to those observed in free-living groups after moving to a naturalistic and enriched enclosure [46]. The same was observed when chimpanzees gained more space per individual in enclosures with similar complexity, showing that providing adequate space is also important for animals [47]. A recent literature review showed how environmental enrichment can positively affect the welfare of birds, modifying behaviours and physiological parameters [48]. Thinking about the Five Domains of Welfare [49][50], the use of environmental enrichment provides experiences not only in the domain of behaviour and interactions, but also in the nutritional, environmental, and health functional domains, positively affecting the mental domain and the overall welfare of the animals.
One idea to increase enclosure complexity is the so-called Zoo360 Concept [51]. In this concept, different enclosures are linked through passages (tunnels or elevated ways), allowing the animals to visit these different enclosures, experiencing varying degrees of complexity [52]. For example, if a tiger is kept in an enclosure designed with passages, it can choose to stay in its original enclosure or walk through the passages until it reaches another enclosure that could be built differently, presenting to the tiger different physical structures and micro-habitats. Along the way, the tiger can also experience different views (in terms of landscape), with more or less visitors, and experience different sounds and smells.
The use of dynamic architecture is another way to increase the complexity of an animal enclosure. The dynamic structure is currently applied to humans and consists of the construction of buildings that can change format in time due to the use of dynamic elements [53]. Stimuli from external sources, such as winds, sun rays, and rain, are able to elicit automated responses from the building, allowing the systems to achieve a high performance, increasing its efficiency, sustainability, and deliverability, and finally, comfort for the users [54]. Animal enclosures built considering dynamic architecture could offer to the animals inside considerable change in complexity, but this technique has not yet been applied [55].
A simpler version of the Zoo 360 concept is to provide rotational zoo exhibits (Jon Coe) or even exhibits whose barriers can be moved. Farmers use moveable barriers to ensure their livestock do not overgraze a particular area of land; this same concept could be used to vary the size and type of habitat that animals in captivity have access to.
Complex habitats also avoid the negative impacts of the visitors (the so-called “visitor effect” [56][57]), as they allow the animals on display to hide from people. Captive Edwards’ pheasants (Lophura edwardsi), for example, decreased feeding and locomotion behaviours because visitors were acting as threats to the birds [58]. In a review study, the authors demonstrated that the existence of hiding areas in the enclosures can reduce the negative effects of the presence of the public for various animal species [59]. The same was observed for five captive felids, where the species housed in enclosures with hiding places preferred to stay hidden when visitors were present, while those housed in enclosures without hiding places exhibited more abnormal behaviours in the presence of visitors [60]. Diurnal mammals and mammals in closed habitats suffered more from the visitor effect than nocturnal and mammals in open habitats [61].
Complex habitats, however, can also cause problems for institutions that maintain captive animals. Firstly, too much stimulation can stress animals in the same way as too little stimulation [62][63]. Thus, stimulation offers should not pass the healthy stress threshold and caretakers need to be aware of this. Secondly, very complex enclosures with many hiding areas can prevent the visiting public from viewing the animals, frustrating them and generating many complaints [64][65], but this can be addressed by using live-streaming cameras that the public can view images from on their smartphone (e.g., San Diego Zoo (USA); Melbourne Zoo (Australia); Houston Zoo (USA) [66][67][68]. This may be because the public fails to realise how important complexity is for the welfare of the animals. Therefore, environmental education activities with the visitors are important to minimise people’s complaints [69][70]. The enclosure can be designed to meet both animal and visitor needs by offering a variety of hiding options such as vegetation, open dens, and shaded elevated platforms while maintaining the animals in view [71].
Furthermore, it is important to evaluate complexity in the different areas of an enclosure. For example, foraging areas might need high levels of changing complexity to improve welfare, whereas sleeping areas might need low and unvarying levels of complexity, depending on the species. Bottlenose dolphins (Tursiops truncatus), for example, had their welfare increased when complex cognitive/foraging enrichment devices were offered, compared to non-cognitive foraging enrichment [72]. Thus, it is important to evaluate which areas of the enclosure will require more complexity and which will not. The best enclosure design will depend on the species and has been debated inside and outside the scientific academy [73].
