Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, L.; Wu, L.; Guo, C.; Guan, Y.; Wang, H.; Lu, J. Fundamentals of the Electroreduction of CO2. Encyclopedia. Available online: https://encyclopedia.pub/entry/43090 (accessed on 01 March 2026).
Wu L, Wu L, Guo C, Guan Y, Wang H, Lu J. Fundamentals of the Electroreduction of CO2. Encyclopedia. Available at: https://encyclopedia.pub/entry/43090. Accessed March 01, 2026.
Wu, Laxia, Lin Wu, Chang Guo, Yebin Guan, Huan Wang, Jiaxing Lu. "Fundamentals of the Electroreduction of CO2" Encyclopedia, https://encyclopedia.pub/entry/43090 (accessed March 01, 2026).
Wu, L., Wu, L., Guo, C., Guan, Y., Wang, H., & Lu, J. (2023, April 16). Fundamentals of the Electroreduction of CO2. In Encyclopedia. https://encyclopedia.pub/entry/43090
Wu, Laxia, et al. "Fundamentals of the Electroreduction of CO2." Encyclopedia. Web. 16 April, 2023.
Copy Citation
Carbon dioxide (CO2) is one of the main greenhouse gases and the major factor driving global climate change. From the viewpoint of abundance, economics, non-toxicity, and renewability, CO2 is an ideal and significant C1 resource, and its capture and recycling into fuels and chemical feedstocks using renewable energy is of great significance for the sustainable development of society. Electrochemical CO2 reduction reactions (CO2RRs) are an important pathway to utilize CO2 resources. Zinc has been demonstrated as an effective catalyst for CO2RRs.
CO2
electrode
metals
electroreduction
1. Electrochemical Behavior of Electrocatalysts
1.1. Cyclic Voltammetry
Cyclic voltammetry experiments require the setting of the three most basic parameters: the potentials of the upper limit, the potentials of the lower limit, and the scan rate. Upper and lower potential limits are determined based on the electrochemical window of the solvent (e.g., water) and the stability of the electrode material. The potential scan rate is determined according to the reaction type and testing method. The scan rate can usually be above 50 mV·s−1 for the liquid phase; however, it should not exceed 20 mV·s−1 during steady-state measurements.
The electrochemical voltametric behavior of electrocatalysts is generally determined as follows: A three-electrode system with platinum mesh is used as a counter electrode. A saturated calomel electrode (SCE) is used as the reference electrode, whereas a catalyst-coated substrate electrode (such as a glassy carbon electrode) is used as the working electrode. These electrodes are used in an undivided cell at normal temperature and pressure. The voltammograms are recorded using an electrochemical workstation under sequential N2 and carbon dioxide (CO2) bubbling.
1.2. Electrochemical Activity Surface Area (ECSA) Characterization
The surface structure of the electrode significantly influences its catalytic performance. Electrodes are usually solid, though the structure of the solid surface is complex. Moreover, there are many types of surface sites (such as platforms, steps, kinks and vacancies, and different structures of the atomic arrangements). The structural information of the electrode surface can be obtained using electrochemical measurements. Atoms, molecules, and ions (such as H, O, CO) that interact strongly with the surface are selected to characterize the surface structure of the electrodes by using their adsorption–desorption characteristics and oxidative removal.
Hydrogen adsorption on the platinum surface consists of monolayer adsorption, which means that one platinum atom corresponds to one adsorbed hydrogen atom. Therefore, the ECSA of platinum can be calculated based on the amounts of charge on the adsorbed and desorbed hydrogen. For platinum alloys, the values of the ECSA calculated using this method tend to be small, because alloying elements can inhibit the adsorption and desorption of hydrogen. Moreover, CO can produce strong adsorption on a variety of metal surfaces. Therefore, CO stripping curves are often used to measure the ECSA of metals, especially platinum group metals and their alloys. For coin elements such as gold, silver, and copper, the adsorption capacities of both H and CO are not strong, and the underpotential deposition of metals such as Pb and Cu is often used to calculate the ECSA.
