Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bo, L.; Wang, Y.; Li, Y.; Wurpel, J.N.D.; Huang, Z.; Chen, Z. The Battlefield of Chemotherapy in Pediatric Cancers. Encyclopedia. Available online: https://encyclopedia.pub/entry/43009 (accessed on 07 February 2026).
Bo L, Wang Y, Li Y, Wurpel JND, Huang Z, Chen Z. The Battlefield of Chemotherapy in Pediatric Cancers. Encyclopedia. Available at: https://encyclopedia.pub/entry/43009. Accessed February 07, 2026.
Bo, Letao, Youyou Wang, Yi-Dong Li, John N. D. Wurpel, Zoufang Huang, Zhe-Sheng Chen. "The Battlefield of Chemotherapy in Pediatric Cancers" Encyclopedia, https://encyclopedia.pub/entry/43009 (accessed February 07, 2026).
Bo, L., Wang, Y., Li, Y., Wurpel, J.N.D., Huang, Z., & Chen, Z. (2023, April 13). The Battlefield of Chemotherapy in Pediatric Cancers. In Encyclopedia. https://encyclopedia.pub/entry/43009
Bo, Letao, et al. "The Battlefield of Chemotherapy in Pediatric Cancers." Encyclopedia. Web. 13 April, 2023.
Copy Citation
The survival rate for pediatric cancers has remarkably improved. Conventional chemotherapy plays a crucial role in treating pediatric cancers, especially in low- and middle-income countries where access to advanced treatments may be limited. The Food and Drug Administration (FDA) approved chemotherapy drugs that can be used in children have expanded, but patients still face numerous side effects from the treatment. In addition, multidrug resistance (MDR) continues to pose a major challenge in improving the survival rates for a significant number of patients.
chemotherapy
pediatric cancers
Multidrug resistance
1. Current Chemotherapy in Pediatric Cancer
There are currently various cancer treatment approaches for cancer. The main four lines of treatment are surgical removal, immunotherapy, radiotherapy, and chemotherapy [1]. Surgical removal has the longest history in cancer treatment. It is an essential treatment option and often is often performed together with other cancer treatments, depending on the type of cancer [2]. Immunotherapy is generally considered to have been applied in 1890 by Dr. Coley, who injected a mixture of live and inactivated Streptococcus and Serratia to achieve responses [3]. It modulates the immune system to act better against cancer, and it includes immune checkpoint inhibitors, T-cell transfer therapy, monoclonal antibodies, and treatment vaccines. Radiotherapy precisely delivers radiation to kill cancer cells and shrink tumors, and was started in 1896 by Emile Grubbé, who treated a breast cancer patient using X-rays [4]. Chemotherapy was first introduced after the Second World War, and works by interfering with cell proliferation. Other cancer treatment approaches include stem cell transplantation, targeted therapy, photodynamic therapy, and hormone therapy.
The efficient treatment of pediatric cancer started after 1945. Sidney Farber treated a 3-year-old patient with acute lymphoblastic leukemia (ALL) with aminopterin [5]. As a conventional cancer therapy, chemotherapy has played an important role in pediatric cancer over the past several decades. In pediatric diagnosis, ALL is the most common type of cancer. The OS of ALL increased from 57% in the 1970s to 96% in recent years [6]. This enormous progress is partly attributed to advances in chemotherapy [7]. Currently, chemotherapy has reached its limit, as severe toxicities have been observed in pediatric patients during treatment. Modifications to chemotherapy are made in order to reduce multiple toxicities. For instance, studies have provided evidence that chronomodulated chemotherapy, based on the body’s intrinsic circadian clock, might minimize toxicity while maintaining an anticancer activity [8]. High-dose chemotherapy has been explored for treating childhood malignant glioma so as to pass the blood−brain barrier (BBB), reduce cell chemoresistance, and achieve a more comprehensive response [9].