Finally, the development of exhibits that allow animals to control or select the level of complexity that they wish could help in the enhancement of welfare. For example, climbing structures can have branches that can be unfolded to create complexity or folded to reduce complexity. This might be important for animal rehabilitation, animals coming from barren enclosures, older individuals, or individuals with offspring [28][74][75].
In conclusion, exhibit planning in any animal facility should consider four important aspects. (1) Size: the enclosure needs to offer a space large enough to provide appropriate complexity and stimulation for the species that allow opportunities to experience positive physical and mental states, with elevated welfare and fitness; (2) design: naturalistic elements (i.e., ideally recreate ecosystems) and environmental enrichment can increase the complexity of the habitat; (3) education: the enclosure’s complexity and environmental enrichment activities must be aligned with education purposes so that visitors have a pleasant experience and feel connected to nature and animals; and (4) architecture: dynamic architecture associated to structures inside the exhibits that can be manipulated by the animals to increase or decrease complexity could help in the welfare of animals and visitors. By implementing these aspects in exhibit planning, zoos can provide a better quality of life for their species, full of positive experiences for their animals, visitors, and staff.
References
- von der Emde, G.W.E. The Ecology of Animal Senses: Matched Filters for Economical Sensing, 1st ed.; Springer: New York, NY, USA, 2016.
- Schmidt-Rhaesa, A. The Evolution of Organ Systems, 1st ed.; Oxford University Press: Oxford, UK, 2007.
- Veilleux, C.C.; Kirk, E.C. Visual Acuity in Mammals: Effects of Eye Size and Ecology. Brain Behav. Evol. 2014, 83, 43–53.
- Boström, J.E.; Dimitrova, M.; Canton, C.; Håstad, O.; Qvarnström, A.; Ödeen, A. Ultra-Rapid Vision in Birds. PLoS ONE 2016, 11, e0151099.
- Debjani, R. Development of Hearing in Vertebrates with Special Reference to Anuran Acoustic Communication. J. Biosci. 1994, 19, 629–644.
- Dooling, R.J.; Prior, N.H. Do We Hear What Birds Hear in Birdsong? Anim. Behav. 2017, 124, 283–289.
- Heffner, H.E.; Heffner, R.S. The Evolution of Mammalian Hearing. AIP Conf. Proc. 2018, 1965, 130001.
- Willemart, R.H.; Gnaspini, P. Comparative Density of Hair Sensilla on the Legs of Cavernicolous and Epigean Harvestmen (Arachnida: Opiliones). Zool. Anz. 2004, 242, 353–365.
- Kasumyan, A.O. Tactile Reception and Behavior of Fish. J. Ichthyol. 2011, 51, 1035–1103.
- Kelley, J.L.; Chapuis, L.; Davies, W.I.L.; Collin, S.P. Sensory System Responses to Human-Induced Environmental Change. Front. Ecol. Evol. 2018, 6, 95.
- Hale, R.; Piggott, J.J.; Swearer, S.E. Describing and Understanding Behavioral Responses to Multiple Stressors and Multiple Stimuli. Ecol. Evol. 2017, 7, 38–47.
- Stephan, H.; Andy, O.J. Quantitative Comparisons of Brain Structures from Insectivores to Primates. Am. Zool. 1964, 4, 59–74.
- Northcutt, R.G. Understanding Vertebrate Brain Evolution. Integr. Comp. Biol. 2002, 42, 743–756.
- Corfield, J.R.; Price, K.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Birkhead, T.; Wylie, D.R. Diversity in Olfactory Bulb Size in Birds Reflects Allometry, Ecology, and Phylogeny. Front. Neuroanat. 2015, 9, 102.
- Ubeda-Bañon, I.; Pro-Sistiaga, P.; Mohedano-Moriano, A.; Saiz-Sanchez, D.; de la Rosa-Prieto, C.; Gutierrez-Castellanos, N.; Lanuza, E.; Martinez-Garcia, F.; Martinez-Marcos, A. Cladistic Analysis of Olfactory and Vomeronasal Systems. Front. Neuroanat. 2011, 5, 3.
