Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marino B. Arnao | -- | 1989 | 2023-04-14 12:06:49 | | | |
| 2 | Rita Xu | Meta information modification | 1989 | 2023-04-18 03:21:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; Mihyaoui, A.E.; Cano, A.; Hernández-Ruiz, J. Biological Melatonin versus Synthetic Melatonin. Encyclopedia. Available online: https://encyclopedia.pub/entry/43060 (accessed on 07 February 2026).
Arnao MB, Giraldo-Acosta M, Castejón-Castillejo A, Losada-Lorán M, Sánchez-Herrerías P, Mihyaoui AE, et al. Biological Melatonin versus Synthetic Melatonin. Encyclopedia. Available at: https://encyclopedia.pub/entry/43060. Accessed February 07, 2026.
Arnao, Marino B., Manuela Giraldo-Acosta, Ana Castejón-Castillejo, Marta Losada-Lorán, Pablo Sánchez-Herrerías, Amina El Mihyaoui, Antonio Cano, Josefa Hernández-Ruiz. "Biological Melatonin versus Synthetic Melatonin" Encyclopedia, https://encyclopedia.pub/entry/43060 (accessed February 07, 2026).
Arnao, M.B., Giraldo-Acosta, M., Castejón-Castillejo, A., Losada-Lorán, M., Sánchez-Herrerías, P., Mihyaoui, A.E., Cano, A., & Hernández-Ruiz, J. (2023, April 14). Biological Melatonin versus Synthetic Melatonin. In Encyclopedia. https://encyclopedia.pub/entry/43060
Arnao, Marino B., et al. "Biological Melatonin versus Synthetic Melatonin." Encyclopedia. Web. 14 April, 2023.
Copy Citation
Melatonin dietary supplements are widely consumed worldwide, with developed countries as the largest consumers, with an estimated annual growth rate of approximately 10% until 2027, mainly in developing countries. The wide use of melatonin against sleep disorders and particular problems, such as jet lag, has been added to other applications, such as anti-aging, anti-stress, immune system activation, anticancer, and others, which have triggered its use, normally without a prescription. The chemical industry currently covers 100% of the needs of the melatonin market.
dietary supplements
GMOs
melatonin
microorganisms
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is widely used around the world as a dietary supplement. In general, melatonin is used as a sleep aid supplement, a mild tranquilizer, a generalist antioxidant, and an anticancer and anti-aging component, among others [1]. According to the American Psychiatric Association (APA), approximately one-third of adults suffer from insomnia during their lifetime [2]. It manifests itself in incessant problems falling asleep and staying asleep. Therefore, it is very likely that the use of synthetic melatonin will spread. In 2019, the global production of synthetic melatonin, which was around 4000 tons, accounted for around 1.3 billion USD. This vast market is fully assisted by chemical melatonin, whose synthesis process is very cheap, effective, and, therefore, lucrative. The melatonin market is expected to grow at a CAGR (compound annual growth rate) of >10% over the next 5 years. With this considerable increase in demand, the insomnia problems generated by the COVID-19 pandemic have been of great relevance [3]. North America has the highest consumption by far, followed by Europe. The global melatonin market is mainly controlled by a few major companies, such as BASF, Aspen Pharmacare Australia, Nature’s Bounty, Pfizer Inc., Natrol LLC, Aurobindo Pharma, and Biotics Research Co. Note that the consumption of melatonin for medical purposes involves about 50% of the synthetic melatonin produced; the rest has chemical and industrial applications [2][4].
Biologically, melatonin is a molecule widely distributed in all kingdoms of living organisms [5]. Discovered in 1958 in the pineal gland of a cow [6] and later in humans [7], it is one of the most studied biomolecules, and its multiple functions are known, mainly in mammals [8][9], but also in fish [10][11][12], poultry [13][14], and invertebrates [15]. In animal and human cells, melatonin acts as an antioxidant—a relevant role attributed to it in 1993 [16][17][18]. Melatonin acts as an interesting cell protector in stressful situations, in various physiological aspects in humans, and, according to multiple studies, benefits an improvement in different diseases and dysfunctions. Figure 1 shows some of the protective and regulatory actions of melatonin in humans and presents melatonin as an interesting pleiotropic molecule, standing out due to its relevance, the role of melatonin in the regulation of lipid and glucose metabolism, inducing nocturnal insulin resistance and diurnal insulin sensitivity. This effect seems to be associated with nocturnal fasting and diurnal feeding, preventing excessive weight gain [19]. Researchers also highlight its role as an anti-oncogenic agent, inhibiting the growth, proliferation, and metastasis of several tumors. The treatment of tumors with melatonin improved chemo- and radiotherapy sensitivity, acting as a synergistic molecule in the control of cancer cells. Additionally, melatonin mitigates acute damage to normal cells, protecting them against drug toxicity, possibly by enhancing immune responses [20][21][22]. Among the dysfunctions and diseases where the beneficial effects of melatonin have been studied are neurological ones, such as Alzheimer’s, Parkinson’s, fibromyalgia, depression, attention-deficit hyperactivity disorder, autism, and migraines; cardiovascular health problems, including hypercholesterolemia, hypertension, metabolic syndrome, and glycemic imbalance; gastrointestinal health problems, such as gastroesophageal reflux, ulcers, and irritable bowel syndrome; immunological health problems, such as multiple sclerosis, autoimmune responses (athletic stress, toxic stress, psoriasis, etc.), sepsis, COVID-19, etc. [3][23][24][25][26]; and also osteopenia [27], sarcopenia [28], pre-eclampsia, fertility, polycystic ovarian syndrome, and menopause, among others [29][30][31][32]. However, even though melatonin is a molecule that has been widely studied since the 1950s, the studies carried out require more clinical and extensive double-blind trials in order to clarify its sometimes confusing pleiotropic action [33][34].
