Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Reza Akbari | -- | 6587 | 2023-04-12 18:28:41 | | | |
| 2 | Conner Chen | Meta information modification | 6587 | 2023-04-14 09:41:18 | | | | |
| 3 | Conner Chen | Meta information modification | 6587 | 2023-04-24 08:01:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, J.W.; Charkhchi, P.; Adekunte, S.; Akbari, M.R. Epidemiology of Young Age of Onset Breast Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/42998 (accessed on 07 February 2026).
Zhu JW, Charkhchi P, Adekunte S, Akbari MR. Epidemiology of Young Age of Onset Breast Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/42998. Accessed February 07, 2026.
Zhu, Jie Wei, Parsa Charkhchi, Shadia Adekunte, Mohammad R. Akbari. "Epidemiology of Young Age of Onset Breast Cancer" Encyclopedia, https://encyclopedia.pub/entry/42998 (accessed February 07, 2026).
Zhu, J.W., Charkhchi, P., Adekunte, S., & Akbari, M.R. (2023, April 12). Epidemiology of Young Age of Onset Breast Cancer. In Encyclopedia. https://encyclopedia.pub/entry/42998
Zhu, Jie Wei, et al. "Epidemiology of Young Age of Onset Breast Cancer." Encyclopedia. Web. 12 April, 2023.
Copy Citation
Breast cancer (BC) in young women is poorly understood and understudied in the literature. We need to design cohorts of breast cancer patients with young age of onset to study this particular group of patients better.
breast cancer
young women
epidemiology
1. Geographic Variations
International variations in breast cancer incidence and mortality are poorly studied in young women compared to the general population. The global incidence of BCYW increased by 16% since the 1990s and breast cancer is currently the most common cancer in young women, with 244,000 cases diagnosed per year [1]. According to an analysis of GLOBOCAN 2018 data, the average risk of developing breast cancer by the age of 40 years is 0.44%. Across continents, the average cumulative risk of developing breast cancer before 40 years varied by approximately two-fold, being highest in Oceania (0.69%), followed by Europe (0.63%), the Americas (0.53%), Africa (0.49%), and lowest in Asia (0.38%) [1]. National comparisons across 185 countries showed that the highest cumulative incidence rate was in South Korea (0.95%), followed by United Kingdom, United States, and Canada (0.77%, 0.61%, and 0.61%, respectively), and lowest in Guinea (0.13%) [1]. Although Asia in general has the lowest average breast cancer incidence before age 40, there is an approximately six-fold difference among Asian countries [1]. These variations may be attributable to a lack of public registries with accurate population data and the increasing “westernization” of lifestyle habits among some of the developing countries (i.e., dietary changes and decreased physical activity) that increase breast cancer risk [2].
Breast cancer mortality for women aged <40 years varies worldwide despite similar incidence rates, suggesting a large disparity in case fatality rates of BCYW by geographic region. For example, although Western Africa and North America had similar age-standardized breast cancer incidence rates in women <40 years at 9.8 and 11.3 per 100,000, respectively, mortality rates differed greatly at 6.4 per 100,000 for Western Africa compared to 1.8 per 100,000 for North America [1]. Globally, the average risk of dying from breast cancer by 40 years of age was 0.08% in 2018. However, Africa had a notably higher risk than the world average at 0.18%. In 2018, worldwide mortality rates varied nearly 6-fold across regions, from 1.1 per 100,000 in Eastern Asia to 6.4 per 100,000 in Western Africa for women aged <45 years [1]. Differences in screening policies are less likely to account for this disparity, as young women aged <40 years generally do not qualify for breast cancer screening in any country. Therefore, other factors, including varying levels of awareness for breast cancer symptoms, time from diagnosis to treatment, and differences in treatment plans and accessibility to care may influence the observed differences, as lower income countries have lesser funding and capacity for cancer treatment [3].
Compared to developed countries, there is a higher breast cancer incidence rate among young women from low- and middle-income countries (LMIC), while the incidence rates show an increasing trend in LMICs. Regression analysis using data from Global Burden of Disease 2019 determined the age-standardized rates of incidence (ASIRs) and mortality in 60 countries from 2000 through 2019 and found that 21 countries showed an increasing incidence of breast cancer in women aged <40 years, while 16 countries demonstrated a decreasing trend [4]. Low HDI (Human Development Index) countries including Ecuador, Fiji, and Mauritius had the most significant increases, while high HDI countries such as Norway showed the largest decrease [4]. The remaining 23 countries showed a stable trend [4][5][6][7]. A population-based analysis of 645,000 premenopausal women diagnosed with breast cancer worldwide in 2018 found that the greatest burden of premenopausal breast cancer occurred in LMICs, with premenopausal (aged <50 years) breast cancer accounting for 55.2% of total breast cancer cases in low HDI countries, compared to 20.7% in very high HDI countries [5]. Although LMICs have a higher proportion of total breast cancer cases diagnosed at <50 years of age, the age-specific incidence rate of premenopausal breast cancer is higher in developed countries. Population analysis found the highest ASIRs for premenopausal breast cancer in Western Europe (38.4 per 100,000) and New Zealand (36.7 per 100,000), which were more than double that of south-central Asia (12.0 per 100,000) and eastern Africa (15.2 per 100,000) [5]. Risk factors contributing to the higher incidence of premenopausal breast cancer in developed countries are incompletely understood. Reproductive factors—fewer children, nulliparity, and childbearing at later ages—more common in developed countries are associated with earlier onset, usually hormone receptor-positive (HR+), breast cancer. Another possible explanation is the varied screening practices across geographical locations. Although North American and European guidelines recommend starting mammography at 50 years, earlier screening may be more accessible to young women in developed countries compared to those in LMICs [5]. In fact, 14% of women aged 18–39 years in the USA at average risk of breast cancer received a mammogram from 2011 to 2015 [6]. Keating et al. found that 19% of the BCs diagnosed during annual screening over 10 years are over-diagnosed and would not have become clinically apparent in the absence of screening [7]. Other observational studies have reported varying overdiagnosis rates ranging up to 54% [8]. Further research is warranted to better elucidate the genetic and environmental risk factors implicated in the incidence rate disparities and increasing incidence in both LMICs and higher income countries.
Compared to developed countries, there is a disproportionate burden of breast cancer incidence and mortality among young women from low- and middle-income countries (LMIC). The age-standardised mortality for premenopausal breast cancer in low HDI countries (8.5 cases per 100,000) was more than double the mortality in very high HDI countries (3.3 cases per 100,000) [5]. For example, although Canada and Nigeria have similar cumulative incidence rates of premenopausal breast cancer (0.61% and 0.64%, respectively), Nigeria had more than six times the cumulative mortality rate at 0.25% vs. 0.04% for Canada [1]. Although the cumulative risk of developing breast cancer by age 40 years is greater (0.67%) in high income countries compared to LMICs (0.34%), the case fatality ratio is almost four-fold higher in LMICs at 0.30% compared to 0.08% in high income countries [1]. Disparities in case-fatality rates for BCYW may be attributable to the treatment advances, early diagnosis, and starting mammography screening programmes earlier in high-income countries that substantially improve survival [9]. Other factors contributing to the current disparities in mortality rates are lower breast cancer awareness and sociocultural barriers to care for women in LMIC regions [3][4][5][6][7][8][10].
2. Ethnic/Racial Differences
Current evidence suggests that there are ethnic disparities in breast cancer incidence and mortality among young women. Black women aged <35 years have a higher breast cancer incidence rate than White women, although the overall age-adjusted breast cancer incidence rate is higher for White compared to Black women [11]. A retrospective analysis of nine SEER cancer registries between 1995 and 2004 found that the age-adjusted incidence rate for Black women aged <40 years was 16% higher compared to White women aged <40 years (incidence rate ratio = 1.16; 95% CI: 1.10–1.23) [12]. In addition, the age-adjusted mortality for Black women aged <40 years was more than twice the rate for White women age <40 (mortality rate ratio = 2.07; 95%CI: 1.99–2.14) [12]. Similarly, a recent analysis using data from the National Cancer Registration and Analysis Service that included 24,022 women aged 30–46 at the time of breast cancer diagnosis found that all ethnic minority groups apart from Indian women had a significantly greater odds of less favourable tumour characteristics compared to White women [13]. Multivariate analysis in the same study for women aged 30–46 years found that Black women had higher odds of having less favourable tumour characteristics compared to White women, including more advanced stage disease (OR = 1.58; 95%CI: 1.29–1.92), high grade disease (OR = 1.40; 95% CI: 1.18–1.66), and ER-negative disease (OR = 1.36; 95% CI: 1.09–1.70) [13]. The POSH prospective study of 2915 breast cancer patients aged 18–40 years also reported similar findings of higher median tumour diameter and higher frequency of triple negative tumours in Black compared to White women (26.1% vs. 18.6%, respectively, p = 0.04) [13][14]. Studies have suggested that lower surveillance attendance rates and increased prevalence of risk factors (i.e., parity and breastfeeding, higher BMI and hormone replacement therapy) in Black women compared to White women contribute to the less favourable tumour characteristics in young women; however, further studies are required [15][16][17][18].
Interestingly, the incidence of BC peaks at age 50 in Eastern and Southeastern Asia compared to 70 years in the United States [19]. The age-specific incidence of BCs in East Asian women aged 59 years and younger had a greater increase compared to US patients in recent decades. In the 40–49 age range, the probability of having an estrogen receptor positive (ER+) BC is significantly higher (OR = 1.50, 95% CI: 1.36–1.67, p < 0.001) although the probability of having a triple-negative breast cancer (TNBC) is lower compared to Americans (OR = 0.79, 95% CI: 0.71–0.88, p < 0.001) [20]. Additionally, the incidence rates of BC in some younger East Asian populations have surpassed those of the United States [21]. The increase in the incidence of BC in East Asian populations has been linked to dietary and reproductive factors that are representative of “westernization”. Such factors include high fat intake, low vegetable consumption, reduced parity, delayed childbearing, less breastfeeding, and late menopause [22]. Further, a body mass index (BMI) increase of 5 kg/m2 is associated with an increased risk for premenopausal BC in Asian women. However, in African and Caucasian women, an inverse association has been observed [23].
In a study of women with BC aged 20–49 years, Black, American Indian or Alaska Native, and Hispanic women had a greater frequency of diagnosis in late stages compared to White and Asian or Pacific Islander women [24]. Additionally, a higher proportion of African American and Hispanic BC patients aged 15–39 experience delayed treatment after diagnosis compared to White Americans (p < 0.001). Further analysis showed that longer treatment delay time was a risk factor for shorter survival (p < 0.001) [25].
3. Risk Factors
Several risk factors are implicated in developing breast cancer in young women (Figure 1). Factors associated with BC development are classified into lifestyle risk factors (i.e., physical activity, body habitus, and alcohol consumption), inherent or genetic risk factors, reproductive risk factors, and iatrogenic risk factors.