- Shumway, C.A. Habitat Complexity, Brain, and Behavior. Brain Behav. Evol. 2008, 72, 123–134.
- Healy, K.; McNally, L.; Ruxton, G.D.; Cooper, N.; Jackson, A.L. Metabolic Rate and Body Size Are Linked with Perception of Temporal Information. Anim. Behav. 2013, 86, 685–696.
- Mayor, S.J.; Schneider, D.C.; Schaefer, J.A.; Mahoney, S.P. Habitat Selection at Multiple Scales. Ecoscience 2009, 16, 238–247.
- Buskirk, S.W.; Millspaugh, J.J. Metrics for Studies of Resource Selection. J. Wildl. Manag. 2006, 70, 358–366.
- Coria-Avila, G.A.; Pfaus, J.G.; Orihuela, A.; Domínguez-Oliva, A.; José-Pérez, N.; Hernández, L.A.; Mota-Rojas, D. The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review. Animals 2022, 12, 928.
- Singh, S.V.; Kumar, S. Circadian Rhythm and Their Significance in Relation to Physiological Functions of Animals: A Review. J. Entomol. Zool. Stud. 2018, 6, 1861–1866.
- Helm, B.; Ben-Shlomo, R.; Sheriff, M.J.; Hut, R.A.; Foster, R.; Barnes, B.M.; Dominoni, D. Annual Rhythms That Underlie Phenology: Biological Time-Keeping Meets Environmental Change. Proc. R. Soc. B Biol. Sci. 2013, 280.
- Jacobs, G.H. Ultraviolet Vision in Vertebrates. Am. Zool. 1992, 32, 544–554.
- McGann, J.P. Poor Human Olfaction Is a 19th-Century Myth. Science 2017, 356, eaam7263.
- Heffner, H. Hearing Ranges of Laboratory Animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–22.
- Poole, T.B. Meeting a Mammal’s Psychological Needs: Basic Principles. In Second Nature: Environmental Enrichment for Captive Animals; Shepherdson, D.J., Mellen, J.D., Hutchins, M., Eds.; Smithsonian Books: Washington, DC, USA, 1998; pp. 83–94.
- Hancocks, D. The History and Principles of Zoo Exhibition. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management; Kleiman, D.G., Thompson, K.V., Baer, C.K., Eds.; The University of Chicago Press: Chicago, IL, USA, 2010; pp. 121–136.
- Hosey, G.; Melfi, V.; Pankhurst, S. Zoo Animals: Behaviour, Management, and Welfare, 2nd ed.; Hosey, G., Melfi, V., Pankhurst, S., Eds.; Oxford University Press: Oxford, UK, 2013.
- Rajchard, J. Ultraviolet (UV) Light Perception by Birds: A Review. Vet. Med. 2009, 54, 351–359.
- House, G.M.; Sobotik, E.B.; Nelson, J.R.; Archer, G.S. Effects of Ultraviolet Light Supplementation on Pekin Duck Production, Behavior, and Welfare. Animals 2020, 10, 833.
- Ross, M.R.; Gillespie, K.L.; Hopper, L.M.; Bloomsmith, M.A.; Maple, T.L. Differential Preference for Ultraviolet Light among Captive Birds from Three Ecological Habitats. Appl. Anim. Behav. Sci. 2013, 147, 278–285.
- Stevens, E.F. Flamingo Breeding: The Role of Group Display. Zoo Biol. 1991, 10, 53–63.
- Rose, P.E.; Brereton, J.E.; Gardner, L. Developing Flamingo Husbandry Practices through Workshop Communication. J. Zoo Aquar. Res. 2016, 4, 115–121.
- Lawrence, K.; Sherwen, S.L.; Larsen, H. Natural Habitat Design for Zoo-housed Elasmobranch and Teleost Fish Species Improves Behavioural Repertoire and Space Use in a Visitor Facing Exhibit. Animals 2021, 11, 2979.
- Jameson, K.A.; Winkler, A.D.; Goldfarb, K. Art, Interpersonal Comparisons of Color Experience, and Potential Tetrachromacy. Hum. Vis. Electron. Imaging 2016, 145, 354–365.