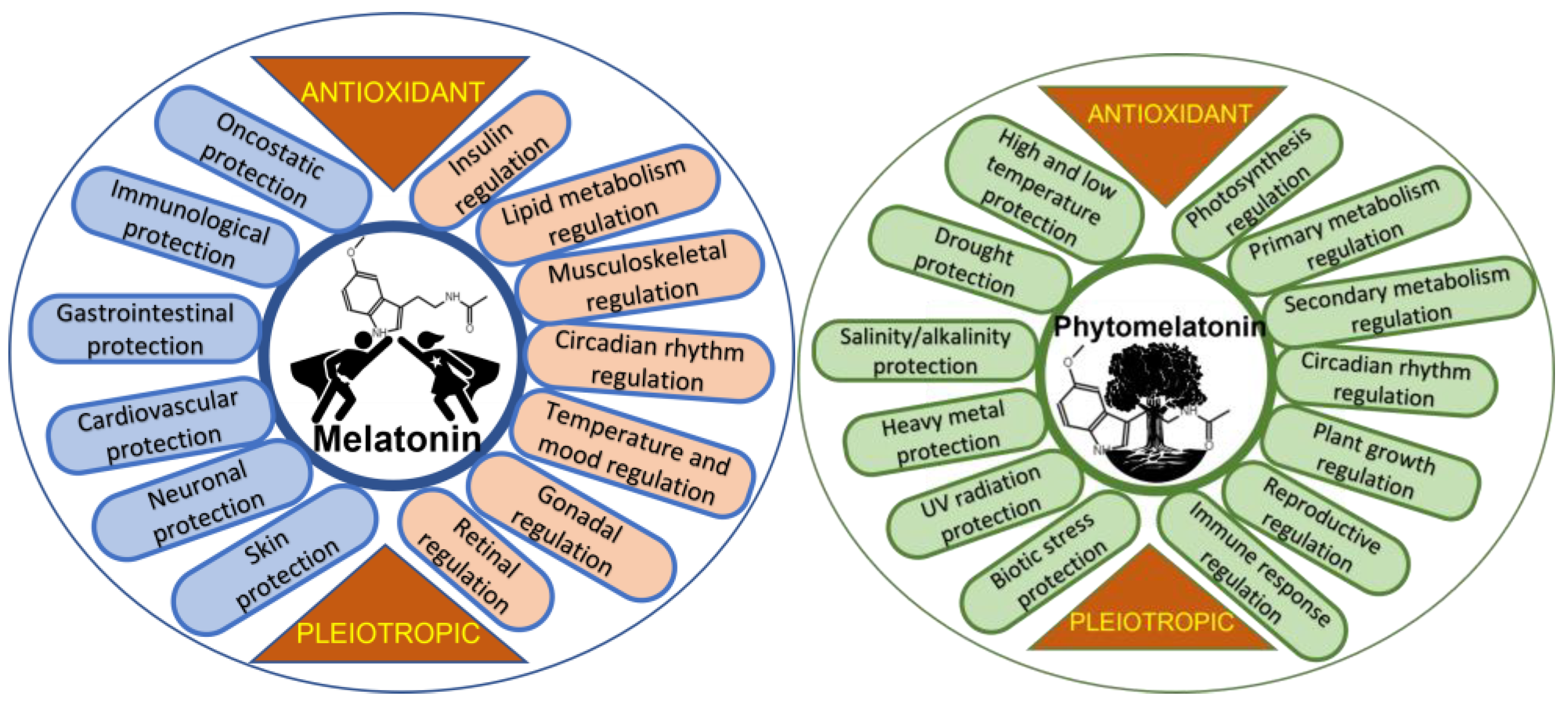
Figure 1. Diagram showing general roles of melatonin in humans and phytomelatonin in plants.
However, melatonin is well known for being the hormone that regulates sleep. Its oscillating levels in the blood flow according to the periods of light and darkness (circadian rhythms) due to the release of melatonin by the pineal gland is one of the most studied and known aspects of this molecule. The increase in blood melatonin levels during the first period of sleep to around 150–220 pmoles/mL acts on sleep initiation, reduces sleep latency and fragmentation, and increases sleep duration and quality [1][35][36]. Melatonin acts as an internal synchronizer of the circadian sleep–wake cycle and seasonal rhythmicity. In this sense, many sleep disorders have been treated with melatonin, including delayed sleep phase syndrome, night shift work sleep disorder, seasonal affective disorder, sleep disorders in the blind and aging, and pathophysiological disorders of children, with notable improvements in sleep quality [37][38][39][40][41]. The most widespread disorder treated with melatonin is jet lag—a de-phasing in sleep–wake rhythms following trans-oceanic flights [42][43][44][45]. Possibly, the emphasis in studies on its role as a sleep regulator has caused a lack of studies on its possible role in many other physiological and clinical aspects.