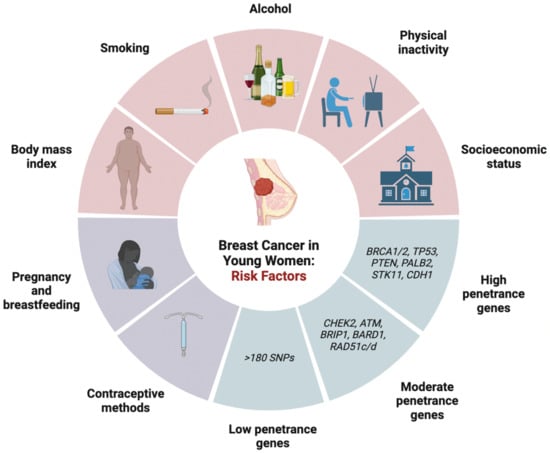
Figure 1. Risk factors associated with breast cancer in young women of onset development including lifestyle risk, genetic risk factors, and reproductive risk factors. SNPs: single nucleotide polymorphisms.
3.1. Lifestyle Risk Factors
Lifestyle risk factors include physical activity, body mass index (BMI), alcohol, smoking, socioeconomic status, and certain occupational conditions. The current evidence suggests that physical activity is associated with a dose-dependent reduction in the risk of early onset breast cancer for all types of activity and should be recommended. Previously, the consensus was that premenopausal BC risk is independent of physical activity levels, following several prospective cohort studies that reported no association [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51]. For example, a large prospective cohort study by Rockhill et al. examining the physical activity levels of 104,468 women over a 6-year follow-up period found that women who were more physically active (i.e., engaged in a strenuous activity at least twice per week for 10–12 months per year in late adolescence) did not have a lower risk of developing breast cancer compared to those who did not engage in physical activity (RR = 1.1; 95% CI: 0.8–1.6) [29]. However, recent data since then have contradicted these findings, with three meta-analyses concluding that physical activity significantly reduced the risk of developing premenopausal breast cancer [52][53][54]. In a meta-analysis of 6 studies that included 2258 cases, Wu et al. compared women in the highest versus lowest categories of physical activity and found that increased physical activity was inversely associated with breast cancer risk and a 23% reduction in breast cancer cases (RR = 0.77; 95%CI: 0.72–0.84) [52]. Similarly, Hardefelt et al. conducted a meta-analysis of 48 cohort studies with 236,955 breast cancer cases and 3,963,367 controls, and concluded that physical activity significantly reduced the overall risk (OR = 0.79; 95% CI: 0.73–0.87) [53]. Chen et al. examined 38 cohort studies with 68,416 breast cancer cases and found a reduced risk of developing premenopausal breast cancer with physical activity (RR = 0.83; 95% CI: 0.79–0.87) [54]. The main mechanism by which physical activity acts as primary prevention for breast cancer development is by lowering the cumulative exposure to circulating ovarian hormones [55][56][57]. Specifically, strenuous physical activity at the pre-pubertal stage delays the onset of regular ovulatory cycles, while activity during reproductive years reduces circulating ovarian hormone levels, frequency of regular cycles, and fat stores where androgen is converted to estrone [55]. Although emerging evidence suggests that increased intensity and duration of exercise are associated with lower breast cancer risk [58], it remains unclear whether specific types of physical activity (e.g., recreational, occupational, or non-occupational) are more strongly associated with a reduced risk of breast cancer in young women.
The effect of BMI on the risk of developing breast cancer differs between pre- and post-menopausal women. In post-menopausal women, there is a positive correlation between increasing BMI and breast cancer risk. In contrast, several studies have demonstrated that there is a modest protective effect of increased BMI that is inversely associated with the risk for developing breast cancer in young women [23][59][60][61][62][63][64][65]. Renehan et al. conducted a meta-analysis with 20 prospective cohort studies and found a dose–response effect with every 5 kg/m2 increase in BMI associated with a significant decrease in the relative risk (RR) of developing breast cancer in premenopausal women (RR = 0.92; 95% CI: 0.88–0.97) [59]. The underlying mechanism is unknown; it is hypothesized that obesity may lead to ovarian suppression and lower levels of circulating estradiol [66]. Although higher BMI is protective in young women, several studies have found that the risk reduction is offset by the larger cumulative post-menopausal risk for developing breast cancer later in life [59][67][68][69][70]. Interestingly, a meta-analysis by Amadou et al. that included nine case-control studies and three cohort studies found a significant dose–response increase in the relative risk of premenopausal BC (RR = 1.08; 95% CI:1.01–1.16) per 0.1 unit increase in waist-to-hip ratio (WHR), even though a simultaneous increase in BMI by 5 kg/m2 was associated with a significantly decreased risk (RR = 0.95; 95%CI: 0.94–0.97) [23]. This suggests that high general adiposity (indicated by BMI) reduces risk, while central adiposity (indicated by WHR) is conversely associated with an increased risk of premenopausal breast cancer. The Carolina Breast Study also found that BMI was inversely correlated with premenopausal breast cancer risk in White but not Black women [71], while a higher WHR adjusted for BMI was associated with increased breast cancer risk in both Black and White premenopausal women [72]. Overall, given that obesity is associated with increased risk for developing other malignancies and health complications, weight gain is not recommended as a method for breast cancer risk prevention.
Many studies examining the effect of alcohol consumption have found an increased risk of developing breast cancer in young women. In 1997, the first study examining the impact of alcohol intake among young women (defined as <45 years of age) by Swanson et al. found that women who drank >14 alcoholic beverages per week were more likely to develop breast cancer than non-drinkers (RR = 1.73; 95% CI: 1.2–2.6) [73]. Since then, several studies reported similar findings [74][75][76]. A pooled multivariate analysis of 3730 premenopausal women suggested a dose–response effect of alcohol on breast cancer and found that an incremental increase in alcohol consumption by 10g per day was associated with an increased risk of breast cancer (RR = 1.03; 95% CI: 0.99–1.08) [77]. Similarly, a meta-analysis conducted in 2018 by the World Cancer Research Fund also found a statistically significant elevated risk for developing breast cancer in premenopausal women, with a 5% increased risk for every 10 g increase in ethanol per day [78]. Interestingly, the same analysis found that different types of alcohol had varying effects on the risk for premenopausal breast cancer, with beer having the highest risk (RR = 1.32; 95%CI: 1.06–1.64) and spirits having the lowest (RR = 1.10; 95%CI: 0.92–1.30) [78].
The role of active smoking in BCYW risk has remained unclear since it was first discussed in 1982 [79][80]. Most studies concur that if smoking is implicated in breast cancer risk, it likely plays a more significant role in premenopausal compared to post-menopausal women [81][82]. Earlier age of smoking initiation appears to have a higher lifetime risk for breast cancer than those who start smoking later in life. Jones et al. conducted a cohort study with 1815 invasive breast cancer cases and found that the hazard ratio for women who started smoking before the age of 17 was 1.24 (95%CI: 1.08–1.43; p = 0.002) compared to non-smokers, which was higher than all “ever” smokers (HR = 1.14; 95%CI: 1.03–1.25) [83]. However, a meta-analysis demonstrated that the effect of smoking on breast cancer risk is confounded by its close association with alcohol consumption [80]. Other studies have also suggested that passive smoking may pose more risk for BCYW than active smoking. Active smoking is hypothesized to exert an anti-estrogenic effect that counteracts the risk associated with exposure to smoking-related carcinogens. In contrast, passive smoking concurs the same risk associated with carcinogen exposure over time without the protective anti-estrogenic effect [82][84][85]. A meta-analysis of 14 studies found that passive smoking was associated with an increased risk (pooled RR = 1.68; 95%CI: 1.88–2.12) [81]. Another study found that passive smoking increased the risk of premenopausal BC in carriers of PARP1 or ESR1 mutations (OR = 1.54; 95%CI: 1.14–2.07) [86]; however, further studies are required to elucidate individual risk levels stratified according to genetic susceptibility.
Data on the impact of socioeconomic status (SES) on population-level breast cancer risk are currently limited. A study by Akinyemiju et al. examining the SES of different ethnicities within a U.S. population found that the combined risk of both early and late breast cancer increased with higher SES [86]. Current evidence suggests that although women of higher SES in childhood or born to a mother with higher educational attainment are at higher risk of developing breast cancer, survival outcomes are better compared to those with lower income [87][88]. One possible explanation for these findings is that individuals from higher SES tend to be older at the time of their first pregnancy and have lower parity compared to those from lower SES [88].
Occupation-related long-term night shifts in young adulthood may be another factor contributing to the increased risk of developing breast cancer among young women. It is hypothesized that working overnight causes circadian rhythm disruption, as the “light at night” induces the suppression of melatonin production from the pineal gland [89]. Pre-clinical trials have demonstrated that melatonin is associated with tumour-suppressive effects through several mechanisms, including modulating estrogen production and exerting an anti-estrogenic effect [90]. As such, dysregulated or decreased melatonin production may promote tumour growth. According to an analysis of the Nurses’ Health Study II that included 116,430 female registered nurses aged 25–42 years with a 24-year follow-up, the risk of developing breast cancer was significantly elevated among those who had ≥20 years of cumulative rotating night shift work (HR = 1.40; 95%CI: 1.00–1.97) compared to those who did not [91]. These findings are supported by another case-control study that found night shift work was associated with higher breast cancer risk among pre-menopausal (OR = 1.33; 95%CI: 0.98–1.79) than post-menopausal women (OR 1.08; 95%CI: 0.82–1.42) [92]. More recent analyses have also reported that the odds ratio of developing pre-menopausal breast cancer was 1.26 (95% CI:1.06–1.51) for women who had ever worked night shifts compared to those who did not, and that the risk increased with both increasing frequency and number of years with night shift work [93][94].
3.2. Genetic Risk Factors
International guidelines recommend that patients younger than 50 years of age or with TNBC should be referred for genetic counselling. Common clinical features suggestive of hereditary breast cancer include high cancer incidence or the same type of cancer within a family, early age of onset (<50 years of age), different cancers in a person, bilateral disease, and multifocality. Timely identification of genetic mutations and screening of family members is crucial for prevention and treatment to improve long-term outcomes. Recent efforts have analyzed the mutational landscape of hereditary breast cancer using next-generation sequencing (NGS) and microarray genotyping. Predisposing mutations may be categorized based on their relative risk for developing a specific type of cancer as high-penetrant mutations (associated with cancer RR > 5) or moderate-penetrant mutations (RR = 1.5–5) and low-penetrant loci (RR < 1.5), with varying risks between different populations and age groups (Table 1) [95]. Approximately 5–10% of breast cancer cases are hereditary, of which 50% are estimated to be caused by deleterious mutations in high or moderate penetrance genes [96][97].