Chemo drugs are grouped by function, chemical structure, and interaction with other medications. Types of chemo drugs include alkylating agents, antimetabolites, antibiotics, topoisomerase inhibitors, mitotic inhibitors, and corticosteroids [10][11]. In 1997, under the Food and Drug Administration Modernization Act2 (FDAMA), a pediatric exclusivity provision was enacted by the U.S. Congress. Few chemo drugs were explicitly approved for treating pediatric cancer. The FDAMA later reauthorized as the Best Pharmaceuticals Act for Children (BPCA), which encourages pediatric drug studies in companies by providing a financial incentive. A total of 16 chemo drugs have been approved by the FDA with pediatric indications in the U.S. Before the FDAMA, only nine chemo drugs were approved for pediatric indications, see Table 1. After the FDAMA (1997–2022), seven chemo drugs were approved to treat childhood cancer (see Table 2).
Table 1. Chemo drugs approved prior to FDAMA, for which labeling includes pediatric indications [12].
| Drug | Original Approval |
Indications |
|---|---|---|
| Doxorubicin Hydrochloride | 7 August 1974 | Wilm’s Tumor and Other Childhood Kidney Cancers |
| Vincristine Sulfate | 10 July 1963 | ALL, Neuroblastoma, Non-Hodgkin Lyphoma, Rhabdomyosarcoma, Wilm’s tumor and other childhood kidney cancers |
| Cytarabine | 17 June 1969 | Acute Nonlymphocytic Leukemia |
| Cyclophosphamide | 16 November 1959 | ALL |
| Methotrexate Sodium (Trexall) | 10 August 1959 | ALL |
| Mercaptopurine (Purinethol, Purixan) | 11 September 1953 | ALL |
| Daunorubicin Hydrochloride (Rubidomycin) | 19 December 1979 | ALL |
| Procarbazine Hydrochloride (Matulane) | 22 July 1969 | Hodgkin Lymphoma |
| Dactinomycin (Cosmegen) | 10 December 1964 | Ewing sarcoma, gestational trophoblastic disease |
Data provided by National Cancer Institute (https://www.cancer.gov/), accessed on 30 January 2023, updated: 20 December 2022, and U.S. FDA (https://www.fda.gov/). The drugs and drug combinations are not listed here.
Table 2. Chemo drugs approved post FDAMA with pediatric specific indications (1997–2022) [12].
| Drugs | Original Approval | Pediatric Approval |
Indications for Pediatric Cancer |
|---|---|---|---|
| (Drugs approved post FDAMA with pediatric specific indications (1997–2022)) | |||
| Azacitidine (Vidaza) | 19 May 2004 | 20 May 2022 | JMML |
| Calaspargase Pegol-mknl (Asparlas) | same | 20 December 2018 | ALL |
| Everolimus | 1 November 2010 | 29 August 2012 | Giant Cell Astrocytoma |
| Asparaginase Erwinia Chrysanthemi (Erwinaze) |
same | 18 November 2011 | ALL |
| Clofarabine (Clolar) | same | 28 December 2004 | ALL |
| Pegaspargase (Oncaspar) | same | 24 July 2006 | ALL |
| Nelarabine (Arranon) | same | 28 October 2005 | Non-Hodgkin Lymphoma |
Data provided by National Cancer Institute (https://www.cancer.gov/), updated: 20 December 2022, and U.S. FDA (https://www.fda.gov/). JMML: juvenile myelomonocytic leukemia. The drugs and drug combinations are not listed here.
There are less drugs with pediatric indications worldwide compared with adult indications [13]. For some chemo drugs, the initial approval was only for adult indications. On 20 May 2022, Azacitidine was newly approved for childhood cancers, while it first approved on 19 May 2004 for treating all subtypes of myelodysplastic syndrome. Moreover, FDA approved these chemo drugs (Clofarabine, Nelarabine, Erwinia, and Mercaptopurine) for both adult and pediatric indications at the same time (Table 2). Although efforts have been made in pediatric chemotherapy, only a few chemo drugs have gained pediatric indications—there is a gap between pediatric and adult approval.
The therapy to treat pediatric cancers requires specific prerequisites and considerations, as patients are still growing and developing. Some adverse effects of cancer treatments are more severe for children than adults, as developing organs are more susceptible. Infection-related serious adverse events are more common in ALL patients receiving chemotherapy, which enhances the risk of death [14]. Therefore, it is necessary to identify drug resistance mechanisms to reduce chemotherapy intensity and toxicity. The counterstrategies against drug resistance are discussed in the following section.