2. Experimental Procedures and Product Analysis
2.1. Experimental Procedures
Three types of electrocatalytic reactors are used for CO2RRs and include the H-cell, the flow cell, and the membrane electrode assembly (MEA) cell [1]. Among them, the H-cell is the most commonly used in fundamental studies mainly because of its low cost and simple operation [2]. In this section, the setup of the H-cell and the experimental procedure for electrocatalysis are briefly described. Typically, a CO2RR is carried out using potentiostatic electrolysis in a two-compartment electrochemical cell using a standard three-electrode system. The working electrode is usually a catalyst-coated carbon paper, a glassy carbon electrode, a glassy carbon plate, or a carbon fiber paper. The working and reference electrodes are placed in the cathode compartment, whereas the counter electrode is placed in the anode compartment. The two compartments are separated by an ion exchange membrane. A proton exchange membrane is taken as an example in Figure 1. Aqueous solutions of NaHCO3 or KHCO3 are often chosen as electrolytes. When saturated with CO2, an electrolyte can effectively buffer the change in pH of the bulk solution and keep it to near-neutral. The reduction in CO2 occurs at the cathode, whereas the oxidation of oxygen (coming from water) occurs at the anode. H+ ions migrate to the cathode through a proton exchange membrane under the action of an electric field, thereby providing a source of hydrogen for the reduction of the carbon dioxide. Thermodynamically, for the electroreduction of CO2 at different potentials, different multiple electron transfer reactions can occur and include the transfer of 2e−, 4e−, 6e−, 8e−, 12e−, and so on while also generating different reduction products. At present, the reported products of the electroreduction of CO2 mainly include carbon monoxide (CO) [3][4][5], methane (CH4) [6], methanol (CH3OH) [7][8], formic acid/formate (HCOOH/HCOO-) [9][10], ethylene (C2H4) [11][12], ethane (C2H6), ethanol (C2H5OH) [13][14][15], acetic acid/acetate (CH3COOH/CH3COO-) [16], and n-propanol (CH3CH2CH2OH) [17]. The electrochemical half reactions generating these products, along with the corresponding standard redox potentials, are listed in Table 1 [18].
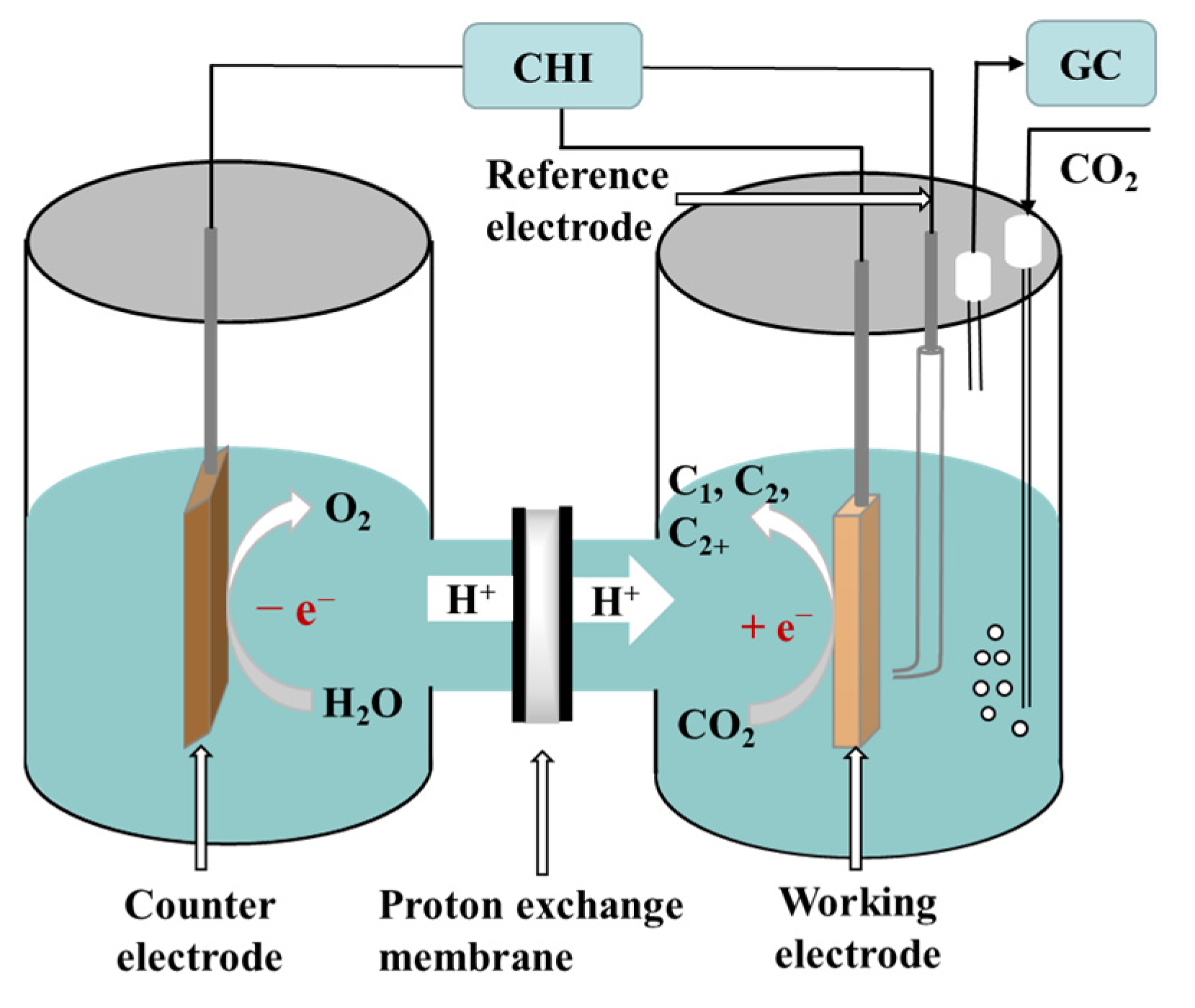