Table 1. Breast cancer susceptibility genes and breast cancer risk in young women from recent major population-based studies.
| Breast Cancer Susceptibility Gene | Study, Year | Patient Population (Age Group) | Odds Ratio | 95% Confidence Interval |
Penetrance |
|---|---|---|---|---|---|
| BRCA1 | Breast Cancer Association Consortium, 2021 [98] |
175 cases, 10 controls with mutation (<40 years) | 32.8 | 16.9–63.4 | High |
| Hu et al., 2021 [99] | 209 cases, 27 controls with mutation (<45 years) | 8.63 | 5.63–13.89 | High | |
| BRCA2 | Breast Cancer Association Consortium, 2021 [98] |
156 cases, 20 controls with mutation (<40 years) | 11.9 | 7.33–19.4 | High |
| Hu et al., 2021 [99] | 296 cases, 38 controls with mutation (<45 years) | 7.65 | 5.47–11.02 | High | |
| PALB2 | Breast Cancer Association Consortium, 2021 [98] |
26 cases, 8 controls with mutation (<40 years) | 5.36 | 2.26–12.7 | High |
| Hu et al., 2021 [99] | 15 cases, 5 controls with mutation (<45 years) | 3.99 | 2.50–6.67 | High | |
| CHD1 | Hu et al., 2021 [99] | 89 cases, 22 controls with mutation (<45 years) | 2.66 * | 1.00–8.38 | High |
| TP53 | Hu et al., 2021 [99] Breast Cancer Association Consortium, 2021 [98] |
NA † | NA † | NA † | High |
| PTEN | Hu et al., 2021 [99] Breast Cancer Association Consortium, 2021 [98] |
NA † | NA † | NA † | High |
| STK11 | Hu et al., 2021 [99] Breast Cancer Association Consortium, 2021 [98] |
NA † | NA † | NA † | High |
| CHEK2 | Breast Cancer Association Consortium, 2021 [98] |
77 cases, 28 controls with mutation (<40 years) | 4.54 | 2.87–7.17 | Moderate |
| Hu et al., 2021 [99] | 218 cases, 72 controls with mutation (<45 years) | 3.06 | 2.32–4.08 | Moderate | |
| ATM | Breast Cancer Association Consortium, 2021 [98] |
21 cases, 17 controls with mutation (<40 years) | 1.77 | 0.87–3.59 | Moderate |
| Hu et al., 2021 [99] | 162 cases, 80 controls with mutation (<45 years) | 1.89 | 1.43–2.53 | Moderate | |
| BARD1 | Breast Cancer Association Consortium, 2021 [98] |
6 cases, 3 controls with the mutation (<40 years) | 4.30 | 1.05–17.7 | Moderate |
| Hu et al., 2021 [99] | 33 cases, 22 controls with the mutation (<45 years) | 1.37 | 0.78–2.43 | Moderate | |
| RAD51C | Breast Cancer Association Consortium, 2021 [98] |
4 cases, 1 control with the mutation (<40 years) | 4.83 | 0.52–45.2 | Moderate |
| Hu et al., 2021 [99] | 26 cases, 20 controls with the mutation (<45 years) | 1.26 | 0.69–2.35 | Moderate | |
| RAD51D | Breast Cancer Association Consortium, 2021 [98] |
4 cases, 3 controls with the mutation (<40 years) | 1.76 | 0.38–8.17 | Moderate |
| Hu et al., 2021 [99] | 16 cases, 6 controls with the mutation (<45 years) | 2.41 | 0.91–7.60 | Moderate | |
| BRIP1 | Hu et al., 2021 [99] | 41 cases, 35 controls with the mutation (<45 years) | 1.22 * | 0.75–1.99 | Moderate |
| RAD51B | Hu et al., 2021 [99] Breast Cancer Association Consortium, 2021 [98] |
NA † | NA † | NA † | Moderate |
| XRCC2 | Hu et al., 2021 [99] | 21 cases, 13 controls with the mutation (<45 years) | 1.37 * | 0.69–2.83 | Moderate |
| XRCC3 | Hu et al., 2021 [99] Breast Cancer Association Consortium, 2021 [98] |
NA † | NA † | NA † | Moderate |
* Value is not statistically significant (p > 0.05). NA † indicates that age-specific odds ratio was not reported.
High-Penetrance Genes
High-penetrance genes include BRCA1, BRCA2, TP53, PTEN, STK11, CDH1, and PALB2 [100].
To date, BRCA1 and BRCA2 are the most common and widely studied breast cancer susceptibility genes, accounting for up to 40% of familial breast cancer. The BRCA1 gene codes for a nuclear phosphoprotein involved in DNA damage response, cell cycle progression, centrosome number, and regulating transcription, while BRCA2 encodes a protein responsible for double-stranded break repair during homologous recombination (HR) [101]. Unlike BRCA1-associated cancers, BRCA2 tumours often express estrogen and progesterone receptors with similar features as sporadic breast cancers. In a pooled analysis, the risk for breast cancer before the age of 40 years in carriers of BRCA1/2 germline mutations was 9.4% to 12% [100]. In an analysis of two population-based case-control studies involving 2013 women diagnosed with breast cancer before age 35 years and no family history and 225 affected women under the age of 45 years and a positive first-degree family history of breast cancer, Malone et al. found that 12% of women aged <45 years with a family history of breast cancer were carriers of BRCA1/2 mutations. The same study found that among women aged <35 years diagnosed with breast cancer and no family history, 9.4% (7.1% for BRCA1 and 4.9% for BRCA2) were carriers of germline mutations [102]. These results are consistent with a prospective cohort study by Copson et al. which found that 12% (338 of 2733) of breast cancer patients aged <40 years were carriers of germline BRCA1/2 mutations [103]. A recent study by the Breast Cancer Association Consortium that examined the risk of breast cancer for protein-truncating germline variants in nine genes found the highest breast cancer risk for age <40 years associated with BRCA1 (OR = 32.8; 95% CI: 16.9–63.4) and BRCA2 (OR = 11.9; 95% CI: 7.33–19.4) [98]. Similarly, the CARRIERS population-based study examined pathogenic variants in predisposition genes and risk of breast cancer in women < 45 years of age and reported the strongest association between BRCA1 (OR = 8.63; 95% CI: 5.63–13.89; p < 0.001) and BRCA2 (OR = 7.65; 95%CI: 5.47–11.02; p < 0.001), and the risk of developing breast cancer before the age of 45 years (Table 1) [99].
TP53 mutations are highly penetrant and associated with several cancers, of which early-onset breast cancer is the most common tumour type among women with germline TP53 mutations [104]. The protein encoded by the TP53 gene responds to cellular stress and is implicated in regulating the expression of genes in different pathways, inducing cell cycle arrest, senescence, apoptosis, and DNA repair [104]. The lifetime risk for developing breast cancer in women who are TP53 mutation carriers is approximately 50% [105]. Among women <35 years of age diagnosed with breast cancer, the frequency of germline TP53 mutations ranges from 1–7% and can reach up to 30% in those diagnosed before the age of 30 [98][105][106][107][108][109][110].
PTEN encodes a protein that suppresses the P13K/Akt/mTOR pathway and regulates cell metabolism, proliferation, and survival [111][112]. Germline pathogenic PTEN mutations are uncommon and associated with a constellation of clinical manifestations, including Cowden syndrome [113]. Breast cancer is the most commonly diagnosed malignancy among women with Cowden syndrome, with a lifetime risk of 85%, typically associated with thyroid and endometrial tumours, and the age of diagnosis between 38 and 46 years [114][115]. Studies have demonstrated that the frequency of PTEN mutations in women <40 years of age is <1% and estimated that the risk of developing breast cancer for all ages is 2- to 5-fold higher among PTEN mutations carriers compared to noncarriers [114][115][116].
STK11/LKBI encodes a tumour suppressor protein involved in cell metabolism, proliferation, and p53-dependent apoptosis. Pathogenic STK11 mutations increase susceptibility to breast, pancreatic, and gastrointestinal cancers, with a cumulative incidence of 45% [116]. However, Hearl et al. found an only 8% risk of breast cancer by the age of 40 among women with STK11/LKB1 pathogenic variants, with a non-significant difference (log-rank test of difference = 0.62; p = 0.43) between carriers of STK11/LKB1 mutations compared to non-carriers [117].
Loss of function mutations in CDH1 that encodes E-cadherin are associated with increased cell proliferation, invasion, and metastasis. CDH1 germline mutations are associated with autosomal dominant hereditary diffuse gastric carcinoma (HDGC), of which approximately 30% of patients present with invasive lobular breast cancer [118][119][120]. Female carriers of pathogenic CDH1 mutations have a 40–54% lifetime risk of developing lobular breast cancer, typically presenting with an early-onset disease with a mean age at diagnosis of 40 years and bilateral breast cancer [105][121][122][123][124].
PALB2 encodes a tumour suppressor protein that recruits BRCA2 to DNA damage sites. Heterozygous germline PALB2 mutations are associated with an increased risk for breast cancer, with previous studies reporting a higher penetrance in younger than older women. Antoniou et al. analyzed the risk of breast cancer among 362 women with identified deleterious PALB2 mutations and found that the risk of developing breast cancer was 8- to 9-fold higher in PALB2 mutation carriers who were <40 years of age and 5- to 8-fold higher in those >40 years of age compared to the general population [125]. The same study reported that the cumulative breast cancer risk among PALB2 mutation carriers was 14% (95% CI: 9–20) by 50 years of age [125]. Further, the odds ratio for PALB2 was reported to be 5.36 (95% CI: 2.26–12.7) for the <40 age group [98].
Moderate-Penetrance Genes
Moderate penetrance genes include CHEK2, ATM, BRIP1, BARD1, RAD51C, and RAD51D, which account for approximately 5% of the hereditary risk [100].
CHEK2 encodes a G2 checkpoint kinase that responds to DNA damage and prevents mitosis. A meta-analysis by Weischer et al. with 26,000 cases and 27,000 controls found that heterozygotes of CHEK2*1100delC had an OR of 2.6 (95% CI, 1.3–5.5) for developing early-onset BC compared to non-carriers [126]. The same study found that approximately 0.64% of all early-onset cases (<51 years) were heterozygous for CHEK2*1100delC, premenopausal and with ER+ disease. Several studies have identified CHEK2 mutations in specific ethnic populations. Rashid et al. assessed the prevalence of CHEK2 germline mutations in 145 BRCA1/2-negative breast cancer patients from Pakistan and found a low frequency (1.4%) of two potentially deleterious missense mutations, c.275C>G (p.P92R) and c.1216C>T (p.R406C) in women aged <40 years [127]. In another study, the CHEK2 Y390C (1169A>G) mutation was found in 8% (12 of 150) of young Chinese women with BC aged <35 years that were significantly higher than controls [128].