2. Multidrug Resistance: The Challenge in Pediatric Cancer Chemotherapy
Cancer cells can develop resistance to one chemotherapeutic drug, as well as other chemotherapeutic drugs that may have different chemical properties and mechanisms of action, which is called multi-drug resistance (MDR) [15]. MDR can occur in both adult and pediatric cancer chemotherapy. Although the mechanisms of MDR have been studied for a few decades, MDR is a very limiting factor to the success of cancer chemotherapies. MDR exists not only in chemotherapy and radiation therapy, but also in newly developed therapies such as targeted therapy and immunotherapy [16][17]. Drug resistance can be divided into intrinsic resistance or extrinsic resistance based on the cause of its occurrence. Cancer cells may have inherent drug resistance before receiving chemotherapy. However, various adaptive responses of cancer cells during the treatment cause extrinsic or acquired drug resistance of cancer cells. Several MDR mechanisms have been discovered, yet they are not yet fully understood. As shown in Figure 1, the factors of MDR include increased drug inactivation, reduction of influx and increased efflux of drugs, decreased activation of prodrugs, epigenetic dysregulation, changes in cell surface markers, tumor microenvironment (TME), epithelial−mesenchymal transition (EMT), altered miRNA, disruption of responses to DNA damage, inhibition of apoptotic pathways, tumor heterogeneity, and cancer stem cells [15][18][19].
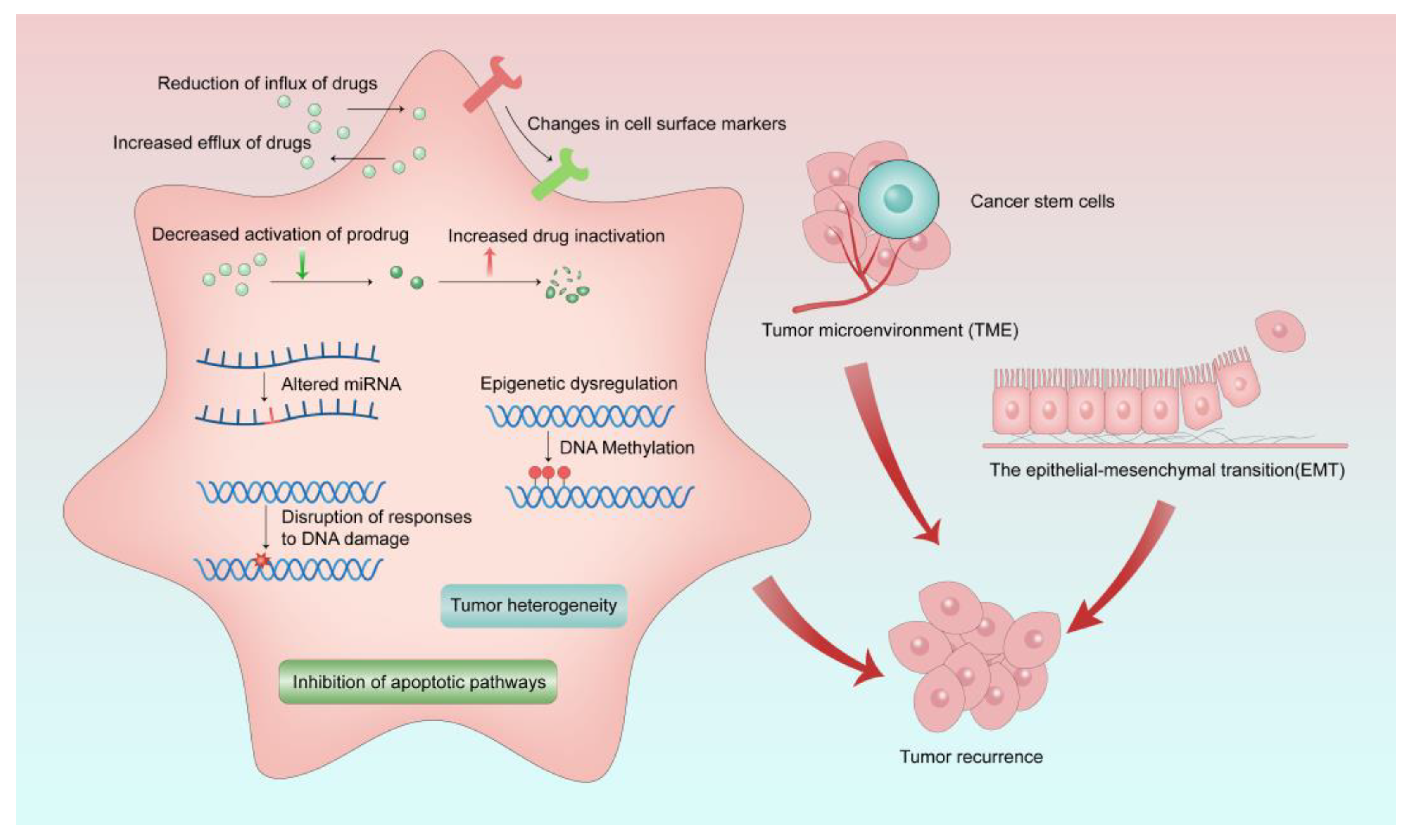
Figure 1. Mechanisms of MDR in cancer treatment. MDR occurs as a result of various causes, which ultimately lead to tumor recurrence.