Figure 1. Schematic of electroreduction of CO2 in the H-cell.
Table 1. Electrochemical potentials of possible CO2RRs in aqueous solutions [18].
| Products | Equation | E⊖ (V vs. RHE) |
|---|---|---|
| Hydrogen | 2H+ + 2e− → H2 | 0.000 |
| Carbon monoxide | CO2 + 2H+ + 2e− → CO + H2O | −0.104 |
| Methane | CO2 + 8H+ + 8e− → CH4 + H2O | 0.169 |
| Methanol | CO2 + 6H+ + 6e− → CH3OH + H2O | 0.016 |
| Formic acid/formate | CO2 + 2H+ + 2e− → HCOOH | −0.171 |
| Ethylene | CO2 + 12H+ + 12e− → C2H4 + 4H2O | 0.085 |
| Ethane | CO2 + 14H+ + 14e− → C2H6 + 4H2O | 0.144 |
| Ethanol | 2CO2 + 12H+ + 12e− → CH3CH2OH + 3H2O | 0.084 |
| Acetic acid/acetate | 2CO2 + 8H+ + 8e− → CH3COOH + 2H2O | 0.098 |
| n-Propanol | 3CO2 + 18H+ + 18e−→ CH3CH2CH2OH + 5H2O | 0.095 |
2.2. Qualitative and Quantitative Analyses of Products
After electrolysis, the catholyte is transferred to a headspace sample injector, while the liquid products, such as methanol, ethanol, and acetone, are detected using gas chromatography. Comparing the product’s peak position with that of the standard sample allows for qualitative judgment of the liquid products. The liquid product can also be quantified using 1H NMR spectroscopy.
The gas products (such as H2, CO, CH4, and C2H4) generated during electrolysis are collected using a gas sampling bag. At the time of detection, gas is injected into the gas chromatograph with a syringe. Comparing the retention times of products obtained using gas chromatography with those of standards allows for qualitative judgment of the gas products. A standard curve is plotted according to the peak area of the produced gas chromatogram of the standard gas and the concentration of each component in the gas. The Faraday efficiency of the products can be quantitatively calculated according to Equation (1) [19].
where φ is the volume fraction of gas products in the total gas, which can be obtained from the standard curve, v is the flow rate of CO2 (L·min−1), t is the electrolysis time (min), z is the number of electrons transferred in a specific electrode reaction, as shown by the data presented in Table 1 (for example, z = 2 for a CO2RR to CO), F is the Faraday constant with the value of 96,485 C·mol−1, Q is the total amount of electricity in the electrolysis process (C), and Vm is the molar volume of gas at 25 °C and standard pressure.