The ATM gene encodes a PI3/PI4-kinase involved in cell cycle checkpoint signaling pathways that are sensitive to DNA damage and it regulates p53, BRCA1, and RAD17, among others. Thompson et al. conducted a prospective study involving 247 heterozygous carriers of ATM mutations and found a higher relative risk for developing breast cancer in carriers <50 years of age (RR = 4.94; 95%CI: 1.9–12.9) compared to the general population [129]. In another study by Maillet et al., which included 94 patients <40 years of age diagnosed with breast cancer without any family history of breast cancer and 140 healthy controls, they identified germline ATM variants among 10 breast cancer cases (10.6%, 95%CI: 5.2–18.7%) with no mutation carrier found in the control group (p = 0.0006) [130]. Similarly, Teraoka et al. analyzed genomic DNA samples of women diagnosed with breast cancer before 45 years of age compared to matched controls, and detected ATM mutations among 11 of the 142 breast cancer cases (7.7%; 95% CI, 3.9 –13.4%) compared to 1 of 81 controls (1.2%; 95% CI, 0.0–6.7%) (p = 0.06) [131]. In this study, all the cases with identified mutations had a first-degree family history of breast cancer (OR:12.1; 95% CI, 6.2–20.6, p = 0.02). Several other studies have reported similar findings, with ATM mutations identified in women <45 years of age and a higher frequency in cases with a positive family history of breast cancer [132][133][134][135][136].
BRIP1 mutations are associated with breast and ovarian cancers and encode a protein that interacts with BRCA1 and is involved in double-stranded DNA break repair. Couch et al. analyzed germline DNA samples from 1824 cases of TNBC and identified BRIP1 mutations in 8 patients with a mean age of diagnosis of 46 years (range 36–68), suggesting that BRIP1 mutations may be more common in women with earlier onset and TNBC [137]. Other studies have also identified BRIP1 mutations (c.2392C>T) in an Irish cohort comprising patients with breast cancer diagnosed at <42 years of age and the p.Q994E mutation in Chinese patients with early-onset breast cancer diagnosed at <35 years of age [138][139]. Overall, studies have estimated that there is a 1.2- to 3.2-fold higher risk for developing breast cancer in patients <40 years of age with identified BRIP1 mutations compared to non-carriers, and that 1% of patients with early-onset or familial breast cancer carried a deleterious BRIP1 mutation [133][134][137].
The BARD1 protein interacts with BRCA1 and is thought to play a critical role in BRCA1-mediated tumour suppression [140]. Studies have reported polymorphic variants associated with a 2.5-fold increased risk for developing early-onset breast cancer [136][141][142]. In addition, the p.Cys557Ser variant was first identified in the analysis of fresh frozen tissue in 5 women with BRCA-negative breast cancer within an Italian family and was absent in controls [143], and later found to have a frequency of 7.4% in 94 Finish breast cancer patients whose family history did not include ovarian cancer [144]. The c.Cys557Ser mutant allele had a 2.8% frequency in an Icelandic population with breast cancer compared to 1.6% in controls (OR = 1.82; 95%CI: 1.11–3.01; p = 0.014) [140]. In this study, the two patients who were homozygous for the p.Cys557Ser variant were diagnosed with breast cancer at the ages of 41 and 47 [140].
The RAD51 family (RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3) transduces the DNA damage signal and is a critical protein in homologous recombination through p53 interactions. In an association analysis that used a panel of 34 putative susceptibility genes to perform sequencing on samples from 60,466 women with breast cancer and 53,461 controls, pathogenic germline variants in RAD51C (OR = 4.83; 95% CI: 0.52–45.2) and RAD51D (OR = 1.76; 95% CI: 0.38–8.17) were both found to be associated with the risk of developing breast cancer before 40 years of age [98]. Another study analyzed data from 7216 families, among which 215 women had RAD51C pathogenic variants and 92 women had RAD51D pathogenic variants. For RAD51C, a higher BC relative risk in younger women aged 20–49 years (RR = 2.42; 95% CI: 1.61–3.63) was observed compared with older women ≥50 years (RR = 1.36, 95% CI: 0.70–2.63). For RAD51D, the RR for the 20–49 age group was 1.84 (95% CI: 1.12–3.02); whereas, the RR for the 50–79 age group was 1.83 (95% CI: 1.02–3.26) [145]. The estimated cumulative risks of developing BC by age 50 years were 4% (95% CI = 3% to 6%) for carriers of pathogenic variants in RAD51C and 4% (95% CI = 2% to 5%) for RAD51D carriers [145].
Low-Penetrance Genes
Over 180 single nucleotide polymorphisms (SNPs) identified by genome wide association studies (GWAS) are considered low penetrance for breast cancer and account for 18% of the hereditary risk [100]. These studies involving large samples of cases and controls have allowed the identification and assessment of low-risk loci and led to the development of a polygenic model for breast cancer [146][147][148]. However, the relevance of polygenic risk score to BCYW has not been studied yet.
2.3.3. Reproductive Risk Factors
Several hormonal and reproductive factors, including hormonal contraceptives, hormonal therapy, pregnancy, and breastfeeding, may influence the risk for BCYW (Table 2). There has been an increased usage of exogenous hormonal medications, which include oral contraceptive pills (OCPs), intrauterine devices (IUDs), and menopausal hormonal therapy (MHT). In the past few decades, there has also been a shift within high-income countries towards having fewer children per household and pregnancies later in life.
Table 2. Comparison between lifestyle and reproductive risk factors in pre-menopausal and post-menopausal women.
| Pre-Menopausal Women | Post-Menopausal Women | |
|---|---|---|
| Lifestyle Risk Factors | ||
| Physical Exercise | Reduces risk of breast cancer | Reduces risk of breast cancer [149] |
| BMI | Increasing BMI has a modest protective effect against breast cancer | Increasing BMI associated with increased risk of breast cancer |
| Alcohol Consumption | Increases risk of breast cancer | Increases risk of breast cancer [150] |
| Smoking | Increases risk of breast cancer, Higher risk in younger age of initiation | Increases risk of breast cancer [85] |
| Socioeconomic status (SES) | Increasing risk with higher SES | Increasing risk with higher SES |
| Occupational related long-term night shifts | Increases risk of breast cancer | Increases risk of breast cancer |
| Reproductive Factors | ||
| Oral contraceptive pills (OCPs) | Increases risk of breast cancer | Increases risk of breast cancer [151] |
| Levonorgestrel-releasing intrauterine system (LNG-IUS) | Increases risk of breast cancer | Increases risk of breast cancer [152] |
| Menopausal hormonal therapy (MHT) | NA | Does not increase the risk of breast cancer |
| Age of pregnancy | Parity before 20 years of age is associated with reduced risk of breast cancer, Parity after 35 years of age is associated with increased risk | Older age of first pregnancy is associated with higher risk of breast cancer [153] |
| Fertility preservation techniques | Does not increase the risk of breast cancer | Does not increase the risk of breast cancer |
| Breastfeeding | Reduces risk of breast cancer | Reduces risk of breast cancer |
NA indicates “Not Available”.
In 2018, OCPs were the most common contraception method used in women aged 15 to 49 [154]. In 1996, a large dataset study first suggested an association between combined OCP (i.e., estrogen and progesterone preparations) and increased breast cancer risk. The analysis included 53,297 breast cancer cases and 1,000,239 controls and found a 1.24-fold elevated risk for breast cancer in combined OCP users (RR = 1.24; 95%CI: 1.15–1.33) compared to controls [155]. For every 20,0000 women aged 20–25 years using OCPs, there is one woman who would develop breast cancer. The study also found that the modestly elevated risk resolved after 10 years (RR = 1.01; 95% CI: 0.96–1.05) [155]. Data for OCP use in high-risk women who have genetic risk factors or positive family history are limited, but the current evidence suggest a similar effect of OCP use on BC risk compared to the overall population [156]. For high-risk individuals with BRCA1 or BRCA2 mutations considering combined OCPs, genetics consultation should be considered to judge the competing impacts of increased breast cancer risk against individual needs and potential protective effects against ovarian cancer [156]. In a prospective cohort study by Mørch et al. that examined the association between breast cancer and OCP use in 1,797,932 Danish women aged 15 to 49 years over a mean follow-up period of 11 years, the authors found a higher risk of breast cancer among those who currently or recently used combined OCPs (RR = 1.20 (95%CI: 1.14 to 1.26) and this increased with duration of use, from 1.09 (95% CI, 0.96 to 1.23) with <1 year of use to 1.38 (95% CI, 1.26 to 1.51) after more than 10 years of use (p = 0.002) [157]. Few studies have examined progestin-only contraceptives and breast cancer risk. One study analyzing a cohort of 93,843 women who used the levonorgestrel-releasing intrauterine system (LNG-IUS) reported a modestly elevated breast cancer risk (RR = 1.19; 95%CI: 1.13–1.25) compared to the general incidence rate among Finnish women younger than 55 years of age [158]. Similarly, a recent systematic review and meta-analysis found an overall increased breast cancer risk (OR = 1.16; 95%CI: 1.06–1.28) in LNG-IUS users aged <50 years, which increased in women over 50 years (OR = 1.52 (95%CI: 1.34–1.72) [152]. However, considering the potential benefits of OCP use with long-term follow-up data on women using the combined OCP suggesting protective effects against ovarian, endometrial, and colorectal cancer, and the clinical indications for progesterone OCPs in controlling menorrhagia, risk–benefit counselling should be provided on an individual basis before commencing hormonal contraception [159].
Premature menopause, defined as occurring <40 years of age without iatrogenic causes, is rare and affects approximately 1% of women. Menopausal hormonal therapy (MHT) is often recommended in this population for symptom relief or bone protection, and may contain either unopposed estrogen or combined estrogen and progestin preparations. In 2019, the Collaborative Group on Hormonal Factors in Breast Cancer conducted a large meta-analysis of international epidemiological evidence for the type and timing of MHT associated with breast cancer risk. This study reported that the use of MHT for 5 to 15 years, starting between the ages of 30–39 years, was not associated with a statistically significant increased risk (RR = 1.07; 95% CI: 0.88–1.31) of breast cancer [17]. Current guidelines recommend that average-risk women with early menopause can be offered either combined HRT if their uterus is intact or estrogen-only HRT if their uterus has been removed up to the natural age of menopause (typically 51–52 years), but more caution should be exercised with the lowest dose and shortest duration, and specialist input for high-risk patients [160].