Melatonin in plants, so-called phytomelatonin, was discovered simultaneously by three research groups in diverse plant material in 1995 [46][47][48]. The term phytomelatonin, which refers to melatonin of vegetable origin (plants and algae), is used to differentiate it from animal and/or synthetic melatonin. This term is very widespread and is used continuously in studies of phytochemistry, plant physiology, botany, food chemistry, etc., on plant melatonin. In plants, phytomelatonin is also a pleiotropic molecule, presenting multiple roles in diverse physiological responses (Figure 1). The regulation by melatonin of aspects such as photosynthesis, including stomatal CO2 uptake and water economy, carbohydrate, lipid, nitrogen, and sulfur metabolism, and simple phenol, flavonoid, and terpenoid metabolism, has demonstrated crucial interest in the basic and technical processes of vegetative (germination, plant growth, rooting, branching, etc.) and reproductive development, including fertility, parthenocarpy, seed and fruit development, ripening, senescence, and the conservation of fruits and cut flowers [49][50][51][52][53]. Generally, melatonin regulates these processes through the action of the plant hormone network, up/down-regulating several biosynthesis, catabolic, and transcription factors that are plant hormone-related [54][55][56]. One of the aspects of greatest agronomic and biotechnological interest is the role of phytomelatonin as a promoter of tolerance against biotic and abiotic stresses [57][58][59][60][61][62][63][64][65][66][67][68] (Figure 1). Currently, phytomelatonin is presented as an interesting eco-friendly tool to control biological diseases and to facilitate the resistance/adaptation of plants to/against climate change.
2. Biosynthesis of Melatonin
Melatonin is an acetylated compound derived from serotonin. Both indolic amines are synthesized from the amino acid tryptophan in a biosynthetic pathway that has been extensively studied in both animals and plants [69][70]. In plants, tryptophan is converted into tryptamine by the enzyme tryptophan decarboxylase (TDC) (Figure 2). Tryptamine is then converted into 5-hydroxytryptamine (serotonin) by tryptamine 5-hydroxylase (T5H), an enzyme that has been extensively studied in rice, and which could act with many substrates, although this has not been studied in depth. Serotonin is N-acetylated by serotonin N-acetyltransferase (SNAT). N-acetylserotonin is then methylated by acetylserotonin methyl transferase (ASMT)—a hydroxyindole-O-methyltransferase—which generates melatonin. In plants, the methylation of N-acetylserotonin can also be performed by caffeic acid O-methyltransferase (COMT), a class of enzyme that can act on a variety of substrates, including caffeic acid and quercetin [71]. Serotonin may also be transformed into 5-methoxytryptamine by ASMT/COMT to generate melatonin after the action of SNAT. This route would occur in senescence and/or stress situations [70][72]. Furthermore, melatonin can be generated through the formation of N-acetyltryptamine by SNAT, which would be converted into N-acetylserotonin by T5H [73], although this route has not been demonstrated, possibly because T5H is the least studied enzyme of the pathway (Figure 2). Interestingly, up to four genes encoding histone deacetylases (DAC) have been identified in rice plants that can reverse the steps from serotonin to N-acetylserotonin and from 5-methoxytryptamine to melatonin. DAC, expressed in chloroplast, exhibited enzyme activity toward N-acetylserotonin, N-acetyltryptamine, and melatonin, with the highest deacetylase activity for N-acetyltyramine [74].
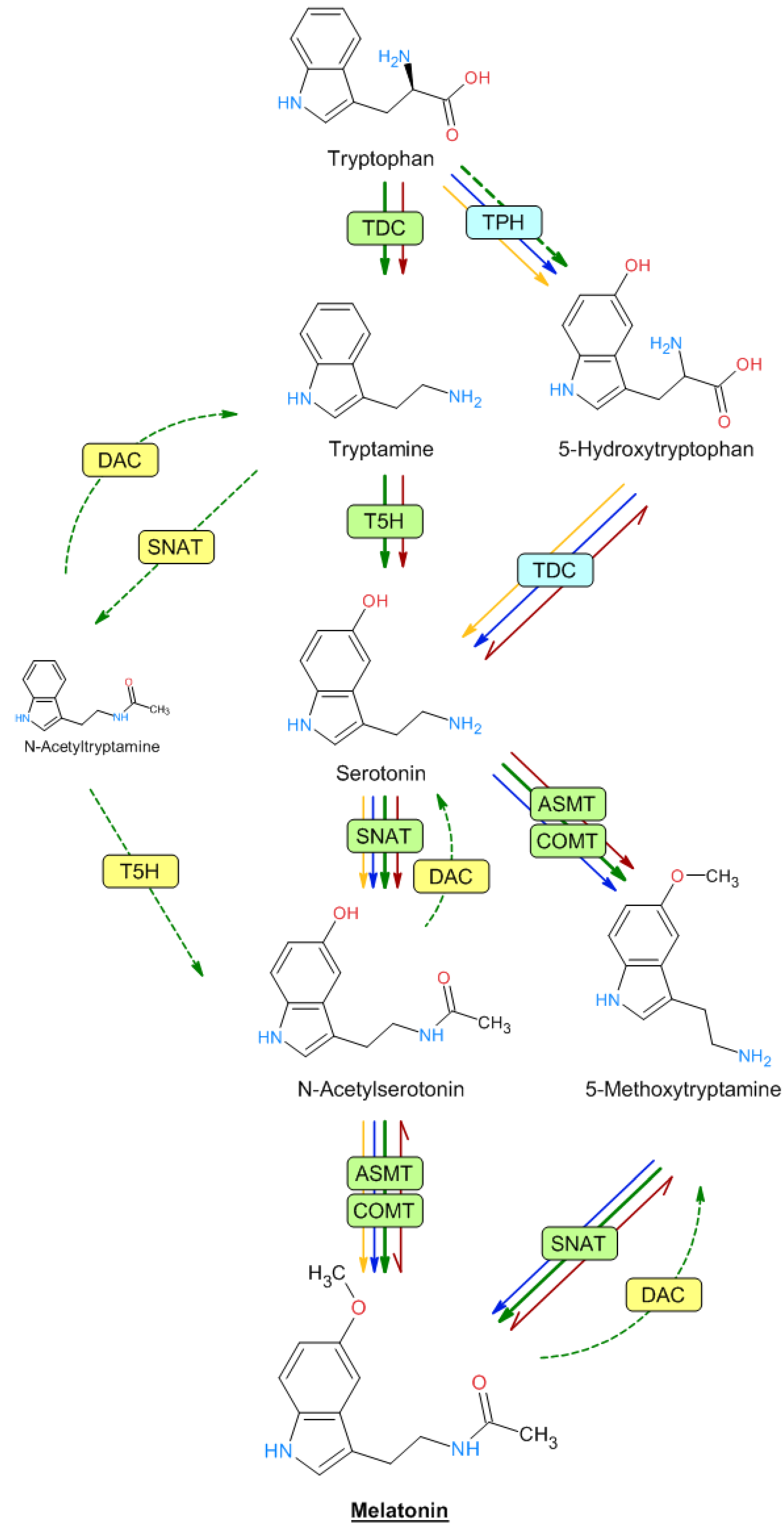
Figure 2. Biosynthetic melatonin pathways in mammals, plants, and microorganisms. The names of the different enzymes are described in the text. The different arrow colors denote plants (green), animals (blue), bacteria (yellow), and yeasts (red). Dashed lines indicate unproven reactions.