3. Multidrug Resistance-Related Drug Transporters and Their Roles in Pediatric Cancers
Drug transporters are membrane proteins involved in the absorption, distribution, and excretion of drugs. Two transporter superfamilies have been identified in humans: the solute carrier (SLC) superfamily and the ABC superfamily [20]. ABC transporters are generally involved with the efflux of drugs, and SLC transporters have been chiefly described as influx transporters [21]. In addition to transporting therapeutic drugs across membranes, these transporters also mediate the transport of endogenous compounds. There is considerable interest in transporters from both families, as they are known to confer MDR to cancer cells.
3.1. Solute Carrier Family Transporters
SLC family transporters are a family that includes more than 300 membrane-bound proteins involved in the influx and efflux of a wide array of substrates, such as ions, metabolites, and drugs [22]. SLC transports substrates based on the electrochemical potential difference between the biological membrane or the ion gradient originally generated by the primary active transporters. Genetic variants of SLC transporters and clinical outcomes of methotrexate (MTX) have been studied in pediatric patients with ALL [23]. Although SLC transporter families are essential in human health, related studies focusing on pediatric cancer therapy are rare. Researchers limited their discussion to ABC transporters.
3.2. ABC Transporters
The ABC transporter superfamily, one of the most prominent transporter families, is responsible for MDR by mediating drug efflux, which subsequently leads to a low intracellular concentration of antineoplastic agents in cancer cells and deteriorates therapeutic outcome [24]. In addition, more than efflux pumps, other critical roles in cancer development of this transporter superfamily have been revealed step by step [25][26][27][28][29]. So far, 7 subfamilies (ABC-A to ABC-G) and at least 48 additional subfamily members have been found and characterized depending on their structural differences and similarities [30][31]. Among these subfamily members, three members have been demonstrated that are closely related to MDR in chemotherapy, including P-glycoprotein (P-gp/ABCB1/MDR1), multidrug resistance protein 1 (MRP1/ABCC1), and breast cancer resistance protein (BCRP/ABCG2) [32][33].
3.2.1. MDR1
The ABCB subfamily involves four full transporters (ABCB1/4/5/11) and 7 half transporters (ABCB2/3/6/7/8/9/10) that can transport a vast variety of molecules, including peptides, drugs, and ions [34]. Half transporters include two polypeptides, each having a transmembrane binding domain (TMD) and (nucleotide-binding domains) NBD to form a homo- or hetero-dimer. Full transporters are characterized as all four domains reside on a single polypeptide [32].
P-gp (also named ABCB1 or MDR1) is the first discovered and well-studied ABCB subfamily transporter that mediates MDR in cancer cells [35]. It is most expressed in the blood−brain barrier, liver, placenta, gallbladder, and endocrine tissues. In 2020, Nosol et al. revealed the protein structure of P-gp and found that inhibitors are bound in pairs and interact with structural features to block the function of P-gp [36]. As P-gp is the substrate of a broad range of antineoplastic agents, overexpressed P-gp has been demonstrated to lead to the development of drug resistance, including chemotherapeutic agents Vinca alkaloids (vinblastine and vincristine), Taxanes (paclitaxel and docetaxel), Anthracyclines (doxorubicin, daunorubicin, and epirubicin), and imatinib mesylate [37][38]. Meanwhile, a high-level expression of P-gp that causes cancer drug resistance has also been found in some tyrosine kinase inhibitors (Imatinib [39], GSK1070916 [40], and WYE354 [41]).