Age of pregnancy has been shown to influence breast cancer risk. In developed countries, approximately 13% of breast cancers are diagnosed during the reproductive years between 20 to 44 years of age, and about 2% of women are diagnosed before 35 years of age [161]. Parity before 20 years of age is associated with the largest long-term risk reduction of 50% compared to nulliparous women [161], while being >35 years of age at the time of first pregnancy is associated with increased breast cancer risk (incidence RR = 1.18; 95%CI: 1.04–1.34) compared to nulliparous women [162]. However, there is a transiently increased breast cancer risk post-partum that is attributed to the involution process within the breast. Breast remodeling is hypothesized to occur after delivery due to the lactational changes in the post-partum period, and the increased risk associated with immune micro-environmental changes may last for up to 10 years [163]. Following this period, the lower breast cancer risk is likely due to a reduction in the ER-sensitive epithelial cells in breast tissue [163]. Parous women have an increased breast cancer risk peaking 5 years after delivery (HR = 1.80; 95%CI: 1.63–1.99) and this decreases to 0.77 (95%CI: 0.67–0.88) 34 years later [164]. The 1993–2001 Carolina Breast Cancer Study included 1809 White and 1505 African American women and found important racial differences in the effect of parity on breast cancer risk in young women between 20 to 49 years of age [165]. Among young African American women, multiparity was associated with increased breast cancer risk (OR for 3–4 pregnancies: = 1.5, 95%CI: 0.9–2.6; OR for ≥5 pregnancies = 1.4; 95% CI: 0.6–3.1) [165]. In contrast, White women did not appear to have increased risk (OR for 3–4 pregnancies = 0.7; 95% CI: 0.4–1.2 and OR for ≥5 pregnancies = 0.8, 95%CI: 0.2–3.0) [165]. A prospective cohort study examining 7152 Chinese women with primary breast cancer also found that younger patients aged <40 years were more likely to be nulliparous compared to patients ≥40 years of age (43.3% vs. 17.8%; p < 0.001) [166]. Future studies are necessary for detailed comparisons of breast cancer risk in young women between different racial and ethnic groups.
Women who have children later in life may opt for fertility techniques such as oocyte harvesting, oocyte cryopreservation, embryo transfer, or in vitro fertilization (IVF). No association (HR = 0.79; 95%CI: 0.46–1.36) has been found between fertility preservation techniques and increased breast cancer risk, including in high-risk patients with BRCA1/2 mutations [167]. However, a systematic review found limited evidence on the effect of IVF on breast cancer in premenopausal women and further research is required [168].
Breastfeeding has been reported to reduce breast cancer risk [169]. Although the risk reduction mechanism is currently unclear, the relative risk reduction of 4% for every 12 months of breastfeeding for women of all ages and a greater reduction of 5.1% for premenopausal breast cancer have been reported [170][171]. Studies have found a similar protective effect of breastfeeding for hormone receptor-positive and negative breast cancer subtypes; although, there was a stronger inverse association reported for TNBC (OR: 0.78; 95% CI: 0.66–0.91) which is more common in younger women [172][173]. According to the World Health Organization recommendations, young mothers should be supported to ensure breastfeeding is continued for at least six months before weaning to benefit from its protective effect [174].
References
- Sopik, V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res. Treat. 2020, 186, 497–507.
- Narod, S.A. Breast cancer in young women. Nat. Rev. Clin. Oncol. 2012, 9, 460–470.
- Bellanger, M.; Zeinomar, N.; Tehranifar, P.; Terry, M.B. Are Global Breast Cancer Incidence and Mortality Patterns Related to Country-Specific Economic Development and Prevention Strategies? J. Glob. Oncol. 2018, 4, 1–16.
- Luo, C.-Y.; Na Li, N.; Bin Lu, B.; Cai, J.; Lu, M.; Zhang, Y.-H.; Chen, H.-D.; Dai, M. Global and regional trends in incidence and mortality of female breast cancer and associated factors at national level in 2000 to 2019. Chin. Med. J. 2021, 135, 42–51.
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037.
- Qin, J.; White, M.C.; Sabatino, S.A.; Febo-Vázquez, I. Mammography use among women aged 18–39 years in the United States. Breast Cancer Res. Treat. 2017, 168, 687–693.
- Keating, N.L.; Pace, L.E. New Guidelines for Breast Cancer Screening in US Women. JAMA 2015, 314, 1569–1571.
- Nelson, H.D.; Pappas, M.; Cantor, A.; Griffin, J.; Daeges, M.; Humphrey, L. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 256–267.
- DeSantis, C.E.; Ma, J.; Jemal, A. Trends in stage at diagnosis for young breast cancer patients in the United States. Breast Cancer Res. Treat. 2018, 173, 743–747.
- Yip, C.H.; Anderson, B.O. The Breast Health Global Initiative: Clinical practice guidelines for management of breast cancer in low- and middle-income countries. Expert Rev. Anticancer Ther. 2007, 7, 1095–1104.
- Smigal, C.; Jemal, A.; Ward, E.; Cokkinides, V.; Smith, R.; Howe, H.L.; Thun, M. Trends in Breast Cancer by Race and Ethnicity: Update 2006. CA Cancer J. Clin. 2006, 56, 168–183.
- Baquet, C.R.; Mishra, S.I.; Commiskey, P.; Ellison, G.L.; DeShields, M. Breast Cancer Epidemiology in Blacks and Whites: Disparities in Incidence, Mortality, Survival Rates and Histology. J. Natl. Med. Assoc. 2008, 100, 480–489.
- Gathani, T.; Reeves, G.; Broggio, J.; Barnes, I. Ethnicity and the tumour characteristics of invasive breast cancer in over 116,500 women in England. Br. J. Cancer 2021, 125, 611–617.
- Copson, E.; Eccles, B.; Maishman, T.; Gerty, S.; Stanton, L.; Cutress, R.I.; Altman, D.G.; Durcan, L.; Simmonds, P.; Lawrence, G.; et al. Prospective Observational Study of Breast Cancer Treatment Outcomes for UK Women Aged 18–40 Years at Diagnosis: The POSH Study. Gynecol. Oncol. 2013, 105, 978–988.
- Lambertini, M.; Santoro, L.; Del Mastro, L.; Nguyen, B.; Livraghi, L.; Ugolini, D.; Peccatori, F.; Azim, H.A. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 2016, 49, 65–76.
- Hidayat, K.; Yang, C.-M.; Shi, B.-M. Body fatness at a young age, body fatness gain and risk of breast cancer: Systematic review and meta-analysis of cohort studies. Obes. Rev. 2017, 19, 254–268.
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168.
- Gathani, T.; Ali, R.; Balkwill, A.; Green, J.; Reeves, G.; Beral, V.; Moser, K.A.; on behalf of the Million Women Study Collaborators. Ethnic differences in breast cancer incidence in England are due to differences in known risk factors for the disease: Prospective study. Br. J. Cancer 2013, 110, 224–229.
- Shin, H.-R.; Joubert, C.; Boniol, M.; Hery, C.; Ahn, S.H.; Won, Y.-J.; Nishino, Y.; Sobue, T.; Chen, C.-J.; You, S.-L.; et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control 2010, 21, 1777–1785.
- Lin, C.-H.; Yap, Y.S.; Lee, K.-H.; Im, S.-A.; Naito, Y.; Yeo, W.; Ueno, T.; Kwong, A.; Li, H.; Huang, S.-M.; et al. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. Gynecol. Oncol. 2019, 111, 1298–1306.
- Sung, H.; Rosenberg, P.S.; Chen, W.-Q.; Hartman, M.; Lim, W.-Y.; Chia, K.S.; Mang, O.W.-K.; Chiang, C.-J.; Kang, D.; Ngan, R.K.-C.; et al. Female Breast Cancer Incidence Among Asian and Western Populations: More Similar Than Expected. Gynecol. Oncol. 2015, 107, djv107.
- Porter, P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N. Engl. J. Med. 2008, 358, 213–216.
- Amadou, A.; Ferrari, P.; Muwonge, R.; Moskal, A.; Biessy, C.; Romieu, I.; Hainaut, P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: A systematic review and dose-response meta-analysis. Obes. Rev. 2013, 14, 665–678.
- Shoemaker, M.L.; White, M.C.; Wu, M.; Weir, H.K.; Romieu, I. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res. Treat. 2018, 169, 595–606.
- Smith, E.C.; Ziogas, A.; Anton-Culver, H. Delay in Surgical Treatment and Survival After Breast Cancer Diagnosis in Young Women by Race/Ethnicity. JAMA Surg. 2013, 148, 516–523.
- Dorgan, J.F.; Brown, C.; Barrett, M.; Splansky, G.L.; Kreger, B.E.; D’Agostino, R.B.; Albanes, D.; Schatzkin, A. Physical Activity and Risk of Breast Cancer in the Framingham Heart Study. Am. J. Epidemiol. 1994, 139, 662–669.
- Thune, I.; Brenn, T.; Lund, E.; Gaard, M. Physical Activity and the Risk of Breast Cancer. N. Engl. J. Med. 1997, 336, 1269–1275.
- Cerhan, J.; Chiu, B.C.-H.; Wallace, R.B.; Lemke, J.H.; Lynch, C.F.; Tomer, J.C.; Rubenstein, L.M. Physical Activity, Physical Function, and the Risk of Breast Cancer in a Prospective Study Among Elderly Women. J. Gerontol. Ser. A 1998, 53, M251–M256.
- Rockhill, B.; Willett, W.C.; Hunter, D.J.; Manson, J.E.; Hankinson, S.E.; Spiegelman, D.; Colditz, G.A. Physical activity and breast cancer risk in a cohort of young women. Gynecol. Oncol. 1998, 90, 1155–1160.
- Sesso, H.D.; Lee, I.-M.; Paffenbarger, R.S.P., Jr. Physical activity and breast cancer risk in the College Alumni Health Study (United States). Cancer Causes Control 1998, 9, 433–439.
- Rockhill, B.; Willett, W.C.; Hunter, D.J.; Manson, J.E.; Hankinson, S.E.; Colditz, G.A. A prospective study of recreational physical activity and breast cancer risk. Arch. Intern. Med. 1999, 159, 2290–2296.
- Luoto, R.; Latikka, P.; Pukkala, E.; Hakulinen, T.; Vihko, V. The effect of physical activity on breast cancer risk: A cohort study of 30,548 women. Eur. J. Epidemiol. 2000, 16, 973–980.
- Wyrwich, K.W.; Wolinsky, F.D. Physical activity, disability, and the risk of hospitalization for breast cancer among older women. J. Gerontol. Ser. A 2000, 55, M418–M421.
- Breslow, R.A.; Ballard-Barbash, R.; Munoz, K.; Graubard, B.I. Long-term recreational physical activity and breast cancer in the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer Epidemiol. Biomark. Prev. 2001, 10, 805–808.
- Lee, I.-M.; Rexrode, K.M.; Cook, N.R.; Hennekens, C.H.; Burin, J.E.; Buring, J.E. Physical activity and breast cancer risk: The Women’s Health Study (United States). Cancer Causes Control 2001, 12, 137–145.
- Moradi, T.; Adami, H.-O.; Ekbom, A.; Wedrén, S.; Terry, P.; Floderus, B.; Lichtenstein, P. Physical activity and risk for breast cancer a prospective cohort study among Swedish twins. Int. J. Cancer 2002, 100, 76–81.
- Rintala, P.E.; Pukkala, E.; Paakkulainen, H.T.; Vihko, V.J. Self-experienced physical workload and risk of breast cancer. Scand. J. Work Environ. Health 2002, 28, 158–162.
- Patel, A.V.; Calle, E.E.; Bernstein, L.; Wu, A.H.; Thun, M.J. Recreational physical activity and risk of postmenopausal breast cancer in a large cohort of US women. Cancer Causes Control 2003, 14, 519–529.