- BBC. The Women with Superhuman Vision. 2023. Available online: https://www.bbc.com/future/article/20140905-the-women-with-super-human-vision (accessed on 12 February 2023).
- Fàbregas, M.C.; Guillén-Salazar, F.; Garcés-Narro, C. Do Naturalistic Enclosures Provide Suitable Environments for Zoo Animals? Zoo Biol. 2012, 31, 362–373.
- Finch, K.; Waterman, J.O.; Cowl, V.B.; Marshall, A.; Underwood, L.; Williams, L.J.; Davis, N.; Holmes, L. Island Life: Use of Activity Budgets and Visibility to Evaluate a Multi-Species Within-Zoo Exhibit Move. Animals 2022, 12, 2123.
- Learmonth, M.J. Dilemmas for Natural Living Concepts of Zoo Animal Welfare. Animals 2019, 9, 318.
- Young, R.J. Environmental Enrichment for Captive Animals, 1st ed.; Blackwell Science Ltda.: Oxford, UK, 2003.
- Azevedo, C.S.; Cipreste, C.F.; Pizzutto, C.S. Fundamentos Do Enriquecimento Ambiental, 1st ed.; Payá: São Paulo, Brazil, 2022.
- Smith, L.E.; Rowe, C.; Mackay, F.; Matthews, C.; Matthews, C.G.G. Aquarium Tank Design Is Integral to the Elimination of Mantle Abrasion in the Captive Curled Octopus (Eledone Cirrhosa): A Case Study at Macduff Marine Aquarium. J. Appl. Anim. Welf. Sci. 2022, 25, 355–361.
- Tan, S.M.L.; Jajou, S.; Stellato, A.C.; Niel, L. Perspectives of Canadian and American Cat Owners on Provision of Uncontrolled Outdoor Access for Owned Domestic Cats. Front. Vet. Sci. 2021, 8.
- Tan, H.M.; Ong, S.M.; Langat, G.; Bahaman, A.R.; Sharma, R.S.K.; Sumita, S. The Influence of Enclosure Design on Diurnal Activity and Stereotypic Behaviour in Captive Malayan Sun Bears (Helarctos Malayanus). Res. Vet. Sci. 2013, 94, 228–239.
- Warwick, C.; Grant, R.; Steedman, C.; Howell, T.J.; Arena, P.C.; Lambiris, A.J.L.; Nash, A.E.; Jessop, M.; Pilny, A.; Amarello, M.; et al. Getting It Straight: Accommodating Rectilinear Behavior in Captive Snakes—A Review of Recommendations and Their Evidence Base. Animals 2021, 11, 1459.
- Jensvold, M.L.A.; Sanz, C.M.; Fouts, R.S.; Fouts, D.H. Effect of Enclosure Size and Complexity on the Behaviors of Captive Chimpanzees (Pan Troglodytes). J. Appl. Anim. Welf. Sci. 2001, 4, 53–69.
- Webb, S.J.N.; Hau, J.; Schapiro, S.J. Captive Chimpanzee (Pan Troglodytes) Behavior as a Function of Space per Animal and Enclosure Type. Am. J. Primatol. 2018, 80, e22749.
- Woods, J.M.; Eyer, A.; Miller, L.J. Bird Welfare in Zoos and Aquariums: General Insights across Industries. J. Zool. Bot. Gard. 2022, 3, 198–222.
- Mellor, D.J.; Reid, C.S.W. Concepts of Animal Well-Being and Predicting the Impact of procedures on Experimental Animals. In Improving the Well-Being of Animals in the Research Environment; WellBeing International: Potomac, MD, USA, 1994.
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870.
- Philadelphia Zoo Philadelphia Magazine. 2016. Available online: https://www.phillymag.com/sponsor-content/philadelphia-zoo-animal-trails/ (accessed on 12 February 2023).
- Bisgrove, D. Zooscape Ecology: A Conceptual Analysis of Zoos and Landscape Ecology. Landsc. Ecol. 2022, 37, 1733–1745.
- Belyaeva, T.V. Dynamic Architecture. Interaction with City, Nature, Man. IOP Conf. Ser. Mater. Sci. Eng. 2019, 687, 055015.