In animal cells, serotonin is formed from 5-hydroxytryptophan after the sequential action of tryptophan hydroxylase (TPH) and TDC. Although TPH has not been detected in plants, the presence of 5-hydroxytryptophan suggested that some enzymatic activity, such as that of TPH, acts to a lesser extent in plant cells. Moreover, melatonin can be generated through the formation of 5-methoxytryptamine, mainly under stress conditions as proposed by several authors, suggesting that the melatonin biosynthesis pathway may follow various alternative routes compared with animal cells, with a greater ability to adapt to metabolic changes in plants [72][75]. All the named enzymes have been detected and characterized in rice and Arabidopsis, except TPH, which is well known in animals but not in plants. Nevertheless, some authors have proposed that T5H can act as a hydroxylase with low substrate specificity and is capable of acting in all the hydroxylation steps described [70][76][77][78][79]. This same broad substrate specificity can also be attributed to SNAT, ASMT, and COMT enzymes. Melatonin intermediates are produced in various subcellular compartments, such as the cytoplasm, endoplasmic reticulum, mitochondria, and chloroplasts, which determine the subsequent enzymatic steps [80][81].
In microorganisms, there are few studies on the melatonin biosynthesis pathway [82]. Saccharomyces and bacteria (Geobacillus, Bacillus, and Pseudomonas) produced both serotonin and melatonin at different concentrations [83][84][85][86][87][88][89]. Moreover, the production of melatonin was evidenced by other authors in the cultures of the yeasts Pichia kluyveri, Saccharomyces cerevisiae, and S. uvarum and bacteria (Agrobacterium, Pseudomonas, Variovorax, Bacillus, and Oenococcus) [85][90][91] and previously in the photosynthetic bacteria Rhodospirillum rubrum [92] and Erythrobacter longus [93] and Escherichia coli [94].
In the yeast Saccharomyces cerevisiae, unlike in plants and animals, it seems that the biosynthesis of 5-hydroxytryptophan from tryptophan does not occur. Interestingly, several of the described stages appear to be reversible in S. cerevisiae, such as between 5-hydroxytryptophan and serotonin, N-acetylserotonin and melatonin, and 5-methoxytryptamine and melatonin [90][95], as detailed in Figure 2. In Bacillus amyloliquefaciens SB-9 and Pseudomonas fluorescens RG11, 5-hydroxytryptophan, serotonin, and N-acetylserotonin, but not tryptamine, were detected [85][86]. So, several genes from bacterial origin were used to build a melatonin-producing Escherichia coli strain. For example, the DDC gene, encoding an aromatic L-amino acid decarboxylase from Candidatus Koribacter versatilis Ellin 345 and Draconibacterium orientale, and the AANAT gene, encoding an aralkylamine N-acetyltransferase from Streptomyces griseofuscus, were assayed [96][97]. Undoubtedly, further studies are needed to elucidate the complete biosynthetic pathways of melatonin in different prokaryotic and eukaryotic microbes [82].
3. Biological Melatonin versus Synthetic Melatonin
Initially, melatonin was obtained for experimental and clinical studies from animal sources (mainly from the pineal gland and urine), with the consequent risk of viral transmission [98][99]. These techniques were withdrawn when melatonin could be obtained by chemical synthesis [100]. Currently, all melatonin used for industrial and medical purposes is obtained by using chemical synthesis methods. These methods, which presented serious problems in the 1980s, including deaths due to the presence of by-products of synthesis from tryptophan [101], are much safer and more efficient today. However, melatonin preparations have described the presence of a whole set of undesirable by-products due to their toxic nature. Figure 3 shows three of the most commonly used chemical synthesis routes for melatonin and the by-products that are generated in its synthesis [102]. The synthesis of melatonin from tryptophan derivatives (Figure 3, Scheme A) generates toxic by-products that have sometimes caused significant diseases, such as eosinophilia myalgia syndrome [101][103][104], while the most current methods (Figure 3, Scheme B) for the synthesis of melatonin from phthalimide [105] raise important doubts about the toxicity of several of the by-products that are generated [106]. In addition, Fischer indole reactions from allylamine (Figure 3, Scheme C) present dangerous and toxic reactants [107].