- Margolis, K.L.; Mucci, L.; Braaten, T.; Kumle, M.; Lagerros, Y.T.; Adami, H.-O.; Lund, E.; Weiderpass, E. Physical Activity in Different Periods of Life and the Risk of Breast Cancer: The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 27–32.
- Bardia, A.; Hartmann, L.C.; Vachon, C.M. Recreational Physical Activity and Risk of Postmenopausal Breast Cancer Based on Hormone Receptor Status. Arch. Intern. Med. 2006, 166, 2478–2483.
- Mertens, A.J.; Sweeney, C.; Shahar, E.; Rosamond, W.D.; Folsom, A.R. Physical activity and breast cancer incidence in middle-aged women: A prospective cohort study. Breast Cancer Res. Treat. 2005, 97, 209–214.
- Silvera, S.A.N.; Jain, M.; Howe, G.R.; Miller, A.B.; Rohan, T.E. Energy balance and breast cancer risk: A prospective cohort study. Breast Cancer Res. Treat. 2005, 97, 97–106.
- Tehard, B.; Friedenreich, C.M.; Oppert, J.-M.; Clavel-Chapelon, F. Effect of Physical Activity on Women at Increased Risk of Breast Cancer: Results from the E3N Cohort Study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 57–64.
- Dallal, C.M.; Sullivan-Halley, J.; Ross, R.K.; Wang, Y.; Deapen, D.; Horn-Ross, P.L.; Reynolds, P.; Stram, D.O.; Clarke, C.A.; Anton-Culver, H.; et al. Long-term Recreational Physical Activity and Risk of Invasive and In Situ Breast Cancer. Arch. Intern. Med. 2007, 167, 408–415.
- Leitzmann, M.F.; Moore, S.C.; Peters, T.M.; Lacey, J.V.; Schatzkin, A.; Schairer, C.; Brinton, L.A.; Albanes, D. Prospective study of physical activity and risk of postmenopausal breast cancer. Breast Cancer Res. 2008, 10, R92.
- Maruti, S.S.; Willett, W.C.; Feskanich, D.; Rosner, B.; Colditz, G.A. A Prospective Study of Age-Specific Physical Activity and Premenopausal Breast Cancer. Gynecol. Oncol. 2008, 100, 728–737.
- Suzuki, S.; Kojima, M.; Tokudome, S.; Mori, M.; Sakauchi, F.; Fujino, Y.; Wakai, K.; Lin, Y.; Kikuchi, S.; Tamakoshi, K.; et al. Effect of Physical Activity on Breast Cancer Risk: Findings of the Japan Collaborative Cohort Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3396–3401.
- Howard, R.A.; Leitzmann, M.F.; Linet, M.S.; Freedman, D.M. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control 2008, 20, 323–333.
- Chang, S.-C.; Ziegler, R.G.; Dunn, B.; Stolzenberg-Solomon, R.; Lacey, J.V.; Huang, W.-Y.; Schatzkin, A.; Reding, D.; Hoover, R.N.; Hartge, P.; et al. Association of Energy Intake and Energy Balance with Postmenopausal Breast Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 334–341.
- Schnohr, P.; Grønbaek, M.; Petersen, L.V.; Hein, H.O.; Sørensen, T.I.A. Physical activity in leisure-time and risk of cancer: 14-year follow-up of 28,000 Danish men and women. Scand. J. Public Health 2005, 33, 244–249.
- McTiernan, A.; Kooperberg, C.L.; White, E.; Wilcox, S.; Coates, R.J.; Adams-Campbell, L.L.; Woods, N.F.; Ockene, J.K. Recreational Physical Activity and the Risk of Breast Cancer in Postmenopausal Women. JAMA 2003, 290, 1331–1336.
- Wu, Y.; Zhang, D.; Kang, S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 137, 869–882.
- Hardefeldt, P.J.; Penninkilampi, R.; Edirimanne, S.; Eslick, G.D. Physical Activity and Weight Loss Reduce the Risk of Breast Cancer: A Meta-analysis of 139 Prospective and Retrospective Studies. Clin. Breast Cancer 2018, 18, e601–e612.
- Chen, X.; Wang, Q.; Zhang, Y.; Xie, Q.; Tan, X. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health 2018, 22, 104–128.
- Endicott, J.A.; Ling, V. The biochemistry of p-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 1989, 58, 137–171.
- Gottesman, M.M.; Hrycyna, C.A.; Schoenlein, P.V.; Germann, U.A.; Pastan, I. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 1995, 29, 607–649.
- Germann, U. P-glycoprotein—A mediator of multidrug resistance in tumour cells. Eur. J. Cancer 1996, 32, 927–944.
- Catsburg, C.; Kirsh, V.A.; Soskolne, C.L.; Kreiger, N.; Bruce, E.; Ho, T.; Leatherdale, S.T.; Rohan, T.E. Associations between anthropometric characteristics, physical activity, and breast cancer risk in a Canadian cohort. Breast Cancer Res. Treat. 2014, 145, 545–552.
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578.
- Van den Brandt, P.A.; Spiegelman, N.; Yaun, S.-S.; Adami, H.-O.; Beeson, L.; Folsom, A.R.; Fraser, G.; Goldbohm, R.A.; Graham, S.; Kushi, L.; et al. Pooled Analysis of Prospective Cohort Studies on Height, Weight, and Breast Cancer Risk. Am. J. Epidemiol. 2000, 152, 514–527.
- Bergström, A.; Pisani, P.; Tenet, V.; Wolk, A.; Adami, H. Overweight as an avoidable cause of cancer in Europe. Int. J. Cancer 2000, 91, 421–430.
- Ursin, G.; Longnecker, M.; Haik, R.W.; Greenland, S. A Meta-analysis of Body Mass Index and Risk of Premenopausal Breast Cancer. Epidemiology 1995, 6, 137–141.
- Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O’Brien, K.M.; Adami, H.-O.; Baglietto, L.; Bernstein, L.; The Premenopausal Breast Cancer Collaborative Group; et al. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771.
- Michels, K.B.; Terry, K.L.; Willett, W.C. Longitudinal Study on the Role of Body Size in Premenopausal Breast Cancer. Arch. Intern. Med. 2006, 166, 2395–2402.
- Harris, H.R.; Willett, W.C.; Terry, K.L.; Michels, K.B. Body Fat Distribution and Risk of Premenopausal Breast Cancer in the Nurses’ Health Study II. Gynecol. Oncol. 2010, 103, 273–278.
- Rose, D.P.; Vona-Davis, L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 2010, 66, 33–38.
- Vrieling, A.; Buck, K.; Kaaks, R.; Chang-Claude, J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: A meta-analysis. Breast Cancer Res. Treat. 2010, 123, 641–649.
- Eliassen, A.H.; Colditz, G.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Adult Weight Change and Risk of Postmenopausal Breast Cancer. JAMA 2006, 296, 193–201.
- Emaus, M.J.; Van Gils, C.H.; Bakker, M.F.; Bisschop, C.N.S.; Monninkhof, E.M.; Bueno-De-Mesquita, H.B.; Travier, N.; Berentzen, T.L.; Overvad, K.; Tjonneland, A.; et al. Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study. Int. J. Cancer 2014, 135, 2887–2899.
- Rosner, B.; Eliassen, A.H.; Toriola, A.T.; Hankinson, S.E.; Willett, W.C.; Natarajan, L.; Colditz, G.A. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res. Treat. 2015, 150, 643–653.
- Hall, I.J.; Newman, B.; Millikan, R.C.; Moorman, P.G. Body size and breast cancer risk in black women and white women: The Carolina Breast Cancer Study. Am. J. Epidemiol. 2000, 151, 754–764.
- Robinson, W.R.; Tse, C.K.; Olshan, A.F.; Troester, M.A. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer Causes Control 2014, 25, 1101–1117.
- Swanson, C.A.; Coates, R.J.; Malone, K.E.; Gammon, M.D.; Schoenberg, J.B.; Brogan, D.J.; McAdams, M.; Potischman, N.; Hoover, R.N.; Brinton, L.A. Alcohol Consumption and Breast Cancer Risk among Women under Age 45 Years. Epidemiology 1997, 8, 231–237.
- Godinho-Mota, J.C.M.; Gonçalves, L.V.; Mota, J.F.; Soares, L.R.; Schincaglia, R.M.; Martins, K.A.; Freitas-Junior, R. Sedentary Behavior and Alcohol Consumption Increase Breast Cancer Risk Regardless of Menopausal Status: A Case-Control Study. Nutrients 2019, 11, 1871.
- Zhang, S.M.; Lee, I.-M.; Manson, J.E.; Cook, N.R.; Willett, W.C.; Buring, J.E. Alcohol Consumption and Breast Cancer Risk in the Women’s Health Study. Am. J. Epidemiol. 2007, 165, 667–676.
- Chen, W.Y.; Rosner, B.; Hankinson, S.E.; Colditz, G.; Willett, W.C. Moderate Alcohol Consumption During Adult Life, Drinking Patterns, and Breast Cancer Risk. JAMA 2011, 306, 1884–1890.
- Jung, S.; Wang, M.; Anderson, K.; Baglietto, L.; Bergkvist, L.; Bernstein, L.; Brandt, P.A.V.D.; Brinton, L.; Buring, J.E.; Eliassen, A.H.; et al. Alcohol consumption and breast cancer risk by estrogen receptor status: In a pooled analysis of 20 studies. Leuk. Res. 2015, 45, 916–928.
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Breast Cancer: A Global Perspective; World Cancer Research Fund: London, UK, 2017.
- MacMahon, B.; Trichopoulos, D.; Cole, P.; Brown, J. Cigarette Smoking and Urinary Estrogens. N. Engl. J. Med. 1982, 307, 1062–1065.
- Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer—Collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br. J. Cancer 2002, 87, 1234–1245.
- Johnson, K.C. Accumulating evidence on passive and active smoking and breast cancer risk. Int. J. Cancer 2005, 117, 619–628.
- Slattery, M.L.; Curtin, K.; Giuliano, A.R.; Sweeney, C.; Baumgartner, R.; Edwards, S.; Wolff, R.K.; Baumgartner, K.B.; Byers, T. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res. Treat. 2007, 109, 101–111.
- Jones, M.E.; Schoemaker, M.J.; Wright, L.B.; Ashworth, A.; Swerdlow, A.J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 2017, 19, 1–14.
- Morabia, A.; Bemstein, M.; Héritier, S.; Khatchatrian, N. Relation of Breast Cancer with Passive and Active Exposure to Tobacco Smoke. Am. J. Epidemiol. 1996, 143, 918–928.
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224.
- Tang, L.-Y.; Chen, L.-J.; Qi, M.-L.; Su, Y.; Su, F.-X.; Lin, Y.; Wang, K.-P.; Jia, W.-H.; Zhuang, Z.-X.; Ren, Z.-F. Effects of passive smoking on breast cancer risk in pre/post-menopausal women as modified by polymorphisms of PARP1 and ESR1. Gene 2013, 524, 84–89.