Altered expression levels of MDR1 have been detected in various tumors, such as neuroblastoma, rhabdomyosarcoma, and Wilm’s tumor. Neuroblastoma is the most common pediatric solid tumor, which accounts for 7–8% of childhood malignancies and 15% of all pediatric cancer deaths [42]. A study showed that the mRNA expression of MDR1 was increased in neuroblastoma patients with previous chemotherapy [43]. In addition, Qiu et al. [44] found that MDR1 hypermethylation expression can be associated with the pathogenesis and progression of neuroblastoma. MDR1 expression was observed to increase after chemotherapy in rhabdomyosarcoma and Wilm’s tumor [45]. These findings may be helpful to understand the role of MDR1 in pediatric malignancies regarding drug resistance and allow researchers to come up with strategies for therapeutic intervention.
3.2.2. Multidrug Resistance Proteins
Multidrug resistance proteins (MRPs) include 9 transporters from 13 members in the ABCC subfamily due to their ability to mediate cancer MDR [46].
Although each of the MRPs have slight differences in structures and amino acid compositions, the mechanism of transport driven by ATP hydrolysis is much the same. Unlike P-gp, which extrudes mostly xenobiotics, MRPs account for the extruding of both endo- and xenobiotics, thus showing its crucial role in regulating MDR processes in cancer development [47]. The structure of MRP1 has shown a novel substrate recruitment mechanism in that substrates are recruited straight from the cytoplasm, whereas P-gp attaches substrates from the inner leaflet of the lipid bilayer [48]. MRPs are distributed in the human body in various tissues, including the blood−brain barrier, brain, lung, kidney, liver, etc. [46].
The expression of MRPs has been investigated in several pediatric malignant tumors as they are a vital factor causing cytotoxic drug resistance and chemotherapy failure. Abnormal MDR expressions have been observed in pediatric malignancies, such as ALL, neuroblastoma, rhabdomyosarcoma, Wilm’s tumor, and retinoblastoma [49]. After chemotherapy, MRP1 expression has been observed to be upregulated in neuroblastoma, hepatoblastoma, and rhabdomyosarcoma patients [45]. Increased expression of MRP2-6 and decreased expression of MRP1 and MRP10 have been observed in ALL patients, which are associated with high doses of three chemotherapies [50]. Henderson et al. found that the inhibition of MPR1 is associated with reduced neuroblastoma development in transgenic mice [51]. These studies indicate their role in the chemotherapeutic drug efflux of MPRs and cancer prognosis.
3.2.3. Breast Cancer Resistance Protein
The human BCRP has 665 amino acid residues with a molecular weight of 72 kDa. It is a half-transporter that is prominently expressed in various tissues, including, but not limited to, the brain, placenta, testis, liver, breast, and BBB [52]. A high overexpression of BCRP can be observed in different drug resistance cancer types, including in solid tumors and hematopoietic malignancies [53][54]. Although the clinical significance of BCRP-mediated drug resistance remains unclear, many studies have shown strong evidence to support that modulating the expression of BCRP could enhance drug sensitivity in chemotherapy [55][56].
BCRP was first identified in 1998, followed by expression studies to explore its potential role in chemoresistance [57]. The expression of the BCRP gene in childhood ALL has been observed at a low expression level [58]. However, compared with a diagnosis when the co-expression of BCRP and MDR1 was observed, a higher RNA level of BCRP was expressed at the relapsed/refractory state in acute myeloid leukemia (AML) [59]. Correlations between BCRP and MRPs have also been reported. The combined high expression of BCRP and MRP4 is correlated with reduced antileukemia drug methotrexate accumulation. Similarly, evaluated expression of the BCRP gene was found in primary neuroblastoma mitoxantrone-resistant cells [60]. These results underscore the potential value of BCRP as a predictor of chemoresistance drug efflux.