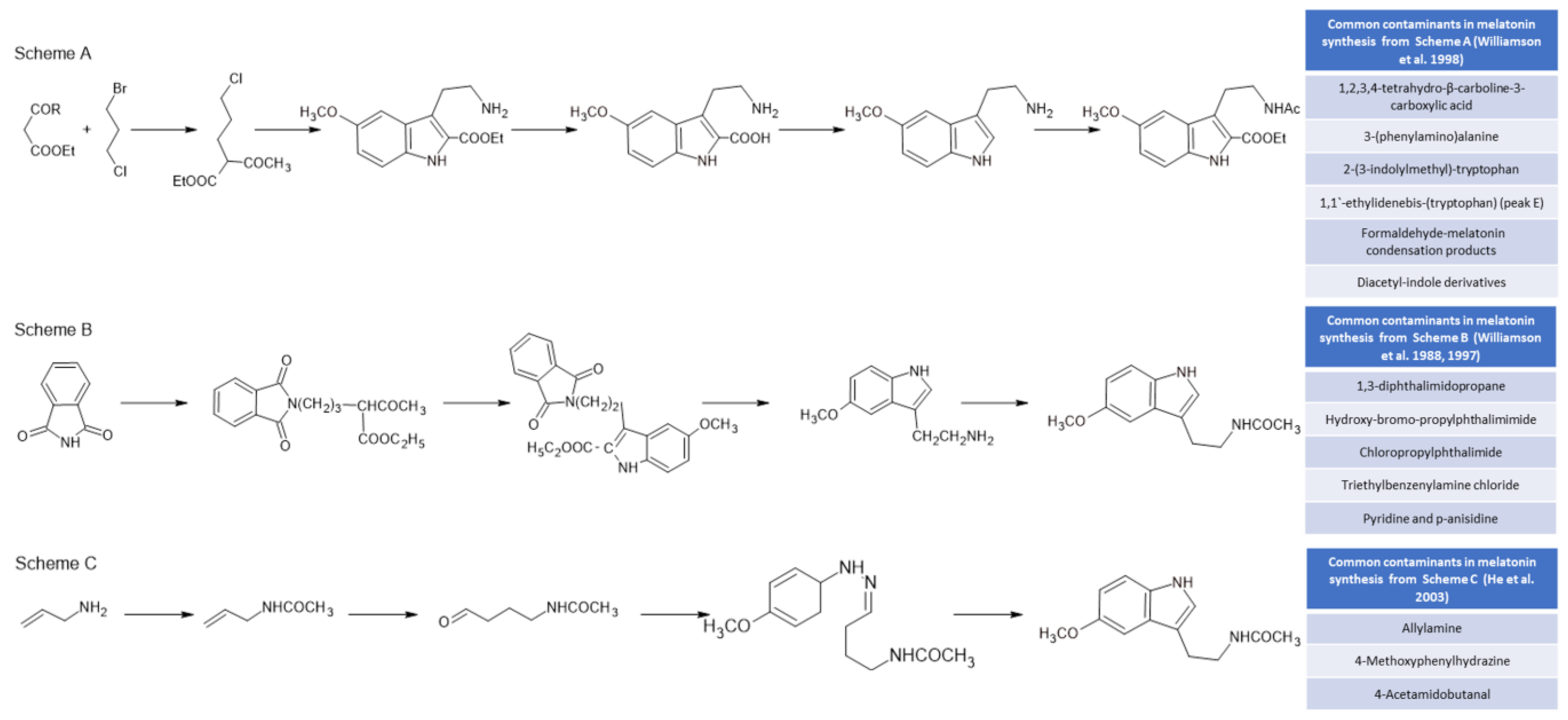
Figure 3. Some chemical melatonin synthesis pathways and their by-products present in synthetic melatonin preparations.
On the other hand, obtaining melatonin from non-animal biological sources is presented as a strong commitment to the future, not to replace synthetic melatonin but to be a more natural complementary and alternative source [108].
References
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199.
- Mordor Intelligence. Melatonin Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/melatonin-market (accessed on 30 October 2022).
- Tan, D.-X.; Reiter, R.J. Mechanisms and Clinical Evidence to Support Melatonin’s Use in Severe COVID-19 Patients to Lower Mortality. Life Sci. 2022, 294, 120368.
- Market Analysis Report Melatonin Market Size, Share & Trends Analysis Report By Application, Regional Outlook, Competitive Strategies, and Segment Forecasts, 2019 to 2025. Available online: https://www.grandviewresearch.com/industry-analysis/melatonin-market (accessed on 30 October 2022).
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996.
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587.
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821.
- Krause, D.; Dubocovich, M. Regulatory Sites in the Melatonin System of Mammals. Trends Neurosci. 1990, 13, 464–470.
- Luo, C.; Yang, Q.; Liu, Y.; Zhou, S.; Jiang, J.; Reiter, R.J.; Bhattacharya, P.; Cui, Y.; Yang, H.; Ma, H.; et al. The Multiple Protective Roles and Molecular Mechanisms of Melatonin and Its Precursor N-Acetylserotonin in Targeting Brain Injury and Liver Damage and in Maintaining Bone Health. Free Radic. Biol. Med. 2019, 130, 215–233.
- Fujii, R. The Regulation of Motile Activity in Fish Chromatophores. Pigment. Cell Res. 2000, 13, 300–319.
- López-Olmeda, J.; Madrid, J.; Sánchez-Vázquez, F. Melatonin Effects on Food Intake and Activity Rhythms in Two Fish Species with Different Activity Patterns: Diurnal (Goldfish) and Nocturnal (Tench). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 180–187.