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32.
- Pudrovska, T.; Anikputa, B. The Role of Early-Life Socioeconomic Status in Breast Cancer Incidence and Mortality. J. Aging Health 2011, 24, 323–344.
- Hansen, J. Night Shift Work and Risk of Breast Cancer. Curr. Environ. Health Rep. 2017, 4, 325–339.
- Nooshinfar, E.; Safaroghli-Azar, A.; Bashash, D.; Akbari, M.E. Melatonin, an inhibitory agent in breast cancer. Breast Cancer 2016, 24, 42–51.
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540.
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragones, N.; Pérez-Gómez, B.; Ardanaz, E.; Altzibar, J.M.; Sanchez, V.M.; Gomez-Acebo, I.; Llorca, J.; et al. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur. J. Epidemiol. 2015, 31, 867–878.
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568.
- Cordina-Duverger, E.; Menegaux, F.; Popa, A.; Rabstein, S.; Harth, V.; Pesch, B.; Brüning, T.; Fritschi, L.; Glass, D.C.; Heyworth, J.S.; et al. Night shift work and breast cancer: A pooled analysis of population-based case–control studies with complete work history. Eur. J. Epidemiol. 2018, 33, 369–379.
- Apostolou, P.; Fostira, F. Hereditary Breast Cancer: The Era of New Susceptibility Genes. BioMed Res. Int. 2013, 2013, 747318.
- Economopoulou, P.; Dimitriadis, G.; Psyrri, A. Beyond BRCA: New hereditary breast cancer susceptibility genes. Cancer Treat. Rev. 2015, 41, 1–8.
- Rich, T.A.; Woodson, A.H.; Litton, J.; Arun, B. Hereditary breast cancer syndromes and genetic testing. J. Surg. Oncol. 2014, 111, 66–80.
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Breast Cancer Association Consortium; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439.
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451.
- Siddig, A.; Din, T.T.; Nafi, S.M.; Yahya, M.; Sulong, S.; Rahman, W.W.A. The Unique Biology behind the Early Onset of Breast Cancer. Genes 2021, 12, 372.
- Narod, S.A.; Foulkes, W. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 2004, 4, 665–676.
- Malone, K.E.; Daling, J.R.; Neal, C.; Suter, N.M.; O’Brien, C.; Cushing-Haugen, K.; Jonasdottir, T.J.; Thompson, J.D.; Ostrander, E.A. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer 2000, 88, 1393–1402.
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180.
- Soussi, T.; Wiman, K.G. TP53: An oncogene in disguise. Cell Death Differ. 2015, 22, 1239–1249.
- Masciari, S.; Dillon, D.A.; Rath, M.; Robson, M.; Weitzel, J.N.; Balmana, J.; Gruber, S.B.; Ford, J.M.; Euhus, D.; Lebensohn, A.; et al. Breast cancer phenotype in women with TP53 germline mutations: A Li-Fraumeni syndrome consortium effort. Breast Cancer Res. Treat. 2012, 133, 1125–1130.
- Lee, D.S.; Yoon, S.-Y.; Looi, L.M.; Kang, P.; Kang, I.N.; Sivanandan, K.; Ariffin, H.; Thong, M.K.; Chin, K.F.; Taib, N.A.M.; et al. Comparable frequency of BRCA1, BRCA2 and TP53 germline mutations in a multi-ethnic Asian cohort suggests TP53 screening should be offered together with BRCA1/2 screening to early-onset breast cancer patients. Breast Cancer Res. 2012, 14, R66.
- Gonzalez, K.D.; Noltner, K.A.; Buzin, C.H.; Gu, D.; Wen-Fong, C.Y.; Nguyen, V.Q.; Han, J.H.; Lowstuter, K.; Longmate, J.; Sommer, S.S.; et al. Beyond Li Fraumeni Syndrome: Clinical Characteristics of Families With p53 Germline Mutations. J. Clin. Oncol. 2009, 27, 1250–1256.
- Damineni, S.; Rao, V.R.; Kumar, S.; Ravuri, R.R.; Kagitha, S.; Dunna, N.R.; Digumarthi, R.; Satti, V. Germline mutations of TP53 gene in breast cancer. Tumor Biol. 2014, 35, 9219–9227.
- Mouchawar, J.; Korch, C.; Byers, T.; Pitts, T.M.; Li, E.; McCredie, M.R.; Giles, G.G.; Hopper, J.L.; Southey, M.C. Population-Based Estimate of the Contribution of TP53 Mutations to Subgroups of Early-Onset Breast Cancer: Australian Breast Cancer Family Study. Cancer Res. 2010, 70, 4795–4800.
- McCuaig, J.M.; Armel, S.R.; Novokmet, A.; Ginsburg, O.; Demsky, R.; Narod, S.A.; Malkin, D. Routine TP53 testing for breast cancer under age 30: Ready for prime time? Fam. Cancer 2012, 11, 607–613.
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296.
- Pulido, R. PTEN: A yin-yang master regulator protein in health and disease. Methods 2015, 77–78, 3–10.
- Hobert, J.A.; Eng, C. PTEN hamartoma tumor syndrome: An overview. Anesth. Analg. 2009, 11, 687–694.
- Tan, M.-H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin. Cancer Res. 2012, 18, 400–407.
- Pradella, L.M.; Evangelisti, C.; Ligorio, C.; Ceccarelli, C.; Neri, I.; Zuntini, R.; Amato, L.B.; Ferrari, S.; Martelli, A.M.; Gasparre, G.; et al. A novel deleterious PTEN mutation in a patient with early-onset bilateral breast cancer. BMC Cancer 2014, 14, 70.
- Turpin, A.; Cattan, S.; Leclerc, J.; Wacrenier, A.; Manouvrier-Hanu, S.; Buisine, M.-P.; Lejeune-Dumoulin, S. Prédisposition héréditaire aux cancers digestifs, mammaires, gynécologiques et gonadiques: État des lieux du syndrome de Peutz-Jeghers. Bull. du Cancer 2014, 101, 813–822.
- Hearle, N.; Schumacher, V.; Menko, F.H.; Olschwang, S.; Boardman, L.A.; Gille, J.J.; Keller, J.J.; Westerman, A.M.; Scott, R.J.; Lim, W.; et al. Frequency and Spectrum of Cancers in the Peutz-Jeghers Syndrome. Clin. Cancer Res. 2006, 12, 3209–3215.
- Pharoah, P.D.; Guilford, P.; Caldas, C.; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001, 121, 1348–1353.
- Brooks-Wilson, A.R.; Kaurah, P.; Suriano, G.; Leach, S.; Senz, J.; Grehan, N.; Butterfield, Y.S.N.; Jeyes, J.; Schinas, J.; Bacani, J.; et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: Assessment of 42 new families and review of genetic screening criteria. J. Med. Genet. 2004, 41, 508–517.
- Suriano, G.; Yew, S.; Ferreira, P.; Senz, J.; Kaurah, P.; Ford, J.M.; Longacre, T.A.; Norton, J.A.; Chun, N.; Young, S.; et al. Characterization of a Recurrent Germ Line Mutation of the E-Cadherin Gene: Implications for Genetic Testing and Clinical Management. Clin. Cancer Res. 2005, 11, 5401–5409.
- Kluijt, I.; Sijmons, R.H.; Hoogerbrugge, N.; Plukker, J.T.; De Jong, D.; van Krieken, J.; Van Hillegersberg, R.; Ligtenberg, M.; Bleiker, E.; Cats, A. Familial gastric cancer: Guidelines for diagnosis, treatment and periodic surveillance. Fam. Cancer 2012, 11, 363–369.
- Petridis, C.; Shinomiya, I.; Kohut, K.; Gorman, P.; Caneppele, M.; Shah, V.; Troy, M.; Pinder, S.E.; Hanby, A.; Tomlinson, I.; et al. Germline CDH1 mutations in bilateral lobular carcinoma in situ. Br. J. Cancer 2013, 110, 1053–1057.
- Xie, Z.M.; Li, L.S.; Laquet, C.; Xie, X.M.; Penault-Llorca, F.; Uhrhammer, N.; Bignon, Y.J. Germline mutations of the E-cadherin gene in families with inherited invasive lobular breast carcinoma but no diffuse gastric cancer. Cancer 2011, 117, 3112–3117.
- Schrader, K.A.; Masciari, S.; Boyd, N.; Salamanca, C.; Senz, J.; Saunders, D.N.; Yorida, E.; Maines-Bandiera, S.; Kaurah, P.; Tung, N.; et al. Germline mutations in CDH1 are infrequent in women with early-onset or familial lobular breast cancers. J. Med. Genet. 2010, 48, 64–68.
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506.
- Weischer, M.; Bojesen, S.E.; Ellervik, C.; Tybjærg-Hansen, A.; Nordestgaard, B.G. CHEK2*1100delC Genotyping for Clinical Assessment of Breast Cancer Risk: Meta-Analyses of 26,000 Patient Cases and 27,000 Controls. J. Clin. Oncol. 2008, 26, 542–548.
- Rashid, M.U.; Muhammad, N.; Faisal, S.; Amin, A.; Hamann, U. Constitutional CHEK2mutations are infrequent in early-onset and familial breast/ovarian cancer patients from Pakistan. BMC Cancer 2013, 13, 312.
- Wang, N.; Ding, H.; Liu, C.; Li, X.; Wei, L.; Yu, J.; Liu, M.; Ying, M.; Gao, W.; Jiang, H.; et al. A novel recurrent CHEK2 Y390C mutation identified in high-risk Chinese breast cancer patients impairs its activity and is associated with increased breast cancer risk. Oncogene 2015, 34, 5198–5205.
- Thompson, D.; Duedal, S.; Kirner, J.; McGuffog, L.; Last, J.; Reiman, A.; Byrd, P.; Taylor, M.; Easton, D.F. Cancer Risks and Mortality in Heterozygous ATM Mutation Carriers. Gynecol. Oncol. 2005, 97, 813–822.
- Maillet, P.; Bonnefoi, H.; Vaudan-Vutskits, G.; Pajk, B.; Cufer, T.; Foulkes, W.; Chappuis, P.O.; Sappino, A.-P. Constitutional alterations of the ATM gene in early onset sporadic breast cancer. J. Med. Genet. 2002, 39, 751–753.
- Teraoka, S.N.; Malone, K.E.; Doody, D.; Suter, N.M.; Ostrander, E.; Daling, J.R.; Concannon, P. Increased frequency of ATM mutations in breast carcinoma patients with early onset disease and positive family history. Cancer 2001, 92, 479–487.
- Morrell, D.; Cromartie, E.; Swift, M. Mortality and Cancer Incidence in 263 Patients With Ataxia-Telangiectasia2. Gynecol. Oncol. 1986, 77, 89–92.