Essentially, MDR can arise because of altered targeted proteins or cell signaling pathways. Changes in cellular or non-cellular processes is also a significant factor of MDR. However, most encountered MDRs are related to drug efflux, which is mainly caused by ABC transporters. Once MDR transporters are overexpressed, the efflux of a chemo drug can increase. For example, the overexpression of P-gp, MRP1, and BCRP decreases the chemosensitivity of cancer cells by limiting exposure to anticancer drugs [61]. The overexpression of P-gp in cancer cells is associated with increased drug resistance to DOX and paclitaxel [62][63]. After anticancer agent treatment, the overexpression of P-gp has been found in acute myeloid leukemia [64]. Decreased chemosensitivity and a high expression of P-gp and BCRP were noticed in MDR patients with chronic lymphocytic leukemia, metastatic breast cancer, and multiple myeloma [65]. BCRP is regulated by proteins such as TGF-β1 and VEGFR-2 [66][67].
References
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682.
- Nonnenmacher, L.; Hasslacher, S.; Zimmermann, J.; Karpel-Massler, G.; La Ferla-Bruhl, K.; Barry, S.E.; Burster, T.; Siegelin, M.D.; Bruhl, O.; Halatsch, M.E.; et al. Cell Death Induction in Cancer Therapy—Past, Present, and Future. Crit. Rev. Oncog. 2016, 21, 253–267.
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923.
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075.
- Fletcher, J.I.; Ziegler, D.S.; Trahair, T.N.; Marshall, G.M.; Haber, M.; Norris, M.D. Too many targets, not enough patients: Rethinking neuroblastoma clinical trials. Nat. Rev. Cancer 2018, 18, 389–400.
- Malczewska, M.; Kosmider, K.; Bednarz, K.; Ostapinska, K.; Lejman, M.; Zawitkowska, J. Recent Advances in Treatment Options for Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 2021.
- Shah, N. Dodging the bullet: Therapeutic resistance mechanisms in pediatric cancers. Cancer Drug Resist. 2019, 2, 428–446.
- Printezi, M.I.; Kilgallen, A.B.; Bond, M.J.G.; Stibler, U.; Putker, M.; Teske, A.J.; Cramer, M.J.; Punt, C.J.A.; Sluijter, J.P.G.; Huitema, A.D.R.; et al. Toxicity and efficacy of chronomodulated chemotherapy: A systematic review. Lancet Oncol. 2022, 23, e129–e143.
- Massimino, M.; Biassoni, V. Use of high-dose chemotherapy in front-line therapy of childhood malignant glioma. Expert Rev. Anticancer. Ther. 2006, 6, 709–717.
- Mir, M.A.; Sofi, S.; Qayoom, H. Chapter 4—Conventional adjuvant chemotherapy in combination with surgery, radiotherapy, and other specific targets. In Combinational Therapy in Triple Negative Breast Cancer; Mir, M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 95–120.
- Principe, D.R.; Kamath, S.D.; Korc, M.; Munshi, H.G. The immune modifying effects of chemotherapy and advances in chemo-immunotherapy. Pharmacol. Ther. 2022, 236, 108111.
- Barone, A.; Casey, D.; McKee, A.E.; Reaman, G. Cancer drugs approved for use in children: Impact of legislative initiatives and future opportunities. Pediatr. Blood Cancer 2019, 66, e27809.
- Nishiwaki, S.; Ando, Y. Gap between pediatric and adult approvals of molecular targeted drugs. Sci. Rep. 2020, 10, 17145.
- Xu, F.L.; Guan, X.M.; Wen, X.H.; Shen, Y.L.; Xiao, J.W.; Guo, Y.X.; Deng, M.Y.; Yu, J. Serious adverse events associated with chemotherapy in children with acute lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 828–833.
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47.
- Singh, M.S.; Tammam, S.N.; Shetab Boushehri, M.A.; Lamprecht, A. MDR in cancer: Addressing the underlying cellular alterations with the use of nanocarriers. Pharmacol. Res. 2017, 126, 2–30.
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166.
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560.
- Girardi, E.; Cesar-Razquin, A.; Lindinger, S.; Papakostas, K.; Konecka, J.; Hemmerich, J.; Kickinger, S.; Kartnig, F.; Gurtl, B.; Klavins, K.; et al. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 2020, 16, 469–478.
- Fang, X.; Liu, Y.; Xiao, W.; Zhao, N.; Zhu, C.; Yu, D.; Zhao, Y. Prognostic SLC family genes promote cell proliferation, migration, and invasion in hepatocellular carcinoma. Acta Biochim. Biophys. Sin. 2021, 53, 1065–1075.