2.3. Reaction Mechanism of CO2RRs
The electroreduction of carbon dioxide is a process in which reduction occurs by CO2 molecules or CO2-solvated ions acquiring electrons from the electrode’s surface within the solution. Electroreduction is a multistep process involving the transfer of multiple electrons, and it consists of CO2 adsorption, electron transfer, and product desorption at the electrode surface. A large number of studies have shown that the current density, species, and selectivity of the CO2RR are largely dependent on the electrode material and the reduction potential. The electrocatalysis of carbon dioxide undergoes different reaction pathways to generate different products. Figure 2 shows the main pathways for the electroreduction of CO2 [20].
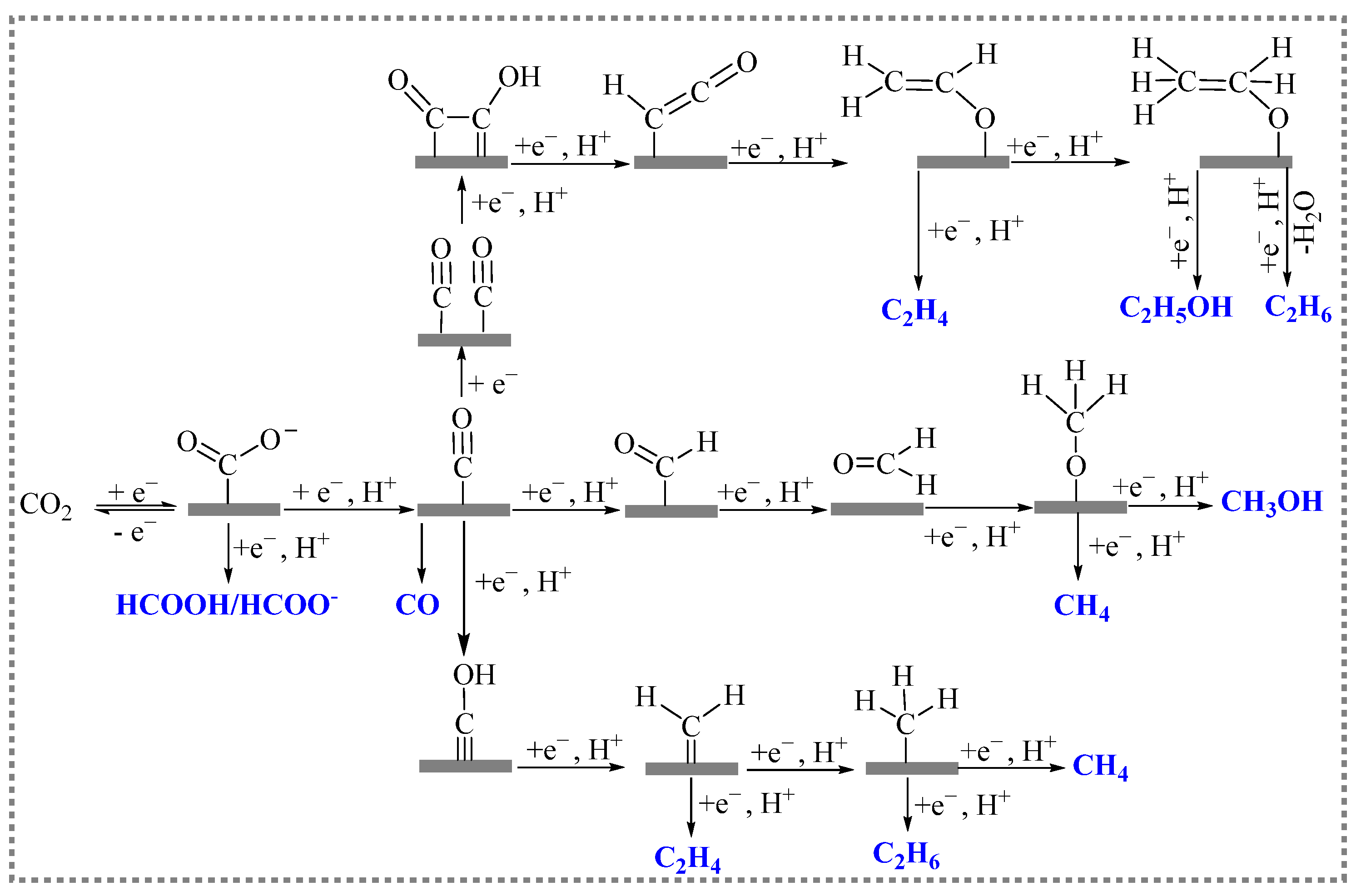
Figure 2. Possible reaction pathways for the electroreduction of CO2.