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Human Diseases. Int. J. Mol. Sci. 2022, 23, 7438.
- Rozov, S.V. Features of Melatonin Catabolism in Chicks. Neurochem. J. 2008, 2, 188–192.
- de Pontes, M.P.; de Souza Khatlab, A.; Del Vesco, A.P.; Granzoto, G.H.; Soares, M.A.M.; de Sousa, F.C.B.; de Souza, M.L.R.; Gasparino, E. The Effect of Light Regime and Time of Slaughter in Broiler on Broiler Performance, Liver Antioxidant Status, and Expression of Genes Related to Peptide Absorption in the Jejunum and Melatonin Synthesis in the Brain. J. Anim. Physiol. Anim. Nutr. 2022.
- Vivien-Roels, B.; Pávet, P. Melatonin: Presence and Formation in Invertebrates. Experientia 1993, 49, 642–647.
- Reiter, R.J.; Poeggeler, B.; Tan, D.X.; Chen, L.; Manchester, L.; Guerrero, J. Antioxidant Capacity of Melatonin. A Novel Action Not Requiring a Receptor. Neuroendocrinol. Lett. 1993, 15, 103–116.
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A Potent, Endogenous Hydroxyl Radical Scavenger. Endocr. J. 1993, 1, 57–60.
- Reiter, R.J. Interactions of the Pineal Hormone Melatonin with Oxygen-Centered Free-Radicals. A Brief Review. Braz. J. Med. Biol. Res. 1993, 26, 1141–1155.
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397.
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, M.A.; Suárez, S.; Puente-Moncada, N.; Anítua, J.M.; et al. Mechanisms Involved in the Pro-Apoptotic Effect of Melatonin in Cancer Cells. Int. J. Mol. Sci. 2013, 14, 6597–6613.
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer Metastasis: Mechanisms of Inhibition by Melatonin. J. Pineal Res. 2017, 62, e12370.
- Loh, D.; Reiter, R. Melatonin: Regulation of Prion Protein Phase Separation in Cancer Multidrug Resistance. Molecules 2022, 27, 705.
- Maqbool, S.; Ihtesham, A.; Langove, M.N.; Jamal, S.; Jamal, T.; Safian, H.A. Neuro-Dermatological Association between Psoriasis and Depression: An Immune-Mediated Inflammatory Process Validating Skin-Brain Axis Theory. AIMS Neurosci. 2021, 8, 340–354.
- Zhao, Y.; Zhang, R.; Wang, Z.; Chen, Z.; Wang, G.; Guan, S.; Lu, J. Melatonin Prevents against Ethanol-Induced Liver Injury by Mitigating Ferroptosis via Targeting Brain and Muscle ARNT-like 1 in Mice Liver and HepG2 Cells. J. Agric. Food Chem. 2022, 70, 12953–12967.
- Kvetnoy, I.; Ivanov, D.; Mironova, E.; Evsyukova, I.; Nasyrov, R.; Kvetnaia, T.; Polyakova, V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. Int. J. Mol. Sci. 2022, 23, 1835.
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a Potential Therapy for Sepsis: A Phase I Dose Escalation Study and an Ex Vivo Whole Blood Model under Conditions of Sepsis. J. Pineal Res. 2014, 56, 427–438.
- Maria, S.; Witt-Enderby, P.A. Melatonin Effects on Bone: Potential Use for the Prevention and Treatment for Osteopenia, Osteoporosis, and Periodontal Disease and for Use in Bone-Grafting Procedures. J. Pineal Res. 2014, 56, 115–125.
- Stacchiotti, A.; Favero, G.; Rodella, F.L. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288.
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The Reduction in Circulating Levels of Melatonin May Be Associated with the Development of Preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671.
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85.
- Alizadeh, M.; Karandish, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Mousavi, R. Metabolic and Hormonal Effects of Melatonin and/or Magnesium Supplementation in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutr. Metab. 2021, 18, 57.
- Cipolla-Neto, J.; Amaral, F.G.; José Maria Soares, J.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Gonçalves, I.; Santos, C.R.A.; Quintela, T. The Crosstalk between Melatonin and Sex Steroid Hormones. Neuroendocrinology 2022, 112, 115–129.
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of Melatonin Supplementation on Sleep Quality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2022, 269, 205–216.
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A Pleiotropic Molecule Regulating Inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852.
- Dahlitz, M.; Alvarez, B.; Vignau, J.; English, J.; Arendt, J.; Parkes, J. Delayed Sleep Phase Syndrome Response to Melatonin. Lancet 1991, 337, 1121–1124.
- Fuller, P.M.; Gooley, J.J.; Saper, C.B. Neurobiology of the Sleep-Wake Cycle: Sleep Architecture, Circadian Regulation, and Regulatory Feedback. J. Biol. Rhythm. 2006, 21, 482–493.
- Jan, J.E.; Reiter, R.J.; Wasdell, M.B.; Bax, M. The Role of the Thalamus in Sleep, Pineal Melatonin Production, and Circadian Rhythm Sleep Disorders. J. Pineal Res. 2009, 46, 1–7.
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; D’Mello, J., Ed.; CAB Intern: Boston, MA, USA, 2015; pp. 390–435. ISBN 978-1-78064-263-5.
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the Efficacy of Melatonin in the Treatment of Primary Adult Sleep Disorders. Sleep Med. Rev. 2017, 34, 10–22.
- Amaral, F.; Silva, J.-A.; Kuwabara, W.; Cipolla-Neto, J. New Insights into the Function of Melatonin and Its Role in Metabolic Disturbances. Expert Rev. Endocrinol. Metabol. 2019, 14, 293–300.