- Hu, Y.H.; Zhou, L.; Wang, S.S.; Jing, X.; Guo, H.L.; Sun, F.; Zhang, Y.; Chen, F.; Xu, J.; Ji, X. Methotrexate Disposition in Pediatric Patients with Acute Lymphoblastic Leukemia: What Have We Learnt from the Genetic Variants of Drug Transporters. Curr. Pharm. Des. 2019, 25, 627–634.
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156.
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269.
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 52–60.
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020, 60, 57–71.
- Cui, Q.; Yang, Y.; Ji, N.; Wang, J.Q.; Ren, L.; Yang, D.H.; Chen, Z.S. Gaseous signaling molecules and their application in resistant cancer treatment: From invisible to visible. Future Med. Chem. 2019, 11, 323–336.
- Nobili, S.; Lapucci, A.; Landini, I.; Coronnello, M.; Roviello, G.; Mini, E. Role of ATP-binding cassette transporters in cancer initiation and progression. Semin. Cancer Biol. 2020, 60, 72–95.
- Juan-Carlos, P.M.; Perla-Lidia, P.P.; Stephanie-Talia, M.M.; Monica-Griselda, A.M.; Luz-Maria, T.E. ABC transporter superfamily. An updated overview, relevance in cancer multidrug resistance and perspectives with personalized medicine. Mol. Biol. Rep. 2021, 48, 1883–1901.
- Wang, J.Q.; Wu, Z.X.; Yang, Y.; Teng, Q.X.; Li, Y.D.; Lei, Z.N.; Jani, K.A.; Kaushal, N.; Chen, Z.S. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J. Evid. Based Med. 2021, 14, 232–256.
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718.
- Szollosi, D.; Rose-Sperling, D.; Hellmich, U.A.; Stockner, T. Comparison of mechanistic transport cycle models of ABC exporters. Biochim. Biophys. Acta Biomembr. 2018, 1860, 818–832.
- Sarkadi, B.; Homolya, L.; Szakacs, G.; Varadi, A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol. Rev. 2006, 86, 1179–1236.
- Nosol, K.; Romane, K.; Irobalieva, R.N.; Alam, A.; Kowal, J.; Fujita, N.; Locher, K.P. Cryo-EM structures reveal distinct mechanisms of inhibition of the human multidrug transporter ABCB1. Proc. Natl. Acad. Sci. USA 2020, 117, 26245–26253.
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234.
- Choudhuri, S.; Klaassen, C.D. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006, 25, 231–259.
- Rajamani, B.M.; Benjamin, E.S.B.; Abraham, A.; Ganesan, S.; Lakshmi, K.M.; Anandan, S.; Karathedath, S.; Varatharajan, S.; Mohanan, E.; Janet, N.B.; et al. Plasma imatinib levels and ABCB1 polymorphism influences early molecular response and failure-free survival in newly diagnosed chronic phase CML patients. Sci. Rep. 2020, 10, 20640.
- Wu, Z.X.; Yang, Y.; Wang, J.Q.; Zhou, W.M.; Chen, J.; Fu, Y.G.; Patel, K.; Chen, Z.S.; Zhang, J.Y. Elevated ABCB1 Expression Confers Acquired Resistance to Aurora Kinase Inhibitor GSK-1070916 in Cancer Cells. Front. Pharmacol. 2020, 11, 615824.
- Wang, J.; Yang, D.H.; Yang, Y.; Wang, J.Q.; Cai, C.Y.; Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Zhao, L.; Chen, Z.S. Overexpression of ABCB1 Transporter Confers Resistance to mTOR Inhibitor WYE-354 in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1387.
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021.
- Bourhis, J.; Benard, J.; Hartmann, O.; Boccon-Gibod, L.; Lemerle, J.; Riou, G. Correlation of MDR1 gene expression with chemotherapy in neuroblastoma. J. Natl. Cancer Inst. 1989, 81, 1401–1405.
- Qiu, Y.Y.; Mirkin, B.L.; Dwivedi, R.S. MDR1 hypermethylation contributes to the progression of neuroblastoma. Mol. Cell. Biochem. 2007, 301, 131–135.
- Oue, T.; Yoneda, A.; Uehara, S.; Yamanaka, H.; Fukuzawa, M. Increased expression of multidrug resistance-associated genes after chemotherapy in pediatric solid malignancies. J. Pediatr. Surg. 2009, 44, 377–380.