- Lin, P.-H.; Kuo, W.-H.; Huang, A.-C.; Lu, Y.-S.; Lin, C.-H.; Kuo, S.-H.; Wang, M.-Y.; Liu, C.-Y.; Cheng, F.T.-F.; Yeh, M.-H.; et al. Multiple gene sequencing for risk assessment in patients with early-onset or familial breast cancer. Oncotarget 2016, 7, 8310–8320.
- Izatt, L.; Greenman, J.; Hodgson, S.; Ellis, D.; Watts, S.; Scott, G.; Jacobs, C.; Liebmann, R.; Zvelebil, M.J.; Mathew, C.; et al. Identification of germline missense mutations and rare allelic variants in the ATM gene in early-onset breast cancer. Genes Chromosomes Cancer 1999, 26, 286–294.
- Byrnes, G.B.; Southey, M.C.; Hopper, J.L. Are the so-called low penetrance breast cancer genes, ATM, BRIP1, PALB2 and CHEK2, high risk for women with strong family histories? Breast Cancer Res. 2008, 10, 208.
- Churpek, J.E.; Walsh, T.; Zheng, Y.; Moton, Z.; Thornton, A.M.; Lee, M.K.; Casadei, S.; Watts, A.; Neistadt, B.; Churpek, M.M.; et al. Inherited predisposition to breast cancer among African American women. Breast Cancer Res. Treat. 2014, 149, 31–39.
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; et al. Inherited Mutations in 17 Breast Cancer Susceptibility Genes Among a Large Triple-Negative Breast Cancer Cohort Unselected for Family History of Breast Cancer. J. Clin. Oncol. 2015, 33, 304–311.
- McInerney, N.M.; Miller, N.; Rowan, A.; Colleran, G.; Barclay, E.; Curran, C.; Kerin, M.; Tomlinson, I.P.; Sawyer, E. Evaluation of variants in the CHEK2, BRIP1 and PALB2 genes in an Irish breast cancer cohort. Breast Cancer Res. Treat. 2009, 121, 203–210.
- Cao, A.-Y.; Huang, J.; Hu, Z.; Li, W.-F.; Ma, Z.-L.; Tang, L.-L.; Zhang, B.; Su, F.-X.; Zhou, J.; Di, G.-H.; et al. Mutation analysis of BRIP1/BACH1 in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res. Treat. 2008, 115, 51–55.
- Stacey, S.N.; Sulem, P.; Johannsson, O.T.; Helgason, A.; Gudmundsson, J.; Kostic, J.P.; Kristjansson, K.; Jonsdottir, T.; Sigurdsson, H.; Hrafnkelsson, J.; et al. The BARD1 Cys557Ser Variant and Breast Cancer Risk in Iceland. PLoS Med. 2006, 3, e217.
- Sun, G.; Wang, J.T.; Ma, B.L.; Geng, Z.L.; Ren, G.H.; Shan, M.H.; Ma, B.; Ma, L.L.; Wang, Y. Association between single nucleotide polymorphisms of BARD 1 gene and susceptibility of early-onset breast cancer in Uygur women in Xinjiang. Zhonghua Zhong Liu Za Zhi 2012, 34, 341–347.
- Young, E.; Feng, B.J.; Stark, A.W.; Damiola, F.; Durand, G.; Forey, N.; Francy, T.C.; Gammon, A.; Kohlmann, W.K.; Kaphingst, K.A.; et al. Multigene testing of moderate-risk genes: Be mindful of the missense. J. Med. Genet. 2016, 53, 366–376.
- Ghimenti, C.; Sensi, E.; Presciuttini, S.; Brunetti, I.M.; Conte, P.; Bevilacqua, G.; Caligo, M.A. Germline mutations of the BRCA1-associated ring domain (BARD1) gene in breast and breast/ovarian families negative for BRCA1 and BRCA2 alterations. Genes Chromosomes Cancer 2002, 33, 235–242.
- Karppinen, S.-M.; Heikkinen, K.; Rapakko, K.; Winqvist, R. Mutation screening of the BARD1 gene: Evidence for involvement of the Cys557Ser allele in hereditary susceptibility to breast cancer. J. Med. Genet. 2004, 41, e114.
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated With Pathogenic Variants in RAD51C and RAD51D. Gynecol. Oncol. 2020, 112, 1242–1250.
- Barnholtz-Sloan, J.S.; Shetty, P.B.; Guan, X.; Nyante, S.J.; Luo, J.; Brennan, D.J.; Millikan, R.C. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis 2010, 31, 1417–1423.
- Michailidou, K.; Beesley, J.; Lindstrom, S.; Canisius, S.; Dennis, J.; Lush, M.J.; Maranian, M.J.; Bolla, M.K.; Wang, Q.; Shah, M.; et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 2015, 47, 373–380.
- Ahsan, H.; Halpern, J.; Kibriya, M.G.; Pierce, B.L.; Tong, L.; Gamazon, E.; McGuire, V.; Felberg, A.; Shi, J.; Jasmine, F.; et al. A Genome-wide Association Study of Early-Onset Breast Cancer Identifies PFKM as a Novel Breast Cancer Gene and Supports a Common Genetic Spectrum for Breast Cancer at Any Age. Cancer Epidemiol. Biomark. Prev. 2014, 23, 658–669.
- Si, S.; Boyle, T.; Heyworth, J.; Glass, D.; Saunders, C.; Fritschi, L. Lifetime physical activity and risk of breast cancer in pre-and post-menopausal women. Breast Cancer Res. Treat. 2015, 152, 449–462.
- Masala, G.; Bendinelli, B.; Assedi, M.; Occhini, D.; Zanna, I.; Sieri, S.; Agnoli, C.; Sacerdote, C.; Ricceri, F.; Mattiello, A.; et al. Up to one-third of breast cancer cases in post-menopausal Mediterranean women might be avoided by modifying lifestyle habits: The EPIC Italy study. Breast Cancer Res. Treat. 2016, 161, 311–320.
- Borghesan, D.H.P.; Agnolo, C.M.D.; Gravena, A.A.F.; Demitto, M.D.O.; Lopes, T.C.R.; Carvalho, M.D.D.B.; Pelloso, S.M. Risk Factors for Breast Cancer in Postmenopausal Women in Brazil. Asian Pac. J. Cancer Prev. 2016, 17, 3587–3593.
- Conz, L.; Mota, B.S.; Bahamondes, L.; Dória, M.T.; Derchain, S.F.M.; Rieira, R.; Sarian, L.O. Levonorgestrel-releasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2020, 99, 970–982.
- Ma, H.; Henderson, K.D.; Sullivan-Halley, J.; Duan, L.; Marshall, S.F.; Ursin, G.; Horn-Ross, P.L.; Largent, J.; Deapen, D.M.; Lacey, J.V.; et al. Pregnancy-related factors and the risk of breast carcinoma in situand invasive breast cancer among postmenopausal women in the California Teachers Study cohort. Breast Cancer Res. 2010, 12, R35.
- Daniels, K.; Abma, J. Current Contraceptive Status among Women Aged 15–49: United States, 2015–2017; NCHS Data Brief, No 327; National Center for Health Statistics: Hyattsville, MD, USA, 2018.
- The Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727.
- Vaz-Luis, I.; Partridge, A.H. Exogenous reproductive hormone use in breast cancer survivors and previvors. Nat. Rev. Clin. Oncol. 2018, 15, 249–261.
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N. Engl. J. Med. 2017, 377, 2228–2239.
- Soini, T.; Hurskainen, R.; Grénman, S.; Mäenpää, J.; Paavonen, J.; Pukkala, E. Cancer Risk in Women Using the Levonorgestrel-Releasing Intrauterine System in Finland. Obstet. Gynecol. 2014, 124, 292–299.
- Zolfaroli, I.; Tarín, J.J.; Cano, A. Hormonal contraceptives and breast cancer: Clinical data. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 212–216.
- Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/cg164 (accessed on 7 October 2022).
- MacMahon, B.; Cole, P.; Lin, T.M.; Lowe, C.R.; Mirra, A.P.; Ravnihar, B.; Salber, E.J.; Valaoras, V.G.; Yuasa, S. Age at first birth and breast cancer risk. Bull. World Health Organ. 1970, 43, 209–221.
- Albrektsen, G.; Heuch, I.; Hansen, S.; Kvåle, G. Breast cancer risk by age at birth, time since birth and time intervals between births: Exploring interaction effects. Br. J. Cancer 2004, 92, 167–175.
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.-A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336.
- Nichols, H.B.; Schoemaker, M.J.; Cai, J.; Xu, B.J.; Wright, M.L.B.; Brook, M.N.; Jones, M.E.; Adami, H.-O.; Baglietto, L.; Bertrand, S.K.A.; et al. Breast Cancer Risk After Recent Childbirth. Ann. Intern. Med. 2018, 170, 22–30.
- Hall, I.J.; Moorman, P.G.; Millikan, R.C.; Newman, B. Comparative Analysis of Breast Cancer Risk Factors among African-American Women and White Women. Am. J. Epidemiol. 2005, 161, 40–51.
- Yeo, W.; Lee, H.-M.; Chan, A.; Chan, E.Y.; Chan, M.C.; Chan, K.-W.; Chan, S.W.; Cheung, F.-Y.; Cheung, P.S.; Choi, P.H.; et al. Risk factors and natural history of breast cancer in younger Chinese women. World J. Clin. Oncol. 2014, 5, 1097–1106.
- Derks-Smeets, I.A.P.; Schrijver, L.H.; de Die-Smulders, C.E.M.; Tjan-Heijnen, V.C.G.; van Golde, R.J.T.; Smits, L.J.; Caanen, B.; van Asperen, C.J.; Ausems, M.; Collée, M.; et al. Ovarian stimulation for IVF and risk of primary breast cancer in BRCA1/2 mutation carriers. Br. J. Cancer 2018, 119, 357–363.
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Use of fertility treatments in BRCA1/2 mutation carriers and risk for ovarian and breast cancer: A systematic review. Arch. Gynecol. Obstet. 2020, 302, 715–720.
- Ambrosone, C.B.; Higgins, M.J. Relationships between Breast Feeding and Breast Cancer Subtypes: Lessons Learned from Studies in Humans and in Mice. Cancer Res. 2020, 80, 4871–4877.
- Britt, K.L.; Cuzick, J.; Phillips, K.-A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. Lancet 2002, 360, 187–195.
- Ursin, G.; Bernstein, L.; Lord, S.J.; Karim, R.; Deapen, D.; Press, M.F.; Daling, J.R.; Norman, S.A.; Liff, J.M.; Marchbanks, P.A.; et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br. J. Cancer 2005, 93, 364–371.
- Islami, F.; Liu, Y.; Jemal, A.; Zhou, J.; Weiderpass, E.; Colditz, G.; Boffetta, P.; Weiss, M. Breastfeeding and breast cancer risk by receptor status—A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2398–2407.
- Global Strategy for Infant and Young Child Feeding. Available online: https://www.who.int/publications-detail-redirect/9241562218 (accessed on 7 October 2022).
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
885
Revisions:
3 times
(View History)
Update Date:
24 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