The CO2 molecule is first adsorbed onto the surface of the catalyst, and, then, it is activated to absorb carbon dioxide (*CO2−), which generates into intermediate *COOH through proton transfer. The intermediate *COOH undergoes another proton transfer and eventually generates HCOOH. The formation pathway of CO is similar to that of formic acid. Meanwhile, the intermediate *COOH is further reduced to form adsorbed CO (*CO). *CO is a relatively important intermediate that undergoes a series of electron transfer and protonation processes to generate different reduction products. For the generation of CH4, the *CO is hydrogenated in C or O to generate *CHO or *COH. In the *CHO pathway, the configurations of adsorbed intermediates change from C binding in *CHO to O binding in *OCH2. Moreover, *OCH3, and gaseous CH4 with *O are obtained on the surfaces of the catalysts. The other pathway of *COH involves the formation of adsorbed C (*C). The *C is further reduced to *CH, *CH2, *CH3, and finally to CH4. The formation of C2H4 or other hydrocarbons requires controlled coupling reactions between CHO* and CH2O* into *OCH-CHO*, *OCH-CH2O*, or *OCH2-CH2O*, which is then followed by hydrogenation/dehydration reactions [21][22].
It is generally believed that the selectivity of the products of the electroreduction of CO2 depends on the binding energy between the electrocatalytic materials and the reaction intermediates such as *CO, CO2−, *COOH. When CO2 is reduced to CO on the surface of electrodes, the binding energy between the electrode material and the CO determines the selectivity of the products generated during electrocatalytic reduction. The electrocatalytic products of electrodes (such as Ag, Au, and Zn) with weak CO binding tend to have high CO selectivity. Moreover, the CO generated during the reduction reaction is easily separated from the electrode surface and does not enter into the next reduction reaction. Various electrode materials (such as Pt, Fe, Co, and Ni) have stronger binding energies for CO, and, therefore, almost no CO2 reduction products are produced when using these materials. This is because, after its generation, CO forms strong interactions with the metal sites on the surface, and the next reduction reaction cannot proceed as a result. Meanwhile, H+ reduction dominates, and a hydrogen evolution reaction occurs. Some electrode materials have moderate binding energies for the intermediates (such as *CO, CO2−, and *COOH) due to the coincidence that the intermediates are stabilized. This, in turn, prompts the generation of reduction products with more than two electron transfers. C-C coupling reaction may also occur to yield C2+ products.
References
- She, X.; Wang, Y.; Xu, H.; Chi Edman Tsang, S.; Ping Lau, S. Challenges and Opportunities in Electrocatalytic CO2 Reduction to Chemicals and Fuels. Angew. Chem. Int. Ed. 2022, 61, e202211396.
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914.
- Yang, H.; Wang, X.; Hu, Q.; Chai, X.; Ren, X.; Zhang, Q.; Liu, J.; He, C. Recent Progress in Self-Supported Catalysts for CO2 Electrochemical Reduction. Small Methods 2020, 4, 1900826.
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648.