- Wang, J.Q.; Yang, Y.; Cai, C.Y.; Teng, Q.X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updat. 2021, 54, 100743.
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117.
- Arana, M.R.; Altenberg, G.A. ATP-binding Cassette Exporters: Structure and Mechanism with a Focus on P-glycoprotein and MRP1. Curr. Med. Chem. 2019, 26, 1062–1078.
- Fruci, D.; Cho, W.C.; Nobili, V.; Locatelli, F.; Alisi, A. Drug Transporters and Multiple Drug Resistance in Pediatric Solid Tumors. Curr. Drug Metab. 2016, 17, 308–316.
- Mehrvar, N.; Abolghasemi, H.; Rezvany, M.R.; Esmaeil Akbari, M.; Saberynejad, J.; Mehrvar, A.; Ehsani, M.A.; Nourian, M.; Qaddoumi, I.; Movafagh, A. Pattern of ABCC Transporter Gene Expression in Pediatric Patients with Relapsed Acute Lymphoblastic Leukemia. Rep. Biochem. Mol. Biol. 2019, 8, 184–193.
- Henderson, M.J.; Haber, M.; Porro, A.; Munoz, M.A.; Iraci, N.; Xue, C.; Murray, J.; Flemming, C.L.; Smith, J.; Fletcher, J.I.; et al. ABCC multidrug transporters in childhood neuroblastoma: Clinical and biological effects independent of cytotoxic drug efflux. J. Natl. Cancer Inst. 2011, 103, 1236–1251.
- Pena-Solorzano, D.; Stark, S.A.; Konig, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med. Res. Rev. 2017, 37, 987–1050.
- Khot, M.I.; Downey, C.L.; Armstrong, G.; Svavarsdottir, H.S.; Jarral, F.; Andrew, H.; Jayne, D.G. The role of ABCG2 in modulating responses to anti-cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101579.
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates with Clinical Implications. Front. Pharmacol. 2019, 10, 208.
- Jain, H.D.; Zhang, C.; Zhou, S.; Zhou, H.; Ma, J.; Liu, X.; Liao, X.; Deveau, A.M.; Dieckhaus, C.M.; Johnson, M.A.; et al. Synthesis and structure-activity relationship studies on tryprostatin A, an inhibitor of breast cancer resistance protein. Bioorg. Med. Chem. 2008, 16, 4626–4651.
- Zattoni, I.F.; Delabio, L.C.; Dutra, J.P.; Kita, D.H.; Scheiffer, G.; Hembecker, M.; Pereira, G.D.S.; Moure, V.R.; Valdameri, G. Targeting breast cancer resistance protein (BCRP/ABCG2): Functional inhibitors and expression modulators. Eur. J. Med. Chem. 2022, 237, 114346.
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670.
- Sauerbrey, A.; Sell, W.; Steinbach, D.; Voigt, A.; Zintl, F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2002, 118, 147–150.
- van den Heuvel-Eibrink, M.M.; Wiemer, E.A.; Prins, A.; Meijerink, J.P.; Vossebeld, P.J.; van der Holt, B.; Pieters, R.; Sonneveld, P. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 2002, 16, 833–839.
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233.
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807.
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441.
- Lal, S.; Wong, Z.W.; Sandanaraj, E.; Xiang, X.; Ang, P.C.; Lee, E.J.; Chowbay, B. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008, 99, 816–823.
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160.
- Wu, C.P.; Hsiao, S.H.; Huang, Y.H.; Hung, L.C.; Yu, Y.J.; Chang, Y.T.; Hung, T.H.; Wu, Y.S. Sitravatinib Sensitizes ABCB1- and ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. Cancers 2020, 12, 195.
- Ahmed, F.; Haass, N.K. Microenvironment-Driven Dynamic Heterogeneity and Phenotypic Plasticity as a Mechanism of Melanoma Therapy Resistance. Front. Oncol. 2018, 8, 173.
- Allen, J.D.; Brinkhuis, R.F.; Wijnholds, J.; Schinkel, A.H. The mouse Bcrp1/Mxr/Abcp gene: Amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999, 59, 4237–4241.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
521
Revisions:
3 times
(View History)
Update Date:
13 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