- Waterhouse, J.; Reilly, T.; Atkinson, G. Jet Lag. Lancet 1997, 350, 1611–1616.
- Takahashi, T.; Sasaki, M.; Itoh, H.; Ozone, M.; Yamadera, W.; Hayshida, K.I.; Ushijima, S.; Matsunaga, N.; Obuchi, K.; Sano, H. Effect of 3 Mg Melatonin on Jet Lag Syndrome in an 8-h Eastward Flight. Psychiatry Clin. Neurosci. 2000, 54, 377–378.
- Takahashi, T.; Sasaki, M.; Itoh, H.; Yamadera, W.; Ozone, M.; Obuchi, K.; Hayashida, K.I.; Matsunaga, N.; Sano, H. Melatonin Alleviates Jet Lag Symptoms Caused by an 11-Hour Eastward Flight. Psychiatry Clin. Neurosci. 2002, 56, 301–302.
- Herxheimer, A. Jet Lag. Clin. Evid 2005, 13, 2178–2183.
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634.
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31.
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm Res. 1995, 26, 406–409.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48.
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87.
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800.
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin During Postharvest of Horticultural Crops. Plant Cell Physiol. 2021, pcab175.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207.
- Arnao, M.B.; Hernández-Ruiz, J. The Multi-Regulatory Properties of Melatonin in Plants. In Neurotransmitters in Plants; Ramakrishna, A., Roshchina, V.V., Eds.; CRC Press: Boca Raton, FL, USA, 2018; p. 448. ISBN 978-0-203-71148-4.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19.
- Arnao, M.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Res. 2019, 2, 152–168.
- Arnao, M.B.; Hernández-Ruiz, J. Regulatory Role of Melatonin in the Redox Network of Plants and Plant Hormone Relationship in Stress. In Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Plant in Challenging Environments; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–272. ISBN 978-3-030-77477-6.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2022, 22, 413–429.
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809.
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359.
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175.
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin Is a Potential Target for Improving Horticultural Crop Resistance to Abiotic Stress. Sci. Hortic. 2022, 291, 110560.
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile Roles of Melatonin in Growth and Stress Tolerance in Plants. J. Plant Growth Regul. 2022, 41, 507–523.
- Yang, X.; Ren, J.; Li, J.; Lin, X.; Xia, X.; Yan, W.; Zhang, Y.; Deng, X.; Ke, Q. Meta-Analysis of the Effect of Melatonin Application on Abiotic Stress Tolerance in Plants. Plant Biotechnol. Rep. 2022.
- Sati, H.; Khandelwal, A.; Pareek, S. Effect of Exogenous Melatonin in Fruit Postharvest, Crosstalk with Hormones, and Defense Mechanism for Oxidative Stress Management. Food Front. 2023, 1–29.
- Ahmad, S. Interactive Effects of Melatonin and Nitrogen Improve Drought Tolerance of Maize Seedlings by Regulating Growth and Physiochemical Attributes. Antioxidants 2022, 11, 359.
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative Effect of Melatonin Improves Drought Tolerance by Regulating Growth, Photosynthetic Traits and Leaf Ultrastructure of Maize Seedlings. BMC Plant Biol. 2021, 21, 368.
- Tan, D.X.; Manchester, C.L.; Esteban-Zubero, E.; Zhou, Z.; Reiter, J.R. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906.
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437.
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic Acid O-Methyltransferase Is Involved in the Synthesis of Melatonin by Methylating N-Acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227.
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40.
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis thaliana Plants. J. Pineal Res. 2014, 57, 408–417.
- Lee, K.; Lee, H.Y.; Back, K. Rice Histone Deacetylase 10 and Arabidopsis Histone Deacetylase 14 Genes Encode N-Acetylserotonin Deacetylase, Which Catalyzes Conversion of N-Acetylserotonin into Serotonin, a Reverse Reaction for Melatonin Biosynthesis in Plants. J. Pineal Res. 2018, 64, e12460.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797.
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391.
- Hwang, O.-J.; Back, K. Functional Characterization of Arylalkylamine N-Acetyltransferase, a Pivotal Gene in Antioxidant Melatonin Biosynthesis from Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1531.
- Hwang, J.O.; Back, K. Simultaneous Suppression of Two Distinct Serotonin N-Acetyltransferase Isogenes by RNA Interference Leads to Severe Decreases in Melatonin and Accelerated Seed Deterioration in Rice. Biomolecules 2020, 10, 141.
- Lee, Y.H.; Lee, K.; Back, K. Knockout of Arabidopsis Serotonin N-Acetyltransferase-2 Reduces Melatonin Levels and Delays Flowering. Biomolecules 2019, 9, 712.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249.
- Tan, D.X.; Reiter, R.J. An Evolutionary View of Melatonin Synthesis and Metabolism Related to Its Biological Functions in Plants. J. Exp. Bot 2020, 71, 4677–4689.
- Danilovich, M.E.; Alberto, M.R.; Juárez Tomás, M.S. Microbial Production of Beneficial Indoleamines (Serotonin and Melatonin) with Potential Application to Biotechnological Products for Human Health. J. Appl. Microbiol. 2021, 131, 1668–1682.
- Al-Hassan, J.M.; Al-Awadi, S.; Oommen, S.; Alkhamis, A.; Afzal, M. Tryptophan Oxidative Metabolism Catalyzed by Geobacillus stearothermophilus: A Thermophile Isolated from Kuwait Soil Contaminated with Petroleum Hydrocarbons. Int. J. Tryptophan Res. 2011, 4, IJTR.S6457.