- Wang, Y.; Huang, N.-Y.; Wang, H.-Y.; Zhang, X.-W.; Huang, J.-R.; Liao, P.-Q.; Chen, X.-M.; Zhang, J.-P. Local Weak Hydrogen Bonds Significantly Enhance CO2 Electroreduction Performances of a Metal-Organic Framework. CCS Chem. 2023, 5, 145–151.
- Zheng, Y.; Wang, Y.; Yuan, Y.; Huang, H. Metal-based Heterogeneous Electrocatalysts for Electrochemical Reduction of Carbon Dioxide to Methane: Progress and Challenges. ChemNanoMat 2021, 7, 502–514.
- Wang, S.; Kou, T.; Baker, S.E.; Duoss, E.B.; Li, Y. Electrochemical Reduction of CO2 to Alcohols: Current Understanding, Progress, and Challenges. Adv. Energy Sustain. Res. 2021, 3, 2100131.
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210.
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2019, 10, 1902338–1902357.
- Zhang, B.; Cao, S.; Wu, Y.; Zhai, P.; Li, Z.; Zhang, Y.; Fan, Z.; Wang, C.; Zhang, X.; Hou, J.; et al. Metal-Organic-Framework-Derived Bismuth Nanosheets for Electrochemical and Solar-Driven Electrochemical CO2 Reduction to Formate. ChemElectroChem 2021, 8, 880–886.
- Akter, T.; Pan, H.; Barile, C.J. Tandem Electrocatalytic CO2 Reduction inside a Membrane with Enhanced Selectivity for Ethylene. J. Phys. Chem. C 2022, 126, 10045–10052.
- Kim, J.; Choi, W.; Park, J.W.; Kim, C.; Kim, M.; Song, H. Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Production by Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 6986–6994.
- Baek, Y.; Song, H.; Hong, D.; Wang, S.; Lee, S.; Joo, Y.-C.; Lee, G.-D.; Oh, J. Electrochemical carbon dioxide reduction on copper–zinc alloys: Ethanol and ethylene selectivity analysis. J. Mater. Chem. A 2022, 10, 9393–9401.
- Chanda, D.; Tufa, R.A.; Aili, D.; Basu, S. Electroreduction of CO2 to ethanol by electrochemically deposited Cu-lignin complexes on Ni foam electrodes. Nanotechnology 2021, 33, 055403.
- Du, J.; Zhang, P.; Liu, H. Electrochemical Reduction of Carbon Dioxide to Ethanol: An Approach to Transforming Greenhouse Gas to Fuel Source. Chem. Asian J. 2021, 16, 588–603.
- Wu, J.X.; Hou, S.Z.; Zhang, X.D.; Xu, M.; Yang, H.F.; Cao, P.S.; Gu, Z.Y. Cathodized copper porphyrin metal-organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019, 10, 2199–2205.
- Yu, J.; Wang, J.; Ma, Y.; Zhou, J.; Wang, Y.; Lu, P.; Yin, J.; Ye, R.; Zhu, Z.; Fan, Z. Recent Progresses in Electrochemical Carbon Dioxide Reduction on Copper-Based Catalysts toward Multicarbon Products. Adv. Funct. Mater. 2021, 31, 2102151.
- Fan, L.; Xia, C.; Yang, F.; Wang, J.; Wang, H.; Lu, Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111.
- Hou, Y.; Jiang, C.J.; Wang, Y.; Zhu, J.W.; Lu, J.X.; Wang, H. Nitrogen-doped mesoporous carbon supported CuSb for electroreduction of CO2. RSC Adv. 2022, 12, 12997–13002.
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021, 50, 4993–5061.
- Li, N.; Wang, X.; Lu, X.; Zhang, P.; Ong, W.J. Comprehensive Mechanism of CO2 Electroreduction on Non-Noble Metal Single-Atom Catalysts of Mo2CS2-MXene. Chemistry 2021, 27, 17900–17909.
- Wang, X.; Hu, Q.; Li, G.; Yang, H.; He, C. Recent Advances and Perspectives of Electrochemical CO2 Reduction Toward C2+ Products on Cu-Based Catalysts. Electrochem. Energy Rev. 2022, 5 (Suppl. S2), 28.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.2K
Revisions:
2 times
(View History)
Update Date:
17 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