- Karanouh, A.; Kerber, E. Innovations in Dynamic Architecture. J. Facade Des. Eng. 2015, 3, 185–221.
- Maulana, R. Architecture for Animals: The Expanding Challenges of Sustainable Development. IOP Conf Ser Earth Environ Sci 2018, 195, 012079.
- Sade, C. Visitor Effects on Zoo Animals. Plymouth Stud. Sci. 2013, 6, 423–433.
- Fernandez, E.J.; Chiew, S.J. Animal-Visitor Interactions: Effects, Experiences, and Welfare. Anim. Behav. Cogn. 2021, 8, 462–467.
- Hoy, R.A.; Brereton, J.E. Does Observer Presence Modify the Behavior and Enclosure Use of Captive Edwards’ Pheasants? J. Zool. Bot. Gard. 2022, 3, 147–157.
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366.
- Suárez, P.; Recuerda, P.; Arias-De-Reyna, L. Behaviour and Welfare: The Visitor Effect in Captive Felids. Anim. Welf. 2017, 26, 25–34.
- Queiroz, M.B.; Young, R.J. The Different Physical and Behavioural Characteristics of Zoo Mammals That Influence Their Response to Visitors. Animals 2018, 8, 139.
- Christakis, D.A.; Ramirez, J.S.B.; Ramirez, J.M. Overstimulation of Newborn Mice Leads to Behavioral Differences and Deficits in Cognitive Performance. Sci. Rep. 2012, 2, 546.
- Townsend, L.; Gee, N.R. Recognizing and Mitigating Canine Stress during Animal Assisted Interventions. Vet. Sci. 2021, 8, 254.
- Moura, D.J.; Naas, I.A.; Pereira, D.F.; Silva, R.B.T.R.; Camargo, G.A. Animal Welfare Concepts and Strategy for Poultry Production: A Review. Braz. J. Poltry Sci. 2006, 8, 137–148.
- Eizaguirre, C.; Baltazar-Soares, M. Evolutionary Conservation-Evaluating the Adaptive Potential of Species. Evol. Appl. 2014, 7, 963–967.
- San Diego Zoo Live Cameras. 2023. Available online: https://zoo.sandiegozoo.org/live-cameras (accessed on 3 March 2023).
- Camstreamer The Melbourne Zoo Is Using QR Codes in Their Live Stream, Forward People from Videos of Sleeping Animals to Other Content. Available online: https://camstreamer.com/resources/melbourne-zoo-pdf (accessed on 3 March 2023).
- Houston Zoo Webcams Houston Zoo. 2023. Available online: https://www.houstonzoo.org/explore/webcams/ (accessed on 3 March 2023).
- Tofield, S.; Coll, R.K.; Vyle, B.; Bolstad, R. Zoos as a Source of Free Choice Learning. Research in Science & Technological Education 2003, 21, 67–99.
- Ivana, G.; Martin, H.; Jitka, Š. Environmental Enrichment in the Awareness of Zoo Visitors and the General Public. Annu Res. Rev. Biol. 2017, 20, 1–5.
- Brando, S.; Buchanan-Smith, H.M. The 24/7 Approach to Promoting Optimal Welfare for Captive Wild Animals. Behav. Process. 2018, 156, 83–95.
- Clegg, I.L.K.; Domingues, M.; Ström, E.; Berggren, L. Cognitive Foraging Enrichment (but Not Non-Cognitive Enrichment) Improved Several Longer-Term Welfare Indicators in Bottlenose Dolphins. Animals 2023, 13, 238.
- Gusset, M.; Chin, S.A. Future of Zoo and Aquarium Design; WAZA Executive Office: Gland, Switzerland, 2016.
- Miller, E.A. Minimum Standards for Wildlife Rehabilitation, 4th ed.; National Wildlife Rehabilitators Association: Minnesota, MN, USA, 2012; ISBN 9781931439282.
- Krebs, B.L.; Marrin, D.; Phelps, A.; Krol, L.; Watters, J.V. Managing Aged Animals in Zoos to Promote Positive Welfare: A Review and Future Directions. Animals 2018, 8, 116.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
2 times
(View History)
Update Date:
17 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