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Validation of an Analytical Method to Determine Melatonin and Compounds Related to L-Tryptophan Metabolism Using UHPLC/HRMS. Food Anal. Methods 2016, 9, 3327–3336.
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant. Sci. 2016, 7, 1387.
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic Bacterium Pseudomonas fluorescens RG11 May Transform Tryptophan to Melatonin and Promote Endogenous Melatonin Levels in the Roots of Four Grape Cultivars. Front. Plant Sci. 2017, 7, 2068.
- Muñiz-Calvo, S.; Bisquert, R.; Guillamón, J.M. Melatonin in Yeast and Fermented Beverages: Analytical Tools for Detection, Physiological Role and Biosynthesis. Melatonin Res. 2020, 3, 144–160.
- Muñiz-Calvo, S.; Bisquert, R.; Fernández-Cruz, E.; García-Parrilla, M.C.; Guillamón, J.M. Deciphering the Melatonin Metabolism in Saccharomyces cerevisiae by the Bioconversion of Related Metabolites. J. Pineal Res. 2019, 66, e12554.
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.-Z. Melatonin and Other 5-Methoxylated Indoles in Yeast: Presence in High Concentrations and Dependence on Tryptophan Availability. Cytologia 1999, 64, 209–213.
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, M.d.C. Production of Melatonin by Saccharomyces Strains under Growth and Fermentation Conditions. J. Pineal Res. 2012, 53, 219–224.
- Fernández-Pachón, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-López, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic Fermentation Induces Melatonin Synthesis in Orange Juice. J. Pineal Res. 2014, 56, 31–38.
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin Immunoreactivity in the Photosynthetic Prokaryote Rhodospirillum rubrum: Implications for an Ancient Antioxidant System. Cell. Mol. Biol. Res. 1995, 41, 391–395.
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin Production in an Aerobic Photosynthetic Bacterium: An Evolutionarily Early Association with Darkness. J. Pineal Res. 1997, 22, 102–106.
- Hardeland, R.; Poeggeler, B. Non-Vertebrate Melatonin. J. Pineal Res. 2003, 34, 233–241.
- Fernández-Cruz, E.; Carrasco-Galán, F.; Cerezo-López, A.B.; Valero, E.; Morcillo-Parra, M.Á.; Beltran, G.; Torija, M.-J.; Troncoso, A.M.; García-Parrilla, M.C. Occurrence of Melatonin and Indolic Compounds Derived from L-Tryptophan Yeast Metabolism in Fermented Wort and Commercial Beers. Food Chem. 2020, 331, 127192.
- Luo, H.; Förster, J. Optimized Microbial Cells for Production of Melatonin and Other Compounds. U.S. Patent US10851365B2, 5 October 2017.
- Luo, H.; Schneider, K.; Christensen, U.; Lei, Y.; Herrgard, M.; Palsson, B.Ø. Microbial Synthesis of Human-Hormone Melatonin at Gram Scales. ACS Synth. Biol. 2020, 9, 1240–1245.
- Bonilla, E.; Valero, N.; Chacin-Bonilla, L.; Medina-Leendertz, S. Melatonin and Viral Infections. J. Pineal Res. 2004, 36, 73–79.
- Kennaway, D.J. Urinary 6-Sulphatoxymelatonin Excretory Rhythms in Laboratory Rats: Effects of Photoperiod and Light. Brain Res. 1993, 603, 338–342.
- Hugel, H.M.; Kennaway, D.J. Synthesis and Chemistry of Melatonin and of Related Compounds. A Review. Org. Prep. Proced. Int. 1995, 27, 1–31.
- Williamson, B.L.; Tomlinson, A.J.; Mishra, P.K.; Gleich, G.J.; Naylor, S. Structural Characterization of Contaminants Found in Commercial Preparations of Melatonin: Similarities to Case-Related Compounds from L-Tryptophan Associated with Eosinophilia-Myalgia Syndrome. Chem. Res. Toxicol 1998, 11, 234–240.
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238.
- Williamson, B.L.; Kenneth, L.J.; Tomlinson, A.J.; Gleich, G.J.; Naylor, S. On-Line HPLC-Tandem Mass Spectrometry Structural Characterization of Case Associated Contaminants of L-Tryptophan Implicated with the Onset of Eosinophilia-Myalgia Syndrome. Toxicol. Lett. 1988, 99, 139–150.
- Williamson, B.L.; Tomlinson, A.J.; Naylor, S.; Gleich, G.J. Contaminats in Commercial Preparations of Melatonin. Mayo Clin. Proceed. 1997, 72, 1094–1095.
- He, L.; Li, J.L.; Zhang, J.J.; Su, P.; Zheng, S.L. Microwave Assisted Synthesis of Melatonin. Synth. Commun. 2003, 33, 741–747.
- OECD-Organisation for Economic Co-operation & Development. Initial Assessment Report on Phthalimide; ID-85-41-6; SIAM 20; Screening Information DataSet (SIDS): Paris, France, 2006.
- Verspui, G.; Elbertse, G.; Sheldon, F.A.; Hacking, M.A.P.J.; Sheldon, R.A. Selective Hydroformylation of N-Allylacetamide in an Inverted Aqueous Two-Phase Catalytic System, Enabling a Short Synthesis of Melatonin. Chem. Commun. 2000, 2000, 1363–1364.
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin: Searching for Plants with High Levels as a Natural Source of Nutraceuticals. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, F.R.S., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2015; Volume 46, pp. 519–545.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
18 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




